Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
The Programmed Cell Death Ligand 1 and Lipocalin 2 Expressions in Primary Breast Cancer and Their Associations with Molecular Subtypes and Prognostic Factors
Authors Ekemen S , Bilir E , Soultan HEA, Zafar S , Demir F, Tabandeh B, Toprak S , Yapicier O, Coban C
Received 24 October 2023
Accepted for publication 21 December 2023
Published 3 January 2024 Volume 2024:16 Pages 1—13
DOI https://doi.org/10.2147/BCTT.S444077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Suheyla Ekemen,1,2 Ebru Bilir,3 Hagar Elsayed Akram Soultan,3 Sadia Zafar,3 Figen Demir,4 Babek Tabandeh,5 Sadik Toprak,6 Ozlem Yapicier,7 Cevayir Coban2,8,9
1Vocational School of Health Services, Acibadem University, Istanbul, Turkey; 2Division of Malaria Immunology, Department of Microbiology and Immunology, Institute of Medical Science (IMSUT), the University of Tokyo, Tokyo, Japan; 3Residency Program, Bahcesehir University School of Medicine, Istanbul, Turkey; 4Department of Public Health, Acibadem University School of Medicine, Istanbul, Turkey; 5Department of General Surgery, Bahcesehir University School of Medicine, Istanbul, Turkey; 6Department of Forensic Medicine, Istanbul University School of Medicine, Istanbul, Turkey; 7Department of Pathology, Bahcesehir University School of Medicine, Istanbul, Turkey; 8Immunology Frontier Research Center (IFReC), Osaka University, Osaka, Japan; 9International Vaccine Design Center, Institute of Medical Science (IMSUT), the University of Tokyo, Tokyo, Japan
Correspondence: Suheyla Ekemen, Division of Malaria Immunology, Department of Microbiology and Immunology the Institute of Medical Science (IMSUT), the University of Tokyo Building 1, 4-6-1 Shirokanedai, Minato-ku, Tokyo, 108-8639, Japan, Email [email protected]; [email protected]
Purpose: Breast cancers exhibit molecular heterogeneity, leading to diverse clinical outcomes and therapeutic responses. Immune checkpoint inhibitors targeting PD-L1 have shown promise in various malignancies, including breast cancer. Lipocalin 2 (LCN2) has also been associated with tumor aggressiveness and prognostic potential in breast cancers. However, the expression of PD-L1 and LCN2 in breast cancer subtypes and their prognostic implications remains poorly investigated.
Methods: A retrospective analysis of 89 primary breast cancer cases was conducted to assess PD-L1 and LCN2 expressions using immunohistochemistry. Cases were classified into four different molecular subtypes based on ER, PR, HER2, and Ki-67 status. Associations between PD-L1 and LCN2 expressions and various prognostic factors were examined.
Results: Although low expression of LCN2 (Allred score of < 3) was observed even in normal breast tissue, LCN2 expression with increasing Allred score (≥ 3) positively correlated with the histological grade, high Ki-67 proliferation index, and ER/PR negativity. Significant elevations of LCN2 and PD-L1 expressions were observed in triple-negative and HER2-positive breast cancers.
Conclusion: The results of the study highlight the association of LCN2 with known prognostic factors and molecular subtypes. To identify potential immunotherapy recipients, it would be useful to evaluate LCN2 as well as PD-L1 immune targets in different subgroups of breast cancer patients. Further studies with larger patient numbers are warranted to validate these observations and establish standardized scoring criteria for LCN2 expression assessment.
Keywords: breast cancer, immunotherapy, PD-L1, LCN2, triple-negative breast cancer, TNBC
Introduction
Breast cancer is a tumor with different morphologies and complex molecular subtypes, and therefore has various prognostic outcomes that require different treatment approaches. A widely accepted breast cancer classification comprise at least four distinct molecular subtypes based on immunohistochemical evaluation of hormone receptors: Luminal A, Luminal B, Human Epidermal Growth Factor Receptor 2 positive (HER2+), and triple-negative breast cancer (TNBC).1 Especially in TNBCs, which are characterized by the absence of Estrogen receptor (ER), Progesterone receptor (PR), and HER2 expressions, chemotherapy has been the sole treatment option for many years due to the lack of response to hormonal therapy.2 However, since the contribution of the immune system in protecting against cancer as well as its involvement in shaping neoplastic disease (cancer immunoediting) has been discovered, immunotherapy has also become a part of treatment protocols for breast cancers.2–5 Immunotherapy is related to the dual function of the immune system in both preventing cancer formation and influencing tumor development.6
In the early phases of carcinogenesis, innate immunity is activated by the acute inflammation present in the breast tissue.7 Acute inflammation stimulates macrophages to respond against the tumor. On the other hand, tumor-associated macrophages (TAMs) release cytokines to help with tumor progression and reduce the effect of tumor-infiltrating lymphocytes (TILs) while increasing the regulatory T-cells which helps the tumor evade immune checkpoints.8 TAMs also modulate the expression of Programmed Cell Death-1 (PD-1), a cell membrane protein, found on T cells and other immune cells. PD-1, when bound to its ligands, PD-L1 and PD-L2, found on macrophages and antigen-presenting cells stimulate the production of regulatory T cells and inhibit the immune system, allowing for tumor progression.9,10 Immunotherapy targeting this protein, its receptor, and ligands, especially PD-L1, has shown promising results in treating breast cancer, especially against TNBC.4,11 Moreover, PD-L1 overexpression in tumor cells has also been associated with poor prognostic features such as high tumor grade, negative ER status, negative PR status, and positive HER2-neu and Ki-67 status.8 There are several studies indicating the immunotherapeutic significance of PD-L1 as an important prognostic marker in TNBC cases.5,12–14
Lipocalin 2 (LCN2, also known as siderocalin or neutrophil gelatinase-associated lipocalin (NGAL)) is a multifunctional soluble protein originally found in specific granules of human neutrophils.15,16 It is known to be involved in various biological processes, including the transport of iron and fatty acids, as well as the induction of apoptosis.15 LCN2 expression can be induced by oxidative stress, hypoxia, anemia, and infection.14,17–19 LCN2 expression also increases in cancer.19,20 In carcinogenesis, besides the accumulation of somatic mutations, immunity associated with the stroma is a significant regulator of tumor growth. Tumor cells create a microenvironment by releasing various mediators to sustain their presence and spread. Due to this microenvironment, including IL10, TGF-β, cell hypoxia, and infiltration of monocytes and leukocytes against the tumor, the iron balance is hypothesized to be disrupted by excessive iron use. This, in turn, may result in the increased expression of LCN2 as an intracellular iron transporter.20,21 The presence of iron in tumor cells and cells in the tumor microenvironment is associated with tumor growth and metastasis, while iron chelation inhibits DNA synthesis, causes G1/S phase arrest, attenuates extracellular matrix (ECM), enhances epithelial-to-mesenchymal transition (EMT) and promotes tumor cell apoptosis.20,22,23 Recent studies have shown the efficacy of chemotherapeutic use of inducing iron chelators in cancer treatment.24
Thus, LCN2 as an iron transporter is thought to be involved in tumor growth, invasion, and metastasis as well as participating in various biological processes including cell migration, cell survival, inflammatory responses, and insulin sensitivity.25,26 Experimental studies on mice have been conducted regarding LCN2 expression in breast tumors and its usability as a prognostic marker.27 Limited studies on human breast cancer tissues have suggested that LCN2 may serve as an indicator of poor prognosis.27,28 Selectively targeting iron in the tumor microenvironment for removal may provide benefits in immunotherapy, and therefore, LCN2 is considered a potential therapeutic target against various cancer types.24–29
We postulate that LCN2, akin to the immunotherapeutic advantages of PD-L1, holds the potential for routine immunotherapeutic utilization in TNBC cases. Therefore, it is imperative to assess the immunohistochemical expression of LCN2 in different molecular subtypes of breast cancer. In this study, we investigated the expressions of PD-L1 and LCN2 in breast cancer molecular subtypes, along with their correlations with other prognostic indicators, including Ki-67, lymph node metastasis, histological grade, tumor-infiltrating lymphocyte (TILs) accumulation, and necrosis. The findings from this research aim to contribute insights into the immunotherapeutic application of LCN2 and its prognostic significance in breast cancer management.
Materials and Methods
Ethics Approval and Study Population
Ethical approval of this retrospective study was obtained from Bahcesehir University Clinical Research Ethics Committee under study number 22481095–020-622 on April 3rd, 2018, and conformed with the Declaration of Helsinki. Because the data were deidentified and collected retrospectively, informed consent was waived by the ethics committee.
The study samples were selected from patients who were diagnosed with breast cancer and underwent mastectomy surgery at Bahcesehir University Faculty of Medicine Hospital between 2017–2018 and did not receive neoadjuvant therapy before surgery. There was a total of 89 patients, and the median age was 55 years, ranging from 31 to 85 years. None of the cases had distant metastasis and multifocality. In addition, all patients had only primary breast cancer and no other cancers or chronic diseases. These cases were morphologically reported as Invasive Ductal Carcinoma of no special type (NST) (73 cases), Invasive Lobular Carcinoma (8 cases), Medullary Carcinoma (2 cases), Invasive Micropapillary Carcinoma (2 cases), NST/Mucinous Carcinoma (mixed carcinoma, 2 cases) and Tubular Carcinoma (2 cases). Tumor diameters were ≥1 cm to <2 cm in 43 cases, and ≥2 cm to <5 cm in 46 cases.
Immunostaining of Breast Cancerous Tissue Samples for Molecular Subtyping
All specimens underwent fixation in a 10% neutral buffered formalin solution for 24 hours before undergoing processing with a Tissue-Tek Vip® 6 AI device (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) to generate paraffin blocks. Three micrometers thick sections were prepared from the blocks and stained with hematoxylin and eosin (H&E) staining using a Shandon Gemini stainer as described previously.30 Immunohistochemical staining was performed in accordance with standard and validated immunohistochemical protocols. Briefly, following antibodies were used: antibodies against ER (ER-6F11-L-CE, Clone 6f11, Leica Biosystems), PR (PGR-312/2-L-CE Clone 16, Leica Biosystems), HER-2/neu4B5 (anti-HER-2/neu (4B5), Rabbit Monoclonal Primary Antibody, Ventana, Roche), and Ki-67 (Monoclonal Mouse anti-Human Clone MIB-1, Dako) using a Ventana Benchmark XT device (Roche Diagnostics, Basel, Switzerland).30
The Nottingham modification of Bloom Richardson Grading was used for tumor histological and nuclear grading.31 In addition, prognostic factors such as tumor diameter, lymphovascular invasion, necrosis, and lymph node metastasis were noted for each case. Ki-67 evaluation was performed in digital pathology (ViraPath program developed by ViraSoft Software, ViraSoft Software Trade Inc, Istanbul, Turkey).32 Based on digital Ki-67 results and ER, PR, and HER2 results, cases were classified as Luminal A (ER+, PR+, HER2-, Ki-67 < %20), Luminal B (ER+, PR ±, HER2-, Ki-67 ≥%20 and ER+, PR ±, HER2+, any Ki-67), HER2+ (ER-, PR-, HER2+, Ki-67 ≥%20), Triple Negative (ER-, PR-, HER2-, Ki-67 ≥% 20) according to four molecular subtypes.33 Because cases with TILs ≥5% have been reported to have increased treatment efficacy,34 we also included the cases with 5% or more reported by evaluation with H&E sections (Table 1). Luminal A and Luminal B cases together comprised about 80% of all cases (40.5% and 40.5%, respectively) and TNBC and HER2+ cases were 14.6% and 4.4%, respectively. Necrosis (+) cases were 12.4%, LNM (+) cases were 30.3%, LVI(+) cases were 19.15%, and TILs ≥5% cases were 13.5% of all cases (Table 1). Necrosis and lymph node metastasis were found mainly in TNBC cases (38.5% for both) (Table 1). Among TNBC cases 92.3% of them were HG III and with TILs (53.8%). All HER2+ cases (n = 4; 100%) and the majority of TNBC cases (92.3%) were HG III, confirming the high aggressiveness of HER2+ and TNBC tumors across all cases.
 |
Table 1 Distribution of Cases According to Molecular Subtypes and Prognostic Factors |
Immunohistochemical Evaluation of PD-L1 and LCN2
The PD-L1 (Clone: CAL10, Dilution: 1/100 Brand: Biocare Medical) and LCN2 (Polyclonal, Dilution: 1/200 Brand: GeneTex) were immunohistochemically applied to the same tumor blocks with the Ventana Benchmark XT device (Roche Diagnostics, Basel, Switzerland).30 Negative and positive controls were used for both immune markers. All pictures were taken by Viracenter Digital Pathology new version.32 Two independent pathologists evaluated and scored all slides (S.E. and O.Y.).
For PD-L1 immunohistochemical evaluation, membranous staining was considered positive, and the Combined Positive Score (CPS) analysis was employed.35 CPS is calculated by dividing the total number of PD-L1 positive cells (lymphocytes, macrophages, and tumor cells) by the number of viable tumor cells and multiplying the result by 100 (score ranges from 0 to 100).36 The CPS cut-off values were determined as CPS < 1 (negative PD-L1 staining), CPS ≥ 1 and CPS ≥ 10 as positive PD-L1 staining as described previously.11 Tonsil tissue was used as a PD-L1 positive control and normal breast tissue was used as a PD-L1 negative control.
Immunohistochemical evaluation of LCN2 was scored according to the intensity and extent of cytoplasmic staining of tumor cells, utilizing the Allred (Quick) scoring system.37–40 The staining percentage was scored as follows: 0 for 0%, 1 for <1%, 2 for 1–10%, 3 for 11–33%, 4 for 34–66%, and 5 for >67%. Staining intensity was scored as follows: 0 for no staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The Allred score was calculated as the sum of the staining percentage score and the staining intensity score, resulting in scores ranging from 0 to 8. Normal breast tissue was utilized for LCN2 negative control, and colon adenocarcinoma was used as the positive control.
Statistical Analysis
The data analysis was performed using SPSS (Statistical Product and Service Solutions) version 28 (IBM Corp., Armonk, NY, USA). The comparisons were performed by non-parametric Mann–Whitney U-test according to non-Gaussian distribution. If more than two groups were analyzed Kruskal–Wallis test followed by Dunn’s multiple comparisons was used. Pearson’s correlation analysis was used to investigate the relationships between variables. A p < 0.05 value was considered statistically significant.
Results
LCN2 Expression in Different Primary Breast Carcinomatous Tissues
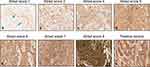
To evaluate LCN2 expression in carcinomatous breast tissues, we newly developed the Allred scoring system (score 1–8) based on the intensity and extent of cytoplasmic LCN2 staining (Figure 1). Among 89 breast tumor tissues, there were no cases with an Allred score <3 for LCN2 expression. Instead, weak LCN2 staining with a score of 0–2 was observed in normal breast tissues (Figure 1a, blue arrow), while all cancer tissues had an Allred score ≥3 for LCN2 expression (Figure 1b-g), comparable to positive control tissue, a colon adenocarcinoma (Figure 1h).
Next, LCN2 expression was scored based on the molecular subtypes and prognostic factors described in Table 1. Most of the Luminal A cases (69.4%) had LCN2 intensity with Allred score 3, and with a lower Ki-67 index (72.2%, <20%) in the cytoplasmic and membranous areas (Table 2). In contrast, the distribution of LCN2 staining steadily increased from Allred score 6 to 8 in TNBC cases (38.4% of TNBC cases had an Allred score 8). This trend was followed in necrosis-positive cases in which 45.5% of them had an Allred score of 8. Similarly, 41.7% TILs ≥ 5% cases had an Allred score of 6.
 |
Table 2 Distribution of Cases into Molecular Subtypes and Prognostic Factors According to LCN2 Expression by Allred Score |
We next sought to compare LCN2 scores quantitatively among subtypes and prognostic factors. Among all cases, within prognostic factors, there was no significant difference in LCN2 staining scores between LNM (+) and LNM (-) cases, and LVI (+) and LVI (-) cases (p > 0.05, and p > 0.05, respectively), while necrosis (+) cases had statistically higher LCN2 scores compared to necrosis (-) cases (p < 0.05) (Table 3). Notably, LCN2 scores and intensity were significantly higher in TILs ≥ 5% cases compared to TILs < 5% cases (p < 0.001). On the other hand, the overall Ki-67 proliferation index (+) (≥20%) cases had significantly higher LCN2 scores (p < 0.0001), along with aggressive molecular subtypes with higher histological grades (HG III), HER2(+) and TNBC cases (p < 0.01 for all three parameters). Notably, relatively less aggressive molecular subtype Luminal A had significantly lower LCN2 scores (p < 0.0001).
 |
Table 3 Comparison of LCN2 Allred Score Expressions in Molecular Subtypes and Prognostic Factors Based on Positive versus Negative Status Among All Cases |
Overall, these statistically significant values among groups were remarkably correlated with the LCN2 intensity in cytoplasmic and membranous regions with Allred score ≥6 that was seen in most of the TNBC (84.6%), TILs ≥ 5% (75%), necrotic (63.6%), HER2 + (50%), and higher histological grade (HG III, 56.1%) cancerous tissues (Table 3). Taken together, these data based on Allred scoring of LCN2 intensity may well indicate that LCN2 intensity is positively correlated with aggressive molecular subtypes such as HG III, TNBC and/or HER2 (+) cases (as ER-negative, PR-negative subtype) with high Ki-67 proliferation index, necrosis, and higher TILs recruitment into the tumor environment. Therefore, we suggest that LCN2 intense staining may be an indicative prognostic factor for the aggressiveness of breast tumors.
PD-L1 Expression in Different Primary Breast Carcinomatous Tissues
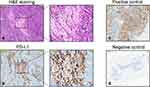
Next, PD-L1 evaluation was performed based on the positive membranous staining and the Combined Positive Score (CPS) (see Materials and Methods). An example of positive membranous PD-L1 expression is shown in Figure 2a and b, together with positive (tonsil tissue) and negative controls (normal breast tissue) (Figure 2c and d, respectively). Accordingly, PD-L1 expression was found to be positive in 46 (51.7%) of all cases. The distribution of PD-L1 positive cases according to molecular subtypes, Ki-67, HG, necrosis, LNM, LVI, and TILs is summarized in Tables 4 and 5. In Table 4 we analyzed PD-L1 levels of all cases based on CPS cut-off values. The cases were categorized based on CPS < 1 (negative PD-L1 staining), CPS ≥ 1 and CPS ≥ 10 (positive PD-L1 staining) values. Overall, among the samples, 91.7% of the TILs ≥ 5% cases were highly positive with PD-L1, followed by 84.6% positivity in TNBC cases, 75.5% positivity in Ki-67 ≥ 20% positive cases, 75% positivity in HER2+ cases, and more than 70% in HG III and necrotic cases (Table 5).
 |
Table 4 Distribution of Cases into Molecular Subtypes and Prognostic Factors According to PD-L1 Expression Based on CPS Cut-Offs |
 |
Table 5 Distribution of Cases into Molecular Subtypes and Prognostic Factors According to PD-L1 Expression |
Among all cases, similar to LCN2 scores, CPS scores of PD-L1 expression were significantly higher in necrosis (+) cases compared to necrosis (-) cases (p < 0.05) (Table 5). In other prognostic factors, there was no significant difference in PD-L1 CPS scores between LNM (+) and LNM (-) cases, and LVI (+) and LVI (-) cases (p > 0.05). However, PD-L1 CPS scores were significantly higher in TILs ≥ 5% cases compared to TILs < 5% cases (p < 0.0001), similar to LCN2 scores. Furthermore, the cases with high Ki-67 proliferation index, higher histological grades (HG III), and with HER2 (+) and TNBC cases significantly had higher PD-L1 expression (p < 0.0001, p < 0.0001, and p < 0.05, respectively) (Table 5). In contrast, Luminal A and HG II cases had significantly lower PD-L1 scores (p < 0.001 and p < 0.0001, respectively).
Association of LCN2 and PD-L1 Intensity Scores with Prognostic Factors and Molecular Subtypes
We therefore followed the above findings by performing an association analysis between LCN2, PD-L1, and breast cancer tissues with different molecular subtypes and prognostic factors. Overall, among 89 cases, there was a statistically significant positive correlation between LCN2 and PD-L1 expression (r = 056, p < 0.01 by Pearson’s correlation test). Next, we performed a detailed analysis to determine how each parameter contributed to this positive correlation. We found that the cases with higher Ki-67 index and higher histological grade (HG III), which had significantly higher LCN2 and PD-L1 expression (Table 3 and Table 5), showed the highest correlation by Pearson’s correlation test (r = 0.46 and r = 0.49, respectively, p < 0.001) (Table 6). Interestingly, LCN2 and PD-L1 expressions were also well correlated in luminal B cases (r = 0.45, p < 0.01), while HG I and HG II cases did not have any correlation (Table 6).
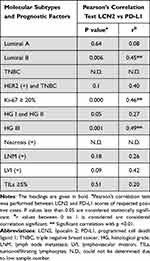 |
Table 6 Association of LCN2 and PD-L1 Staining Scores with Prognostic Factors and Molecular Subtypes |
Although approximately 50% of LNM (+) and LVI (+) cases had PD-L1 positivity (51.8% and 41.2%, respectively, Table 5), only approximately 30% of them (29.6% and 29.4%, respectively) were stained with higher LCN2 (≥6 Allred score) (Table 3). Therefore, there was no correlation between LCN2 and PD-L1 in terms of LNM and LVI positivity (Table 6). Surprisingly, although TILs ≥ 5% cases had significantly higher LCN2 and PD-L1 expressions independently, there was no correlation between them (Table 6). Unfortunately, it was not possible to determine the correlation for necrosis (+) cases due to limited numbers in those groups, although they had a similar trend with TILs ≥ 5%.
Although the case numbers were limited, based on the lack of association between LCN2 and PD-L1 scores in cases where ER and PR were negative (TNBC and HER2-positive cases), we investigated the immunohistochemical expressions of LCN2 and PD-L1 in these cases. Figure 3 shows representative expression pattern of LCN2 and PD-L1 in cancerous tumors of a TNBC case. In this case, LCN2 is expressed in tumor tissue, while PD-L1 is expressed in membranes of infiltrating immune cells. Taken together, these data may suggest that breast cancer tissues only with higher histological grade and high proliferation index may co-express both LCN2 and PD-L1.
Discussion
In the current study, we evaluated LCN2 and PD-L1 expressions in primary human breast cancer tissues before the neoadjuvant therapy. We have conducted an analysis of the relationship between LCN2 and PD-L1 expressions in molecular subtypes (Luminal A, Luminal B, HER2+, and TNBC subtypes) and prognostic factors of breast cancer (proliferation index with Ki-67 positivity, histological grade, necrosis, lymph node metastasis, tumor-infiltrating lymphocyte percentages, and lymphovascular invasion). Despite the low expression of LCN2 even in normal breast tissue (Allred score <3), higher LCN2 expressions (100% positivity with Allred score ≥3) were observed in all cancerous tissues, and LCN2 expression was positively correlated with histological grade, high Ki-67 proliferation index, and ER/PR negativity. Statistically significant positive correlations were observed for elevated LCN2 and PD-L1 expressions with higher histological grade and with higher proliferation index cases.
A direct correlation between the intensity of LCN2 expression and tumor aggressiveness has been reported in previous studies evaluating LCN2 expression in human and mouse breast cancer tissues.15,27–29,37 Our findings are in line with those earlier studies. In the study by Yang et al LCN2 expression was significantly elevated in mouse breast cancer and in a limited number of human breast cancer tissues compared to normal breast tissue.27 Especially in metastatic breast cancer cases, a high level of LCN2 expression was also observed in serum and urine.27 Since our main aim was to evaluate LCN2 expression in molecular subtypes, we selected primary breast cancer cases without distant metastasis, the presence of other tumors, and chronic inflammatory diseases in order to exclude conditions that may affect LCN2 expression. Therefore, it is likely that we did not find a significant association between LCN2 expression and lymph node metastasis in our study, in contrast to previous studies.28,29 Previous studies reported a correlation between strong HER2 positivity and LCN2 expression.28,29 Unfortunately, in the current study, the number of cases with HER2 positivity was very low (4 cases), which resulted in a lack of statistically significant results. However, in our study, the rate of aggressive TNBC cases was 14.6% (13 cases), which usually accounts for 12–17% of all breast cancers,11 and LCN2 expression was significantly higher in TNBC cases. Notably, we observed an independent statistically significant increase in LCN2 and PD-L1 levels in our ER- and PR-negative (HER2+ and TNBC). In contrast, in cases with higher histological grade (HG III) breast cancer, there was a strong association between PD-L1 and LCN2, which may be indicative of tumor aggressiveness.
On the other hand, it is interesting to note that Kurozumi et al observed the loss of nuclear expression of LCN2 as a poor prognostic value in breast cancer.28 In our study, we observed sparse nuclear expression of LCN2 in normal breast tissue and within the tumor regardless of histological grade. For this reason, we did not consider further evaluation of nuclear expression. In addition, Kurozumi et al28 also observed a mild expression of LCN2 in the stroma. In our study, we observed weak expression in the stroma and intensity scores of 1 or 2 even in normal mammary duct cells. Therefore, we scored LCN2 expression only according to the intensity and extent of cytoplasmic staining in tumor cells.
Several studies have been conducted to explore the potential benefits of PD-L1 in immunotherapy for the development of alternative treatments, particularly in TBNC aggressive breast cancer.3,5,12–14,41–43 PD-L1 positivity is highly associated with TILs.43,44 In our study, we found a strong association between PD-L1 and TILs with a rate of 91.7%. TILs are found in an average of 11% of breast cancer cases and TNBC (average 20%) is the most common subtype.43 In our study, TILs were high in 7 (53.8%) of our TNBC patients, and all these 7 patients were PD-L1 and LCN2 positive. It was also noteworthy that PD-L1 CPS score (mean 40.5), LCN2 Allred score (mean 6.4), Ki-67 (mean 63%), and TILs percentages (mean 33.5) were higher than the mean expression in these cases. PD-L1 positivity greater than 1% is considered sufficient for a TNBC patient to be a candidate for immunochemotherapy.42 Despite the small number of cases, we experienced high levels of PD-L1 CPS and TILs in our TNBC cases. In the study by Sobral-Leite et al, PD-L1 and TILs were found to be higher both together and in TNBC cases,44 as in our study. Their ER+ HER-, HER2+ and TNBC cases had 53.1%, 73.3%, 84.4%, and 63.9% PD-L1 positivity, respectively, while our cases had 63.9%, 75%, and 84.6% PD-L1 positivity, respectively.44 They did not see a difference between HG and PD-L1 expression, but in our study, PD-L1 positivity was observed in 73.2% of patients with HG III and 34% of patients with HG II, while PD-L1 was negative in HG I.44
While these studies demonstrate the benefit of immunotherapy in PD-L1-expressing TNBC, they also highlight the need to identify and further study alternative new biomarkers for predicting clinical benefit and for PD-L1-negative patients.3,11,34 The main reason for the search for new markers in immunotherapy is that some of the TNBC cases are PD-L1 negative. PD-L1 expression was not observed in 2 of our 13 TNBC cases. The LCN2 Allred score in these cases was 8.
Nevertheless, to the best of our knowledge, our study investigating the relationship between PD-L1 and LCN2 expression while considering molecular subtypes in human breast cancer tissue is the first of its kind in the literature. In our study, we observed a significant increase and correlation of LCN2 and PD-L1 expression in cases with histological high-grade and higher Ki-67 index status, and our series of 89 cases yielded statistically significant results. While keeping in mind that we always found LCN2 staining in tumor tissues, but PD-L1 staining could be more common in broader cell populations, we think that the elevation of LCN2 and PD-L1 in TNBC and HER2(+) cases still highlights the potential of LCN2 as a therapeutic target in aggressive breast cancer cases. We propose that the evaluation of LCN2 expression in breast cancer is meaningful and, like PD-L1, may benefit immunotherapy in high-grade and aggressive breast tumors.
Study Limitations
Our study has several limitations. We found that LCN2 is expressed in normal breast tissue. LCN2 is expressed by many tissues including adipocyte tissues45 as an extracellular transport protein and is found abundantly in normal human and mouse serum.16 Although LCN2 is expressed at low levels in most human tissues, it is abundant in aggressive subtypes of cancer29 and in the current study. Therefore, we recommend the use of the Allred scoring system to evaluate LCN2 expression in various breast cancer tissues.
Although this study had a limited number of cases, statistically significant results were obtained. Therefore, we suggest that further studies with large case series should be conducted to objectify the evaluation criteria of LCN2 expression and evaluate its clinical significance in breast cancer together with PD-L1 expression.
Abbreviations
CPS, Combined Positive Score; ECM, Extracellular matrix; EMT, Epithelial-to-mesenchymal transition; ER, Estrogen receptor; HER2, Human epidermal growth factor receptor 2; HG, Histological Grade; LCN2, Lipocalin 2; LNM, Lymph node metastasis; LVI, Lymphovascular invasion; PD-L1, Programmed cell death Ligand 1; PR, Progesterone receptor; TILs, Tumor-infiltrating lymphocytes; TNBC, Triple-negative breast cancer.
Data Sharing Statement
The data supporting this study’s findings are available at a reasonable request from the corresponding author, SE.
Acknowledgments
We sincerely thank Prof. Takagi from IFReC for his help during the logistic design of this study, and Ms. Hatice Cirakoglu, from the Department of Pathology, Bahcesehir University Faculty of Medicine, for her excellent technical support in immunohistochemical studies. S.E. is a Clinical Fellow of the Takeda Science Foundation and greatly acknowledges support from them. Cevayir Coban acknowledges support from JST-CREST and AMED (JP223fa627001 and 223fa727001), MUFJ Vaccine Development Project, and the International Joint Usage/Research Center, IMSUT, the University of Tokyo.
Funding
This study was evaluated by the Bahcesehir University Scientific Research Projects (BAP) Commission in December 2020 and was accepted and supported as a BAU-BAP project (BAP.2020-02.07).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of Breast Cancer. Breast Cancer. 2022. doi:10.36255/exon-publications-breast-cancer-subtypes
2. Semiglazov V, Tseluiko A, Kudaybergenova A, Artemyeva A, Krivorotko P, Donskih R. Immunology and immunotherapy in breast cancer. Cancer Biol Med. 2022;19(5):609–618. doi:10.20892/j.issn.2095-3941.2021.0597
3. Emens LA. Immunotherapy in Triple-Negative Breast Cancer. Cancer J. 2021;27(1). doi:10.1097/PPO.0000000000000497
4. Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. PD-L1 and intratumoral immune response in breast cancer. Oncotarget. 2017;8(31). doi:10.18632/oncotarget.18305
5. Li CW, Lim SO, Chung EM, et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell. 2018;33(2):187–201.e10. doi:10.1016/j.ccell.2018.01.009
6. Prestwich RJ, Errington F, Hatfield P, et al. The Immune System - is it Relevant to Cancer Development, Progression and Treatment? Clin Oncol. 2008;20(2):101–112. doi:10.1016/j.clon.2007.10.011
7. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):56.
8. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi:10.1189/jlb.0609385
9. Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014
10. Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PDL1 blockade. Oncotarget. 2017;8(66):110693–110707. doi:10.18632/oncotarget.22690
11. Badve SS, Penault-Llorca F, Reis-Filho JS, et al. Determining PD-L1 Status in Patients with Triple-Negative Breast Cancer: lessons Learned from IMpassion130. J Natl Cancer Inst. 2022;114(5):664–675. doi:10.1093/jnci/djab121
12. Botti G, Collina F, Scognamiglio G, et al. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci. 2017;18(2):459. doi:10.3390/ijms18020459
13. Oner G, Önder S, Karatay H, et al. Clinical impact of PD-L1 expression in triple-negative breast cancer patients with residual tumor burden after neoadjuvant chemotherapy. World J Surg Oncol. 2021;19(1). doi:10.1186/s12957-021-02361-9
14. Tarantino P, Gandini S, Trapani D, Criscitiello C, Curigliano G. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2021;159. doi:10.1016/j.critrevonc.2021.103223
15. Yang J, Moses MA. Lipocalin 2: a multifaceted modulator of human cancer. Cell Cycle. 2009;8(15):2347–2352. doi:10.4161/cc.8.15.9224
16. Zhao H, Konishi A, Fujita Y, et al. Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe. 2012;12(5):705–716. doi:10.1016/j.chom.2012.10.010
17. Li C, Chan YR. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine. 2011;56(2):435–441. doi:10.1016/j.cyto.2011.07.021
18. Candido S, Abrams SL, Steelman LS, et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim Biophys Acta Mol Cell Res. 2016;1863(3):438–448. doi:10.1016/j.bbamcr.2015.08.010
19. Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta Rev Cancer. 2012;1826(1). doi:10.1016/j.bbcan.2012.03.008
20. Mertens C, Mora J, Ören B, et al. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology. 2018;7(3):e1408751. doi:10.1080/2162402X.2017.1408751
21. Cheng Z, Akatsuka S, Li GH, Mori K, Takahashi T, Toyokuni S. Ferroptosis resistance determines high susceptibility of murine A/J strain to iron-induced renal carcinogenesis. Cancer Sci. 2022;113(1):65–78. doi:10.1111/cas.15175
22. Mertens C, Schnetz M, Rehwald C, et al. Iron-bound lipocalin-2 from tumor-associated macrophages drives breast cancer progression independent of ferroportin. Metabolites. 2021;11(3):180. doi:10.3390/metabo11030180
23. Jung M, Mertens C, Bauer R, Rehwald C, Brüne B. Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol Res. 2017;120. doi:10.1016/j.phrs.2017.03.018
24. Atiya HI, Frisbie L, Goldfeld E, et al. Endometriosis-Associated Mesenchymal Stem Cells Support Ovarian Clear Cell Carcinoma through Iron Regulation. Cancer Res. 2022;82(24):4680–4693. doi:10.1158/0008-5472.CAN-22-1294
25. Ören B, Urosevic J, Mertens C, et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol. 2016;239(3):274–285. doi:10.1002/path.4724
26. Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11. doi:10.2147/OTT.S181223
27. Yang J, Bielenberg DR, Rodig SJ, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106(10):3913–3918. doi:10.1073/pnas.0810617106
28. Kurozumi S, Alsaeed S, Orah N, et al. Clinicopathological significance of lipocalin 2 nuclear expression in invasive breast cancer. Breast Cancer Res Treat. 2020;179(3):557–564. doi:10.1007/s10549-019-05488-2
29. Santiago-Sánchez GS, Pita-Grisanti V, Quiñones-Díaz B, Gumpper K, Cruz-Monserrate Z, Vivas-Mejía PE. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int J Mol Sci. 2020;21(12):4365. doi:10.3390/ijms21124365
30. Ekemen S, Comunoglu C, Kayhan CK, et al. Endometrial Staining of CD56 (Uterine Natural Killer), BCL-6, and CD138 (Plasma Cells) Improve Diagnosis and Clinical Pregnancy Outcomes in Unexplained Infertility and Recurrent IVF Failures: standardization of Diagnosis with Digital Pathology. Diagnostics. 2023;13(9):1557. doi:10.3390/diagnostics13091557
31. Elston CW, Ellis IO. pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology. 1991;19(5):403–410. doi:10.1111/j.1365-2559.1991.tb00229.x
32. Yildiz P, Aydin Ulgen O, Yol C, Demirkesen C. Proliferating Pilar Tumors: can Immunohistochemistry Differentiate Benign and Malignant Forms? Am J Dermatopathol. 2021;43(3):198–201. doi:10.1097/DAD.0000000000001743
33. do NRG, Otoni KM. Histological and molecular classification of breast cancer: what do we know? Mastology. 2020;30. doi:10.29289/25945394202020200024
34. Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021;21(2):135–148. doi:10.1080/14737140.2021.1840984
35. Ambrosini-Spaltro A, Limarzi F, Gaudio M, Calpona S, Meccariello G. PD-L1 expression in head and neck carcinoma by combined positive score: a comparison among preoperative biopsy, tumor resection, and lymph node metastasis. Virchows Archiv. 2022;481(1):93–99. doi:10.1007/s00428-022-03322-7
36. Akhtar M, Rashid S, Al-Bozom IA. PD−L1 immunostaining: what pathologists need to know. Diagn Pathol. 2021;16(1). doi:10.1186/s13000-021-01151-x
37. Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108(3):389–397. doi:10.1007/s10549-007-9619-3
38. Ahmad Fauzi MF, Ahmad WSHM W, Jamaluddin MF, et al. Allred Scoring of ER-IHC Stained Whole-Slide Images for Hormone Receptor Status in Breast Carcinoma. Diagnostics. 2022;12(12):3093. doi:10.3390/diagnostics12123093
39. Rai PD, Vagha S, Shukla S, Bhake A. Comparison of various scoring systems by immunohistochemistry for evaluating hormone receptors (Estrogen receptor and progesterone receptor) in carcinoma of breast. J Datta Meghe Inst Med Sci Univ. 2020;15(2):202. doi:10.4103/jdmimsu.jdmimsu_7_20
40. Yamashita H, Ando Y, Nishio M, et al. Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer. 2006;13(1):74–83. doi:10.2325/jbcs.13.74
41. Buisseret L, Garaud S, De Wind A, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-l1 expression are linked in breast cancer. Oncoimmunology. 2017;6(1):e1257452. doi:10.1080/2162402X.2016.1257452
42. Won KA, Spruck C. Triple-negative breast cancer therapy: current and future perspectives. Int J Oncol. 2020;57(6):1245–1261. doi:10.3892/ijo.2020.5135
43. Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: a Systematic Review. JAMA Oncol. 2016;2(10):1354. doi:10.1001/jamaoncol.2016.1061
44. Sobral-Leite M, Van de Vijver K, Michaut M, et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1 -like status, tumor-infiltrating immune cells and survival. Oncoimmunology. 2018;7(12):e1509820. doi:10.1080/2162402X.2018.1509820
45. Kamble PG, Pereira MJ, Almby K, Eriksson JW. Estrogen interacts with glucocorticoids in the regulation of lipocalin 2 expression in human adipose tissue. Reciprocal roles of estrogen receptor α and β in insulin resistance? Mol Cell Endocrinol. 2019;490. doi:10.1016/j.mce.2019.04.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.