Back to Journals » Infection and Drug Resistance » Volume 17
The Potentiation Activity of Azithromycin in Combination with Colistin or Levofloxacin Against Pseudomonas aeruginosa Biofilm Infection
Authors Wang Y, Li C, Zhang H, Chi Y, Cai Y
Received 5 November 2023
Accepted for publication 8 March 2024
Published 28 March 2024 Volume 2024:17 Pages 1259—1266
DOI https://doi.org/10.2147/IDR.S438576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yuhang Wang,1,* Chunsun Li,2,* Huan Zhang,1 Yulong Chi,1 Yun Cai1
1Center of Medicine Clinical Research, Department of Pharmacy, Medical Supplies Center of PLA General Hospital, Beijing, People’s Republic of China; 2Laboratory of Department of Pulmonary and Critical Care Medicine, PLA General Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Cai, Center of Medicine Clinical Research, Department of Pharmacy, Medical Supplies center of PLA General Hospital, Postal Address: 28 Fu Xing Road, Beijing, 100853, People’s Republic of China, Tel +86-10-6693-7166, Fax +86-10-8821-4425, Email [email protected]
Objective: Pseudomonas aeruginosa (PA) often displays drug resistance and biofilm-mediated adaptability. Here, we aimed to evaluate the antibiofilm efficacy of azithromycin-based combination regimens.
Methods: Minimum inhibitory concentrations (MICs), minimal biofilm eradication concentrations (MBECs), and MBEC-combination of azithromycin, colistin, amikacin, and levofloxacin to bioluminescent strain PAO1 and carbapenem-resistant PAO1 (CRPAO1) were assessed. An animal biofilm infection model was established and detected using a live animal bio-photonic imaging system.
Results: In vitro, PAO1 and CRPAO1 were susceptible to colistin, amikacin, and levofloxacin, while they were unsusceptible to azithromycin. The combinations based on azithromycin have no synergistic effect on biofilm in vitro. In vivo, azithromycin plus colistin or levofloxacin could shorten the PAO1 biofilm eradication time, which totally eradicates the biofilm in all mice on the 8th or 6th day, while monotherapy only eradicate biofilm in 70% or 80% mice on the 8th day. For CRPAO1 biofilm, only azithromycin–colistin combination and colistin monotherapy eradicated the bacteria in 60% and 40% of mice at the 6th day.
Conclusion: Azithromycin-based combinations containing levofloxacin or colistin had no synergistic effect in vitro, and they are promising for clinical applications due to the good synergistic activity against PAO1 biofilms in vivo.
Keywords: Pseudomonas aeruginosa, biofilm, azithromycin, combinations
Introduction
Pseudomonas aeruginosa (PA) is a common opportunistic pathogen responsible for urinary tract infections, burn wound infections, and some hospital-acquired infections, like ventilator-associated pneumonia (VAP).1 PA often displays drug resistance and biofilm-mediated adaptability, which makes the PA-related infections difficult to treat.2 Pathogens form biofilms, which are dynamic and complex structures that cause chronic persistent and recurrent infections.3 Pathogenic biofilms often lead to the chronic infections that make it difficult for the immune system to fully protect the host. In the presence of biofilms, macrophages have difficulty traversing the extracellular matrix and are easily affected by enzymes secreted by bacteria.4 Besides, exaggerated or long-term neutrophil activity leads to the overflow of harmful compounds in the medium, which are responsible for consecutive tissue damages.5 In addition, most antibiotics have poor effectiveness against biofilm-embedded bacteria, such as β-lactams. In biofilms, the extracellular polymeric substance (EPS) provides a physical barrier that can be difficult for antibiotics to penetrate.6 Additionally, monotherapy could induce resistance through the increased formation of biofilms, such as colistin.7 Thus, PA biofilm infections are difficult to treat.
Azithromycin is commonly practiced in the treatment of patients with cystic fibrosis (CF) and other chronic lung infections with PA. Macrolide azithromycin, although without bactericidal activity against PA, can disrupt quorum sensing and block alginate production, which are important for biofilm function.8 In addition, macrolides modulate the immune function of the host.9 In the initial host defense stage, the drug appears to perform stimulatory effects on neutrophil and dendritic cells.10 Later, the biphasic action of azithromycin helps to reduce bystander tissue injury and promote inflammation resolution.11 Although these properties do not make it possible for azithromycin to eradicate unsusceptible strains-formed biofilms alone, there is a hope for azithromycin in combination with other antimicrobial agents. In some studies, combination therapies show a good activity on biofilm.12 Azithromycin in combination with ceftazidime or ciprofloxacin has been reported to increase therapeutic efficacy against PA biofilms in an animal model of ureteral stent infection.13
Hereby, we aimed to assess the potentiation activity of azithromycin with three promising antimicrobial agents against PA biofilms. Levofloxacin is uncharged molecules and diffuses easily through the biofilm matrix. Colistin has been shown to be specifically potent against bacteria with low metabolic activity, which is difficult to eradicate. For amikacin, PA is known to develop resistance to it very slowly due to its complex structure. MDR-PA remains highly susceptible to it.
Methods
Strains and Agents
The bioluminescent strain PAO1 carrying luxCDABE gene operon was purchased from Caliper Life Sciences, USA. The carbapenem-resistant and bioluminescent strain PAO1 (CRPAO1) was cultured as previously described.14 PA ATCC 27853 was used as a quality control strain.
Amikacin, meropenem, levofloxacin (Shanghai Macklin Biochemical Co., Ltd., China), and colistin sulfate (Sigma, China) were used in our present study. Mueller-Hinton Agar and adjusted Mueller-Hinton Broth (MHB) were used to culture bacteria that were purchased from Becton, Dickinson and Company. Isoflurane (Shenzhen RWD Life Science Company, China) and pentobarbital (Shanghai Rongchuang Biotechnology Company, China) were used to anesthetize mice.
Fractional Inhibitory Concentration Indexes (FICIs) Assay
A CLSI standards broth-microdilution method was employed to assess the minimum inhibitory concentrations (MICs).15 Synergistic effects of antibiotics were assessed using the checkerboard broth microdilution method as previously described.16 FICIs were calculated to test the interactions between two antibiotics. FICI = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). The FICIs were interpreted as follows: FICIs ≤ 0.5, synergy; 0.5 < FICIs < 1.0, additivity; 1.0 ≤ FICIs ≤ 2.0, indifference; and FICIs > 2.0, antagonism.
Minimum Biofilm Eradication Concentrations (MBECs) in vitro
The MBECs were determined as previously described.16 Briefly, disks were cut from a medical drainage tube with a diameter of 0.5 cm. Then, the biofilm was cultivated on disks in 24-well plates. The 24-well plates were incubated in MHB at 37∘C for 1, 3, and 7 days, respectively, and MHB was renewed daily. Subsequently, the disks were washed thrice with fresh MHB and put into new 24-well plates with different concentrations of antibiotics. After 24 h of cultivated at 37 ∘C, the disks were washed thrice with saline to remove planktonic bacteria. The ultrasonic cleaning bath was then used to collect the bacteria in biofilm. The bacterial solution was vigorously mixed, plated on agar plates as 10-fold serial dilutions, and cultured for 24 h. Then, colony forming unit (CFU) was counted, and MBECs were calculated as the minimum concentration of tested antibiotics which were able to eradicate the bacteria in biofilm (CFU=0). MBECs were defined as the minimum concentrations of tested antibiotics that were able to eradicate the bacteria thoroughly in biofilm.
Biofilm Infection Mouse Model and Treatment Regimen
The animal biofilm infection model was determined as previously described.16 PAO1 and CRPAO1 biofilms were grown on disks as described in the in vitro experiment. Then, 25–30g male mice were anesthetized with intraperitoneal injection of 1% pentobarbital (0.005 mL/g). The disks with biofilms were then washed with sterile physiological saline solution and implanted subcutaneously at the dorsal midline. Mice were randomly divided into 10 groups. That were as follows: control group (sterile physiological saline solution 10 mL/kg/24 h); AZI (azithromycin 64 mg/kg/24 h); LEV (levofloxacin 32 mg/kg/24 h); LEV-2 (levofloxacin 64 mg/kg/24 h); AZI+LEV (azithromycin 64 mg/kg/24 h plus levofloxacin 32 mg/kg/24 h); AZI+LEV-2 (azithromycin 64 mg/kg/24 h plus levofloxacin 64 mg/kg/24 h); COL (colistin 20 mg/kg/12 h); AZI+COL (azithromycin 64 mg/kg/24 h plus colistin 20 mg/kg/12 h); AMI (amikacin 135 mg/kg/24 h); and AZI+AMI (azithromycin 64 mg/kg/24 h plus amikacin 135 mg/kg/24 h). The dosage of colistin was consistent with relevant literature reports.17 The dosages of azithromycin, amikacin, and levofloxacin were determined according to the conversion of clinical human dosage to mice according to FDA and SmPC labels.18–20 The antibiotics were injected intraperitoneally 24 h after implantation and continued for 5 days. The animal experiment procedures were approved by the Animal Ethics Committee of Chinese PLA General Hospital (SQ2020095). The guidelines of the Declaration of Helsinki and the National Institute of Health (NIH) Guide for care and use of laboratory animals were followed for animal care.
Bio-Photonic Imaging of Mouse Biofilm Model
After implantation of disks with PA biofilms, mice were anesthetized for imaging with IVIS Lumina Series III live animal biophotonic imaging system immediately and every 24h. A good correlation between the bacterial counts and luminescence of bacteria has been demonstrated in previous studies.21,22 From 0 h post-implantation to the end of therapy or disappearance of flux intensities, signals were collected from a defined region of interest using the contour tool. Using the Living Image Software 4.3.7, total flux intensities (photons/s) were calculated. Ten days was the maximum observation period, because the sutures of mice would be dehiscent for persistent infection at about 10th day. The radiance of bacteria could not be observed when the total flux intensities were less than 105. The implantations would be taken out to count the bacteria in biofilm after the last imaging. The biofilm bacteria counting method was the same as the in vitro test. Figure 1A depicts the timeline of the trial.
Statistical Analysis
Data were presented as Mean ± Standard Error for the Sample Mean (SEM). A one-way analysis of variance (ANOVA) method was used to assess the differences in log10 (CFU) and log10 (p/s, photons/second) among groups. The Mante–Cox method was used to assess the differences in the number of mice showing luminescence. Statistical graphs and analyses were made using the GraphPad Prism 8.0.2. P < 0.05 was considered statistically significant.
Results
The Activity of Antibiotics Against Planktonic and Biofilm PA in vitro
Planktonic PAO1 and CRPAO1 were susceptible to colistin, levofloxacin, and amikacin. The MICs of azithromycin to PAO1 and CRPAO1 were 16 and 32 mg/L, respectively.
Table 1 summarizes the MBECs of azithromycin, colistin, levofloxacin, and amikacin to PAO1 and CRPAO1 biofilms. The MBECs of each antibiotic to PAO1 and CRPAO1 were more than 8 times of their MICs. The MBECs of 3-day-grown and 7-day-grown biofilms were higher compared with the 1-day-grown biofilm. Azithromycin had an extremely high MBEC (>128 mg/L). Even in combination with azithromycin at a concentration of 128 mg/L, the MBECs of colistin, levofloxacin, or amikacin did not decrease.
 |
Table 1 MBECs and MBEC-Combinations of Antibiotics Against PA Biofilm |
Synergistic Effects of Antimicrobial Combinations on PA Biofilm in vivo
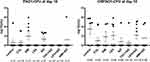
Figure 1E and Figure 2D illustrate the dorsal images of biofilm-infected mice. For PAO1-infected mice, the luminescence of 50% and 60% mice in the colistin and levofloxacin treatment groups on the 6th day could not be detected, respectively, while such a proportion was only 30% in the amikacin treatment group. In addition, luminescence of 100% and 80% of mice in the LEV+AZI treatment group and COL+AZI treatment group could not be detected on the 6th day. The photons of azithromycin in combination with levofloxacin or colistin groups decreased faster than each antibiotic alone. However, amikacin in combination with azithromycin barely showed any synergistic effect in reducing luminescence in all mice during the whole observational period (Figure 1B–D).
For CRPAO1, therapeutic effects were not so obvious in both monotherapy and combination regimen of amikacin and levofloxacin (luminescence could be detected in 90% or 100% of mice on the 6th day). Only the COL+AZI treatment group showed slightly better therapeutic effects than the colistin treatment group alone on the 6th day. The luminescence could not be detected of 60% of mice in COL+AZI treatment group vs 40% in colistin treatment group alone. Besides, the average photons/second of COL+AZI treatment group decreased to the lower limit of detection at 6th day. The LEV+AZI and AMI+AZI treatment groups showed no significant difference compared with the control and monotherapy treatment groups (Figure 2A–C).
The correlation between the radiance and the bacterial counts in the biofilm was good (n=71, r2 =0.7960, P<0.00001). The CFU counts of biofilm on the 10th day were listed in Figure 3. When the total flux was less than 105, no living bacteria were found in the biofilm. Because the suture ruptured on the 10th day due to the persisting infections, the CFU counts in those mice were not included.
Discussion
The pathogens’ ability to form biofilms exacerbates the crisis of antibiotic resistance. Biofilm protected pathogen from immune system attacks, causing persistent infections of implanted medical devices and tissues. Treatment for biofilm infection with any individual drug always requires several times the MIC of a given antibiotic.23 In the present study, the MBECs of single antibiotic to PAO1 immature biofilm were 8 to 64 times higher than those of MICs.
PA is naturally resistant to macrolides. Azithromycin had high MICs (≥16 mg/L) and MBECs (>128 mg/L) against PA strains in our study, which far exceeded the Cmax (0.5 mg/L) of azithromycin after oral administration of 500 mg clinically recommended dose.20 Previous studies show that macrolides could modify the inflammatory response to infection and decrease the virulence of PA.24 However, in our study, azithromycin monotherapy did not show therapeutic effects on biofilms in vivo either. The bacteria clearance time of azithromycin monotherapy did not significantly reduce compared with control. Without the bactericidal effect, azithromycin is not sufficient to eliminate biofilms that only rely on immune regulation and virulence inhibition. Therefore, we speculate that the immune regulation and virulence inhibition of azithromycin is the reason for its synergistic effect with other antibiotics.
Planktonic PA retains a high sensitivity rate to colistin in surveillance program.25 In biofilms, colistin has a positive effect on subpopulations located at the substratum, which are unsusceptible to most of the antibiotics.26 However, colistin monotherapy shows poor effect on biofilms for cap subpopulations surviving due to the migration of the colistin-resistant subgroup.26 Bacterial quorum sensing could modulate the susceptibility of PA to polymyxin antibiotics.27 Azithromycin could inhibit quorum sensing and has been proven to be useful as an adjuvant to polymyxins.28 Our study showed that immature biofilm infection needed at least 8 mg/L colistin to kill the living bacteria in vitro, and mature biofilm infection needed at least 16 mg/L colistin. Such concentrations are difficult to achieve due to the adverse events caused by high concentrations in vivo.29 In our in vivo study, the bacteria in PAO1 or CRPAO1 biofilms were only eradicated in 40–50% of mice with a 5-days of colistin monotherapy. After, in combination with azithromycin, the proportions of mice without biofilm 20–30% higher than colistin monotherapy.
Levofloxacin interferes with bacterial biofilms through electrostatic interference of the activation, adhesion, or release of enzymes to disturb the exopolysaccharide, and inhibits the formation of new exopolysaccharides.30 Thus, the synthesis and mature form of biofilm could all be interfered.31 In addition, as the main reason for recurrence, stationary-phase pathogens could be eradicated by fluoroquinolones.32 However, mature biofilms indeed influence the penetration of levofloxacin. In the present study, 4 mg/L or 8 mg/L of levofloxacin alone was needed to kill the bacteria in 1-day grown (immature) and 3 and 7-day-grown (mature) PAO1 biofilms in vitro. Five days of levofloxacin monotherapy eradicated the bacteria in biofilm in 60% mice, while the combination containing azithromycin eradicated the bacteria in biofilms of 100% mice. However, for CRPAO1, 16 mg/L levofloxacin alone was needed to kill the bacteria in the mature biofilm. Based on the results in vitro, it was not surprising that levofloxacin alone or in combination with azithromycin showed no biofilm-eradicating effect when treating CRPAO1 biofilm in vivo. And because of the higher MIC of CRPAO1 to levofloxacin, the ability of AZI+LEV combination to kill CRPAO1 was weaker than PAO1 in biofilm. According to China Antimicrobial Surveillance, the meropenem-resistant strain is often resistant to levofloxacin.25 So, the application of AZI+LEV in CR-PA may be of limited benefit.
Amikacin has a good PK/PD. PA remains highly susceptible to it.33 However, the slow-growth phenotype bacteria in biofilm shows a weak response to it. The subinhibitory levels of aminoglycosides induce the formation of biofilms.34 In the present study, 64 mg/L amikacin was needed to kill the PA in mature biofilms in vitro. In vivo, no therapeutic effect was detected after 5-days amikacin monotherapy and AMI+AZI therapy.
Previous study has confirmed that twenty-four-week regimen of azithromycin improves lung function and decreases pulmonary exacerbations in CF patients infected with PA.35 Considering that the tissue penetrants of azithromycin in lung and tonsil are nearly 10 times of in soft tissue, the therapeutic effect of our azithromycin-based regimes may be enhanced with prolonged treatment time.20 In our study, azithromycin-based combinations showed weak activity in CRPAO1 biofilm infections. The development of new antibiotics with different modes of action may show a good antimicrobial activity monotherapy or in combination with conventional antibiotics.36
Conclusion
Although azithromycin-based combinations containing levofloxacin or colistin had no synergistic effect in vitro, they exhibited good synergistic activity on PAO1 biofilms in vivo, which is expected to be a promising treatment approach against biofilm infections.
Funding
This work was supported by the National Natural Science Foundations of China (82073894 and 81770004), Cultivation Project of PLA General Hospital for Distinguished Young Scientists (2020-JQPY-004) and New Medicine Clinical Research Fund (4246Z512).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Litwin A, Rojek S, Gozdzik W, Duszynska W. Pseudomonas aeruginosa device associated - healthcare associated infections and its multidrug resistance at intensive care unit of university hospital: polish, 8.5-year, prospective, single-centre study. BMC Infect Dis. 2021;21:180. doi:10.1186/s12879-021-05883-5
2. Ghssein G, Ezzeddine Z. Pseudomonas aeruginosa review of metallophores: pyoverdine, pyochelin and pseudopaline. Biology. 2022;2022:11.
3. Blanco-Cabra N, Paetzold B, Ferrar T, et al. Characterization of different alginate lyases for dissolving pseudomonas aeruginosa biofilms. Sci Rep. 2020;10:9390. doi:10.1038/s41598-020-66293-2
4. Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013;121(s136):1–51. doi:10.1111/apm.12099
5. Olivares E, Badel-Berchoux S, Provot C, Prévost G, Bernardi T, Jehl F. Pseudomonas aeruginosa clinical impact of antibiotics for the treatment of biofilm infections. Front Microbiol. 2019;10:2894. doi:10.3389/fmicb.2019.02894
6. Seebach E, Kubatzky K. Chronic implant-related bone infections-can immune modulation be a therapeutic strategy? Front Immunol. 2019;10:1724. doi:10.3389/fimmu.2019.01724
7. Jalil A, Alrawe R, Al-Saffar M, et al. The use of combination therapy for the improvement of colistin activity against bacterial biofilm. Braz J Microbiol. 2023;55(1):411–427. doi:10.1007/s42770-023-01189-7
8. Martin I, Waters V, Grasemann H, Romero MP. Approaches to targeting bacterial biofilms in cystic fibrosis airways. Int J Mol Sci. 2021;23(1):22. doi:10.3390/ijms23010022
9. Pons S, Arrii E, Arnaud M, et al. Immunomodulation of endothelial cells induced by macrolide therapy in a model of septic stimulation. Immun inflam dis. 2021;9(4):1656–1669. doi:10.1002/iid3.518
10. Polancec D, Munic Kos V, Banjanac M, et al. Azithromycin drives in vitro GM-CSF/IL-4-induced differentiation of human blood monocytes toward dendritic-like cells with regulatory properties. J Leukoc Biol. 2012;91(2):229–243. doi:10.1189/jlb.1210655
11. Parnham M, Erakovic Haber V, Giamarellos-Bourboulis E, Perletti G, Verleden G, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi:10.1016/j.pharmthera.2014.03.003
12. Katip W, Uitrakul S, Oberdorfer P. Acinetobacter baumannii A Comparison of Colistin versus Colistin Plus Meropenem for the Treatment of Carbapenem-Resistant in Critically Ill Patients: A Propensity Score-Matched Analysis. Antibiotics. (Basel, Switzerland):MDPI; 2020:9.
13. Wang X, Cai Y, Xing H, et al. Increased therapeutic efficacy of combination of azithromycin and ceftazidime on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. BMC Microbiol. 2016;16:124. doi:10.1186/s12866-016-0744-1
14. Wang Y, Li C, Wang J, et al. The efficacy of colistin combined with amikacin or levofloxacin against pseudomonas aeruginosa biofilm infection. Microbiol Spectr. 2022;10(5):e0146822. doi:10.1128/spectrum.01468-22
15. Weinstein MP, Bobenchik IIJSL, Campeaus AM. Performance standards for antimicrobial susceptibility testing. Clinical Lab Stand Inst. 2020;2020:M100.
16. Chai D, Liu X, Wang R, Bai Y, Cai Y. Efficacy of linezolid and fosfomycin in catheter-related biofilm infection caused by methicillin-resistant staphylococcus aureus. Biomed Res Int. 2016;2016:6413982. doi:10.1155/2016/6413982
17. Poulakou G, Renieris G, Sabrakos L, et al. Daptomycin as adjunctive treatment for experimental infection by Acinetobacter baumannii with resistance to colistin. Int J Antimicrob Agents. 2019;53:190–194. doi:10.1016/j.ijantimicag.2018.10.024
18. FDA. Approved Drug Product LEVAQUIN; 2024. Available from: https://wwwaccessdatafdagov/drugsatfda_docs/label/2020/020634s073lblpdf.
19. SmPC. Amikacin summary of product characteristics (SmPC); 2020.
20. FDA. Approved drug product for zithromax. 2024. Available from: https://s3-us-west-2amazonawscom/drugbank/cite_this/attachments/files/000/003/154/original/zithromax_fdapdf?1548452062.
21. Krespi Y, Kizhner V, Nistico L, Hall-Stoodley L, Stoodley P. Laser disruption and killing of methicillin-resistant Staphylococcus aureus biofilms. Am J Otolaryngol. 2011;32:198–202. doi:10.1016/j.amjoto.2010.01.010
22. Xiong Y, Willard J, Kadurugamuwa J, Yu J, Francis K, Bayer A. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat staphylococcus aureus endocarditis Model. Antimicrob Agents Chemother. 2005;49(1):380–387. doi:10.1128/AAC.49.1.380-387.2005
23. Roudashti S, Zeighami H, Mirshahabi H, Bahari S, Soltani A, Haghi F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J Microbiol Biotechnol. 2017;33(3):50. doi:10.1007/s11274-016-2195-0
24. Cirioni O, Ghiselli R, Silvestri C, et al. Effect of the combination of clarithromycin and amikacin on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. J Antimicrob Chemother. 2011;66:1318–1323. doi:10.1093/jac/dkr107
25. Yang Y, Guo Y, Yin D, et al. In vitro activity of cefepime-zidebactam, ceftazidime-avibactam, and other comparators against clinical isolates of, pseudomonas aeruginosa, and Acinetobacter baumannii: results from china antimicrobial surveillance network (CHINET) in 2018. Antimicrob Agents Chemother. 2020;2020:65.
26. Herrmann G, Yang L, Wu H, et al. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm pseudomonas aeruginosa. J Infect Dis. 2010;202:1585–1592. doi:10.1086/656788
27. Pang Z, Raudonis R, Glick B, Lin T, Cheng Z. Antibiotic resistance in pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37:177–192. doi:10.1016/j.biotechadv.2018.11.013
28. Bulman Z, Ly N, Lenhard J, Holden P, Bulitta J, Tsuji B. rhlR influence of and on polymyxin pharmacodynamics in pseudomonas aeruginosa and implications for quorum sensing inhibition with azithromycin. Antimicrob Agents Chemother. 2017;2017:61.
29. Katip W, Oberdorfer P, Kasatpibal N, Singhvi G. Acinetobacter baumanniiEffectiveness and nephrotoxicity of loading dose colistin-meropenem versus loading dose colistin-imipenem in the treatment of carbapenem-resistant Infection. Pharmaceutics. 2022;15(1):14. doi:10.3390/pharmaceutics15010014
30. Yassien M, Khardori N. Interaction between biofilms formed by Staphylococcus epidermidis and quinolones. Diagn Microbiol Infect Dis. 2001;40(3):79–89. doi:10.1016/S0732-8893(01)00253-X
31. Drago L, Mattina R, Legnani D, et al. Modulation of biofilm of strains isolated from patients with chronic obstructive pulmonary disease by levofloxacin, moxifloxacin, ciprofloxacin, amoxicillin/clavulanic acid and ceftriaxone. Int J Immunopathol Pharmacol. 2011;24(4):1027–1035. doi:10.1177/039463201102400420
32. Eng R, Padberg F, Smith S, Tan E, Cherubin C. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother. 1991;35(9):1824–1828. doi:10.1128/AAC.35.9.1824
33. Goto T, Nakame Y, Nishida M, Ohi Y. In vitro bactericidal activities of beta-lactamases, amikacin, and fluoroquinolones against Pseudomonas aeruginosa biofilm in artificial urine. Urology. 1999;53(5):1058–1062. doi:10.1016/S0090-4295(98)00649-9
34. Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–868. doi:10.1093/jac/dkn059
35. Saiman L, Marshall B, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–1756. doi:10.1001/jama.290.13.1749
36. Ezzeddine Z, Ghssein G. Towards new antibiotics classes targeting bacterial metallophores. Microb Pathog. 2023;182:106221. doi:10.1016/j.micpath.2023.106221
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.