Back to Journals » Journal of Inflammation Research » Volume 17
The Mechanism of Pyroptosis and Its Application Prospect in Diabetic Wound Healing
Authors Al Mamun A , Shao C, Geng P, Wang S , Xiao J
Received 17 November 2023
Accepted for publication 13 February 2024
Published 6 March 2024 Volume 2024:17 Pages 1481—1501
DOI https://doi.org/10.2147/JIR.S448693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Abdullah Al Mamun,1,2 Chuxiao Shao,1 Peiwu Geng,1 Shuanghu Wang,1 Jian Xiao2,3
1Central Laboratory of the Sixth Affiliated Hospital of Wenzhou Medical University, Lishui People’s Hospital, Lishui City, Zhejiang, 323000, People’s Republic of China; 2Molecular Pharmacology Research Center, School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 3Department of Wound Healing, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Jian Xiao, Email [email protected]
Abstract: Pyroptosis defines a form of pro-inflammatory-dependent programmed cell death triggered by gasdermin proteins, which creates cytoplasmic pores and promotes the activation and accumulation of immune cells by releasing several pro-inflammatory mediators and immunogenic substances upon cell rupture. Pyroptosis comprises canonical (mediated by Caspase-1) and non-canonical (mediated by Caspase-4/5/11) molecular signaling pathways. Numerous studies have explored the contributory roles of inflammasome and pyroptosis in the progression of multiple pathological conditions such as tumors, nerve injury, inflammatory diseases and metabolic disorders. Accumulating evidence indicates that the activation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome results in the activation of pyroptosis and inflammation. Current evidence suggests that pyroptosis-dependent cell death plays a progressive role in the development of diabetic complications including diabetic wound healing (DWH) and diabetic foot ulcers (DFUs). This review presents a brief overview of the molecular mechanisms underlying pyroptosis and addresses the current research on pyroptosis-dependent signaling pathways in the context of DWH. In this review, we also present some prospective therapeutic compounds/agents that can target pyroptotic signaling pathways, which may serve as new strategies for the effective treatment and management of diabetic wounds.
Keywords: diabetes mellitus, diabetic wound healing, pyroptosis, NLRP3, caspase-1, GSDMD, inflammation, inflammasome
Introduction
Diabetes mellitus (DM) is a chronic glucose metabolism condition. However, chronic organ and tissue damage from elevated blood glucose levels in DM patients results in the development of retinopathy, nephropathy, neuropathy and diabetic wound healing (DWH). Accumulating evidence indicates that DWH is caused by chronic inflammation due to impaired tissue repair mechanisms in people with DM.1,2 In addition, diabetic foot ulcers (DFUs) are a severe and common complication of DM that results in significant morbidity and mortality worldwide.3 Mortality rates associated with the development of DFUs are estimated to be 5% in the first 12 months and 5-year mortality rates have been estimated at 42%.4 The standard practices in DFU management include surgical debridement, dressings to facilitate a moist wound environment and exudate control, wound off-loading, vascular assessment and infection and glycemic control.5 Treatment of DFUs accounts for approximately one-third of the total cost of diabetic care, which was estimated to be US $176 billion in direct healthcare expenditures in 2012.6 Despite these high healthcare costs, about 20% of patients have unhealed DFUs at 1 year.7 Although there are well-established principles for managing DFUs, the treatment of DFUs is often challenging. A broad spectrum of novel interventions is being studied to improve wound healing. Persistent wounds in DM typically result in chronic inflammatory responses and accumulate several pro-inflammatory mediators. Extensive studies have also indicated that the NLR family pyrin domain containing 3 (NLRP3) inflammasome is activated in patients with DM.8–10 Inflammasome pathways can be triggered by several metabolic impairments including hyperglycemia, hyperlipidemia and hyperuricemia.11–14 NACHT domains, leucine-rich repeats (LRRs) and NLRP3 may impair angiogenesis and ulcer healing in T2DM.15,16 Pyroptosis, a specific type of cell death, functions as a wide-range immune system that defends against pathogenic microbial infections.17 Numerous studies have shown that excessive pyroptosis can lead to the progression of neurological, cardiovascular and inflammatory diseases.18–21 Pyroptosis exacerbates metabolic conditions including hyperglycemia by inducing persistent inflammation and insulin-resistant mediators.22 Moreover, current studies suggest that pyroptosis plays a significant role in developing diabetes complications, particularly in DWH.23,24
Molecular Mechanisms of Pyroptosis
Pyroptosis is an alternative manifestation of programmed cell death distinguished by cytoplasmic swelling and denaturation, which further facilitates the secretion of intracellular components and triggers a robust inflammatory response. In addition, pyroptosis is a predominant cellular mechanism to harmful stimuli including pathogen ligands, excessive amounts of host substance and external stimuli.
Canonical Pyroptosis
Microbial infections, pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns (DAMPs) activate inflammasome multiprotein complex. The innate immune system depends on the activation of inflammasomes.25 Several intracellular sensor molecules including NOD-like receptors (NLRP1b, NLRC4 and NLRP3), a member of the HIN200/AIM2-like receptor family (AIM2) and a TRIM family member (Pyrin/TRIM20) sense exogenous pathogens and endogenous damage such as bacterial infection, cytosolic double-stranded DNA (dsDNA), crystals and toxins to assemble the canonical inflammasomes.26 Sensor proteins aggregate and recognize the inflammasome apoptosis-associated speck-like protein containing CARD (ASC), which includes a CARD domain. This interaction further promotes the activation of inflammasome sensors and the stimulation of pro-caspase-1 through self-cleavage.27,28 Gasdermin D (GSDMD) acts as a direct substrate of inflammatory Caspases in pyroptosis.29 Gasdermin-N domains are released from the plasma membrane by the Caspase-1 complex, cleaving GSDMD at the connector protein. Active Caspase-1 splits the pro-inflammatory cytokines, resulting in the enzymatic maturation of interleukin-1β (IL-1β) and interleukin-18 (IL-18).31 Rapid/excessive pore creation in the cytoplasmic membrane region releases several pro-inflammatory factors into the extracellular microenvironment, which further allows a variety of immune cells to penetrate and trigger inflammation.32,33 The creation of GSDMD-mediated cytoplasmic pores facilitates ion penetration into cells, resulting in osmotic pressure alterations, cell enlargement, cellular component release and pyroptosis.34 Accumulating evidence indicates that several factors such as potassium efflux, mitochondrial malfunction, ROS and mtDNA release, lysosome disruption, chloride efflux and calcium flux can trigger pyroptosis.35–41 Recent research investigations have demonstrated that NLRP3 inflammasome activation involves thioredoxin interacting protein (TXNIP), NIMA-related kinase 7 (NEK7), pannexin-1 and P2X7 receptor (P2X7R).28,42 Both pannexin-1 and P2X7R have been linked to the transport of potassium ions and fluctuations in adenosine triphosphate (ATP) levels.43,44 Therefore, the activation of canonical inflammasome pathway is a key driver in inducing pyroptosis-dependent cell death.
Non-Canonical Pyroptosis
The non-canonical pyroptotic signaling pathway has been extensively investigated alongside the canonical pyroptotic signaling pathway. In 2011, Caspase-11-infected macrophages with E. coli, Citrobacter corynii, or Vibrio cholera triggered pyroptosis without inflammatory bodies.45 Extracellular lipopolysaccharides (LPS) produced by Gram-negative bacteria can stimulate the transcription of cytokines via the toll-like receptor 4 (TLR-4).46 Caspase-4/5/11 is activated by LPS in macrophages and is transported to the cytoplasm with cholera toxin B.47 LPS induces Caspase-11 pathway activation in a TLR4-independent manner, indicating that Caspase-11 immediately responds to LPS.47 Pyroptosis can be triggered by the activation of Caspase 11 and 4 pathways, which bind directly to LPS. However, the precise role and function of LPS in activating Caspase-11 and its human homologs is still unknown. Caspase-4/5/11 mediates pyroptosis in mouse macrophages through GSDMD.48 According to Aglietti et al, p30 protein fragments from GSDMD splitting after Caspase-11 activation may attach to the membrane, resulting in pyroptosis and cellular rupture.49 Therefore, the activation of Caspase-4/5/11 signaling pathway induces cell swelling and cytoplasmic membrane denaturation, leading to pyroptosis (Figure 1). Caspase-11 cleaves and lyses membrane channel protein pannexin-1, deteriorating the channel for membrane small molecule release, triggering intracellular ATP efflux, activating P2X7 and mediating macrophage pyroptosis.50 Exogenous ATP activation of P2X7 induces the K+ efflux channel, thereby promoting the activation ofNLRP3 inflammasome complex.51 The Caspase-11-mediated non-canonical inflammasome can increase the efflux of potassium ions through pannexin-1, which facilitates the activation of NLRP3 inflammasome and Caspase-1, resulting in pyroptosis, maturation and outflow of several inflammatory mediators.35
Other Signaling Pathways
Caspase-3/8 and granzyme-mediated pathways are implicated in pyroptosis. Caspase-3/8 was once regarded as the primary trigger of apoptosis.52 Caspase-3-mediated cleavage of gasdermin E (GSDME) in tumor cells induced by chemotherapy and Caspase-8-mediated cleavage of GSDMD in mouse macrophages after infection with Yersinia reinforced this theory.53 Programmed cell death ligand 1 (PD-L1) modulates TNF-mediated apoptosis into pyroptosis in breast cancerous cells through the formation of GSDMCs.54 Zhang and co-workers revealed that PD-L1 is transported to the nucleus and upregulated by gasdermin C (GSDMC) with p-Stat3 during hypoxia. Caspase-8 selectively cleaves GSDMC, leading to the activation of pyroptosis.55 A combination of daunorubicin, actinomycin D, doxorubicin (DOX) and epirubicin activates Caspase-3/8-dependent signaling pathways and pyroptosis in breast cancer.54 Previous studies have revealed that granzyme can trigger pyroptosis without inflammatory or pro-apoptotic caspases.56,57 The research by Zhang et al showed that cytotoxic lymphocyte serine protease granzyme B (GzmB) directly cleaves GSDME in the targeted cells.58 In a further investigation, Zhou and his colleagues discovered that cytotoxic lymphocyte-released granzyme A (GzmA) could directly cleave GSDMB protein molecules and result in pyroptosis in the targeted cells.59
This review will discuss the molecular mechanisms and contributory role of pyroptotic signaling pathways in the pathogenesis and progression of DWH. This review also summarizes articles on the potential novel therapies for improving DWH by targeting pyroptosis-related signaling pathways.
Pyroptosis-Dependent Signaling Pathways in the Progression of DWH
Numerous research investigations indicate that reduced inflammatory response impairs skin wound healing in long-term wound healing models.60–64 Pyroptosis is associated with an inflammatory reaction characterized by elevated expression level of inflammasomes, GSDMD pore-forming protein and pro-inflammatory cytokines including IL-18 and IL-1β.65 The NLRP3 inflammasome, Caspase-1 multi-protein complex and ASC are the main regulatory pathways involved in triggering inflammation and pyroptosis following cellular injury. Recently, Yang et al showed that hyperglycemia (HG) activates NLRP3 inflammasome and increases the production of IL-1β in HaCaT cells.24 The activation of Caspase-1, NLPR3, ILβ and IL18 can further induce pyroptosis-dependent cell death. NLPR3 inflammasome signaling is crucial to the natural healing process of wounds. Elevated pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), IL-1β and interleukin 6 (IL-6) further facilitate the process of wound healing. Numerous research investigations have shown that the NALP3 inflammasome signaling pathway is crucial to skin wound healing.66,67 Moreover, robust inflammation significantly contributes to the development of chronic wounds.68 The activation of intracellular inflammasomes triggers massive inflammatory responses, resulting in pyroptotic cell death and the extensive release of several pro-inflammatory mediators. Cytoplasmic LPS further activates the Caspase-11-dependent non-canonical inflammasome signaling pathways. Growing evidence suggests that Caspase-11 activates Caspase-1 via the NLRP3 inflammasome pathway, resulting in the proteolytic maturation of pro-IL-1β and IL-18.45,47 Ao et al found elevated protein and mRNA expression level of GSDMD/Caspase-11/NLRP3/IL-1β in LPS-induced skin tissues.69 The study conducted by Lu et al revealed that pyroptosis mediated by nucleotide-binding oligomerization domain 1 (NOD1), NOD2 and GSDMD-N impairs skin wound healing in diabetic rats.70 Hyperglycemia disrupts injured skin tissue repair by the overactivation of NLRP1 inflammasome and pyroptosis-driven inflammation. The published work by Chen et al has indicated that the activation of canonical pyroptosis and excessive inflammatory reaction in fibroblasts impair wound healing of DFUs.71 Accumulating evidence indicates that72,73 the upregulation of mir-374a-5p reduces viability and activates apoptosis and pyroptosis of DFU fibroblasts.71 Metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) has been identified as a pyroptosis-related long non-coding RNA (lncRNA).74–76 NLRP3 and NEK7 are involved in the assembly of inflammasomes and the pyroptotic process.77 Reduced MALAT1 expression further activates NEK7/NLRP3/Caspase-1 signaling pathways, resulting in pyroptosis in fibroblast cells during DFUs. Gasdermin D (GSDMD) is a cytoplasmic protein that triggers inflammatory cell death through the formation of cytoplasmic pores.78 The activation of canonical and non-canonical inflammasome in macrophages induces the cleavage of GSDMD, cytoplasmic membrane pores and pyroptosis, a lytic pro-inflammatory cell death phenomenon.79 Pyroptosis is characterized by the excessive activation of NLRP3 cascade and Caspases, resulting in pore formation in the cytoplasmic membrane and the subsequent release of several pro-inflammatory factors such as IL-1β and IL-18. Current research indicates that the overactivation of NLRP3 inflammasome may play a contributory role in the development of DM and its related complications, particularly DWH.80 Recently, Liu and colleagues have shown that NETs activate the NLRP3 inflammasome and trigger inflammatory reactions in diabetic wounds.15 Yang et al reported that mice induced with diabetes by STZ exhibited increased activation of the NLRP3/Caspase-1/GSDMD signaling pathway in wound tissues.23 The examination of wound healing at macroscopic level revealed a significant improvement in wound closure in Gsdmd−/− mice with STZ-induced diabetes compared to the delayed wound healing observed in the STZ-induced diabetic animal. Interestingly, the deficiency of NLRP3/Caspase-1 alleviated the release of NETs and accelerated the process of wound healing. Neutrophil extracellular traps (NETs) in DFUs impair wound healing through the activation of GSDMD. Previous studies found that AIM2 and Casp-11-gene knockout mice did not exhibit significant protection against impaired diabetes-induced wound healing. To explore the process of GSDMD activation in neutrophils during the healing of DFU, the authors investigated both canonical (AIM2/NLRP3/Caspase-1) and non-canonical pathways for inflammasome activation (Caspase-11).23 The authors showed that hyperglycemia promotes the formation of neutrophil extracellular traps (NETs) and activates the NLRP3/Caspase-1/GSDMD signaling pathway in wounds of both humans and mice. Xu et al revealed that downregulated expression level of NLRP3, GSDMD, GSDMD-N, pro-caspase-1 and pro-IL-1β proteins contributes to the impairment of wound healing in hyperglycemic rats.81 A prominent study by Bitto and co-workers has demonstrated that NLRP3 inflammasome activation impairs the process of wound healing in diabetic mice.9 Prior research has also demonstrated that the persistent activation of the NLRP3 inflammasome in macrophages has a detrimental effect on the process of wound repair in T2D patients and mouse models.11 In addition to Caspase-1, the enzyme NE cleaves GSDMD to produce an active fragment.82 NE mediates the activation of GSDMD and the formation of NET. Hyperglycemia provides an inflammatory environment, which increases the expression of protein kinase R (PKR) and NALP3 in diabetic wounds.11 Diabetic wounds and macrophages treated with LPS and high glucose showed elevated levels of NALP3 and PKR.83 Caspase-1 and NALP3 gene-knockout mice exhibited diminished inflammatory response during the early stages of injury, resulting in impaired healing.84 PKR was activated by the Caspase-1 and NALP3 inflammasome signaling axis in mice with diabetes and delayed wound healing. The activated NLRP3 inflammasome in diabetic wounds induces elevated pro-inflammatory cytokines, which further contribute to local hyperglycemia, accumulation of AGEs and the formation of reactive oxygen species (ROS).85,86 ROS formation can induce premature senescence in endothelial progenitor cells (EPCs) in hyperglycemic conditions, which further disrupts the repair of damaged tissues and inhibits angiogenesis.87 Previous studies have revealed that inflammasome activation in macrophages impairs insulin sensitivity and angiogenesis, resulting in delayed DFUs healing.2,88,89 Emerging evidence also indicates that exogenous irritants significantly reduce the activity of NLRP3 inflammasome in diabetic wounds, which further inhibits the proliferation and migration of keratinocytes.90,91 Innate immune response to infections and tissue injury relies on the NLRP3 inflammasome, which triggers extensive inflammation.92 Recently, Koh and colleagues have revealed that NLRP3 inflammasome activation may also contribute to the progression of chronic wound inflammation and low skin wound healing in DM.93 Moreover, NLRP3 inflammasome activity at sustained levels leads to impaired epidermal and dermal healing. Koh et al also showed that deficient NLRP3 and Caspase-1 animals showed lower levels of IL-1β, TNF-a and neutrophils (Np) and macrophages (Mp) in wound healing.84 Therefore, the activation of canonical pyroptosis plays a contributory role in the progression of DWH (Figure 2).
Oxidative stress (OS) is a significant contributor to the pathogenesis of DM.94 TXNIP is an endogenous negative modulator of TXN, which regulates cellular redox homeostasis.95 Several studies have revealed that TXNIP has a dual effect of exacerbating OS and triggering inflammation via activating the NLRP3 inflammasome.96–98 The activity of the TXNIP-NLRP3 inflammasome induces GSDMD-dependent pyroptosis, which may contribute to excessive inflammation. Dysregulation of TXNIP is implicated in the progression of diabetes and insulin resistance.99 Increasing evidence indicates that high glucose and O2 production and impaired nitric oxide (NO) signaling trigger inflammation via triggering TXNIP.100–102 TXNIP interacts with NLRP3 protein molecules in a ROS-dependent manner. Previous research indicates that ROS overproduction leads to elevated TXNIP levels, NLRP3 activation, Caspase-1 activation and IL-1β generation.103,104 Angiogenesis and vascular repair may be adversely affected by elevated IL-1β.105 Deng et al observed a significant increase in the protein expression levels of TXNIP/NLRP3/Caspase-1 in diabetic mice and HG-stimulated endothelial progenitor cells (EPCs). Taken together, the above findings suggest that activation of the pyroptotic signaling pathway contributes to the progression of DWH.106
Hypoxia impairs angiogenesis, which further induces excessive cell death.107,108 Apoptosis and necroptosis are classic mechanisms underlying this effect. Pyroptosis is an important type of cell death that is related to inflammation. Multiple studies have revealed that pyroptosis impairs cell proliferation and in vitro functions such as migration and adhesion, whereas hypoxia can lead to pyroptosis.109,110 Recently, Zhang et al reported that PCSK9 induces hypoxia-induced vascular EC pyroptosis via Smac mitochondrion-cytoplasm translocation in critical limb ischemia.111 Even though ECs directly perceive changes in blood oxygen, few studies have focused on the contributory role of hypoxia-induced pyroptosis in the progression of DWH. In addition, the exact contributory role of ischemia-mediated pyroptosis in the progression of DWH is entirely elusive. Thus, further studies are highly warranted to explore the underlying molecular mechanisms of the impacts of hypoxia and ischemia on the activation of inflammasome and pyroptosis in DWH.
Suppressing Pyroptosis-Dependent Signaling in Therapeutic Regulation of DWH
Disulfiram (DIS), developed in the 19th century, inhibits aldehyde dehydrogenase and is an effective therapeutic agent for the treatment and management of alcoholism.112 A recent study has revealed that DIS is an effective inhibitor of GSDMD pore-forming protein.113 Previous studies suggest that DIS mitigates robust inflammation and the formation of fibrosis by inhibiting the activation of canonical pyroptosis in a variety of diseases.114–117 GSDMD is a well-established cytoplasmic pore-forming protein that induces pyroptosis-regulated cell death. This pore-forming protein in the cytoplasmic membrane allows the massive release of IL-1β, IL-18 and other inflammatory mediators. Yang et al recently found that the administration of DIS significantly accelerated wound healing in DFUs by lowering the release of NETs.23 Mechanistically, DIS hinders the formation of NET by inhibiting the activation of NLRP3/Caspase-1/GSDMD signaling pathways in rat models of DFUs. GSDMD serves as the effector of NETosis, a process that forms neutrophil extracellular traps (NETs). Potential inhibitors can be used to treat immunopathological disorders involving NETs. Therefore, the GSDMD inhibitor DIS exhibits the therapeutic potential for improving wound healing in patients with DFUs and could be utilized in clinical applications.
Several stem cells derived from umbilical cords, bone marrow and hair follicles have garnered considerable attention in the field of regenerative medicine.118 Numerous studies have confirmed that transplanted stem cells promote tissue repair through paracrine action.119–121 In addition, exosomes produced by stem cells during the paracrine process have attracted considerable attention in the field of regenerative medicine.122 Studies have revealed that exosomes produced by stem cells can facilitate kidney damage repair, attenuate myocardial infarction areas, modulate immune responses and accelerate the healing of skin woundss. Recent studies have identified a variety of long non-coding RNAs (lncRNAs) in exosomes.123,124 Li et al have indicated that mesenchymal stem cells-derived lncRNA H19 accelerates wound healing by enhancing PTEN via microRNA-152-3p in DFUs.125 Yang et al also showed that HF-MSC-Exo markedly suppressed the production of IL-1β and IL-18 and activation of Caspase-1 and decreased the presence of the NLRP3 inflammasome in HaCaT cells.125 The previous work also demonstrated that HF-MSC exosomal lncRNA H19 facilitates fibroblast proliferation and migration and attenuates pyroptosis, which further accelerates wound healing and DFU-induced tissue damage. Based on a mechanistic analysis, HF-MSC-Exo-expressing lncRNA H19 accelerates the healing of skin wounds by inhibiting the canonical pyroptotic signaling pathway in vitro and in vivo. Therefore, H19-overexpressing exosomes derived from HFMSCs could be considered a potential component in therapeutic approaches aimed at alleviating pyroptosis and enhancing the healing of skin wounds in the treatment of diabetic complications. However, the underlying molecular mechanism of HF-MCSs targeting non-canonical pyroptosis remains unknown. Thus, further studies must be executed to analyze the therapeutic efficacy of HF-MCSs in improving wound healing of diabetic patients.
Mesenchymal stem cells (MSCs) are a distinct type of cells that can undergo self-renewal and differentiate into a variety of bone cells.126 Bone marrow-derived MSCs have been widely utilized in the field of regenerative medicine.127,128 Numerous research studies have been executed to explore the application of MSC-CM in the treatment and management of skin wounds.129–131 BM concentrate-induced MSCs conditioned medium accelerates wound healing and attenuates the formation of hypertrophic scarring.132 A study showed that the application of concentrated hypoxia-preconditioned adipose mesenchymal stem cell-conditioned medium accelerated skin wound healing in a rat model with full-thickness skin defects.133 Moreover, bone marrow-derived mesenchymal stem cell-conditioned medium (BMMSC-CM) in rats improved keratinocyte proliferation and migration in a diabetes-induced microenvironment by suppressing ROS overproduction and reversing MEK 1/2 and Erk 1/2 phosphorylation.134 A new study has revealed that the application of adipose-derived stem cell conditioned medium (CM) can enhance wound healing and promote hair growth in SD rats with burn wounds on their dorsal region.135 Pioneering research by Xu et al indicated that the application of BMMSC-CM mitigates inflammation, enhances autophagy and alleviates NLRP3/Caspase-1/GSDMD-dependent pyroptosis in DFUs (Table 1).81 Therefore, BMMSC-CM can be used as a new cell-free therapeutic approach in suppressing pyroptosis-dependent cell death to accelerate the healing process of DFUs.
 |
Table 1 Compounds/Agents Targeting Pyroptosis-Related Signaling Pathways for the Therapeutic Regulation of DWH |
Histone deacetylase (HDAC6) may deacetylate substrates such as α-tubulin, peroxiredoxin-1 and 2 and HSP90. Past research has indicated that inhibition of HDAC6 possesses promising anti-oxidant and anti-inflammatory actions without acetylating histones or altering epigenetics.148 TSA is a selective inhibitor of HDAC6, as compared to other HDACs. Xu et al reported that TSA suppressed the activation of NLRP3-mediated pyroptosis by enhancing the signaling of transcription factor EB.149 Pioneering research conducted by Kulkarni and co-workers showed that topical application of HDAC6 inhibitor TSA accelerated wound healing through alleviating Caspase-1-dependent pyroptosis in diabetic mice.136 Accumulating studies indicate that suppressing IL-1β and inflammasome components may accelerate wound healing by increasing the levels of IL-10.11,15,150 In addition, the administration of TSA resulted in improved wound healing in diabetic mice through the suppression of pro-inflammatory cytokines and the promotion of pro-healing factors. Therefore, TSA gel could be a new therapeutic drug candidate for accelerating DWH via the regulation of pyroptosis-mediated cell death (Figure 3). More clinical studies are highly warranted to determine the therapeutic efficacy and explore the anti-pyroptotic targets of TSA in the treatment and management of DWH.
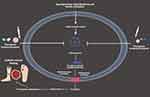 |
Figure 3 Compounds/agents targeting pyroptosis-related signaling pathways for the therapeutic regulation of DWH. |
PKR is upregulated in metabolic conditions and mediates metabolic signaling and inflammation.151 Multiple studies have elucidated the functions of PKR in modulating insulin signaling and enhancing glucose homeostasis in DM.152–155 There is evidence that PKR interacts with NALP3 protein molecules and is necessary for assembling inflammasomes.156 Kulkarni and colleagues evaluated the therapeutic potential of PKR inhibitors in delayed wound healing of diabetic patients using C16 hydrogel.83 Selective PKR inhibition with C16 fostered faster wound healing, decreased levels of p-PKR and p-eIF2α, enhanced angiogenesis, alleviated inflammatory cell accumulation and improved wound strength.83 Therefore, C16 can deliver a new therapeutic effect for treating DWH via the regulation of pyroptosis.
The pleiotropic vitamin D (VD) is derived from dietary supplements or produced endogenously in the skin by UV radiation (Baeke).157 Growing evidence indicates that vitamin D deficiency impairs insulin synthesis and secretion, while vitamin D-supplemented diets remarkably improve insulin production.158,159 In addition, vitamin D regulates plasma calcium and tissue inflammation during wound healing.160 Lu et al reported that mice lacking the VD receptor (VDR− /-) exhibited impaired corneal epithelial wound healing and minimized tight junction integrity at the wound margin. Topically administered vitamin D impairs corneal epithelial wound healing in wild-type mice.161 Calcium-rich diet partially improves corneal wound healing in diabetic mice with VDR− /- and VD deficiency.162 Vitamin D regulates the differentiation of epidermal and hair follicles. Recently, Zhou and co-workers have revealed that the topical application of calcitriol (CAL) enhances the healing of diabetic corneal wounds and the regeneration of peripheral nerves by impeding the activation of NLRP3 inflammasome.137 It was also found that the inhibition of NLRP3/IL-1β accelerates diabetic corneal wound healing and reinnervation. Therefore, CAL may be an anti-pyroptotic agent that accelerates the process of wound healing in DM.137 Therefore, the topical application of CAL provides a potentially effective administrative route in the treatment of DWH. However, further studies are highly required to analyze the pharmacological target of CAL alleviating GSDMD/GSDME-dependent pyroptosis in accelerating wound healing in patients with DM.
Current studies indicate that targeting inflammasome delivers a potential therapeutic avenue for improving DWH.163 The consistent activation of NLRP3 has been considered to impede the process of wound healing in patients with DFUs.164 USP30 regulates the viability and migration of skin fibroblasts by targeting the NLRP3 inflammasome. Furthermore, NLRP3 is implicated in the modulation of IL-1β/IL-18 and MMP-2/MMP-9 by USP30. The NLRP3 inflammasome modulates the level of Caspase-1 p20 protein by regulating USP30. Li et al showed that MF-094 is a strong and specific USP30 blocker, which modulates the NLRP3 inflammasome to promote DWH.138 MF-094 inhibits USP30 and its downstream target Caspase-1 p20 in diabetic rats, accelerating wound healing and reducing the levels of NLRP3, demonstrating the physiological significance of USP30-NLRP3. Further studies are required to explore and confirm the physiological relevance of USP30-NLRP3 regulation by manipulating the NLRP3 inflammasome in vivo. In summary, these findings suggest that MF-094 could be a promising drug candidate for suppressing NLRP3 inflammasome-mediated pyroptosis in the treatment and management of DWH.
Inflammasomes regulate the innate immune response and trigger inflammatory reactions.66 The NLRP3 inflammasome is well-recognized as the predominant inflammasome. It comprises three key components: NOD-like receptor protein 3, ASC and Caspase-1. Recent research indicates that the NLRP3 inflammasome plays a crucial role in the progression of wound healing.165,166 Growing evidence suggests that inhibition of the NLRP3 inflammasome and cleaved IL-1β mitigates inflammation and accelerates wound healing.141,166 Intriguingly, Chiu et al revealed that the modulation of NLRP3 inflammasome by the activation of autophagy resulted in the mitigation of burn wound development and enhancement of wound healing in a rat burn model.167 The modulation of M2 macrophage polarization by inhibiting the activities of NLRP3 inflammasome might potentially serve as a novel therapeutic strategy to enhance the wound-healing process.9 Topical pharmacological inhibitors suppressed the functions of the inflammasome in mice wounds, induced a switch from pro-inflammatory (M1 macrophage) to healing-associated (M2 macrophage) phenotypes and increased the levels of pro-healing growth factors.168 Metformin (MTF), a biguanide, is widely prescribed for the treatment of T2DM. It is well known that MTF, an AMPK activator, inhibits wound healing in patients with diabetes.169 MTF enhanced wound healing, promoted angiogenesis and elevated circulating endothelial progenitor cell (EPC) count.170 Fatma et al suggest that MTF accelerates the healing of cutaneous wounds in STZ-induced diabetic rats.139 Zhao and his colleagues found that MTF locally enhanced the epidermis, hair follicles and collagen deposition, accelerating the process of wound healing.171 Many studies have shown that MTF can significantly inhibit pyroptosis-dependent cell death in alleviating the progression of multiple diseases including diabetic complications.99,172–176 Intriguingly, Qing et al have suggested that MTF increases the polarization of M2 macrophage to accelerate wound healing by modulating the AMPK/mTOR/NLRP3 inflammasome pathways.177 The aforementioned studies have contributed novel perspectives on the molecular mechanism behind MTF treatment and its prospective therapeutic applications in the context of wound healing.
Bletilla striata (Thunb.) Reichb. f. belongs to the group of plants commonly used in traditional Chinese medicine. Bletilla striata has been documented in Chinese historical literature as a folk medicine to stop bleeding and enhance tissue regeneration and wound healing in the skin.178 Bletilla striata are primarily composed of polysaccharides.179 Bletilla striata polysaccharide (BSP) has a wide range of pharmacological actions including anti-inflammatory, anti-oxidant, immunomodulatory, anti-aging and wound and ulcer healing.180–184 Due to these promising properties, BSP is a viable material for wound dressing, hydrogel, tissue engineering scaffolds and drug delivery vehicles.178,185–187 According to Yu et al, BSP promotes fibroblast infiltration and collagen formation in wounds in diabetes with DFUs.188 Further research revealed that BSP mitigated the formation of angiotensin II–induced ROS and the release of pro-inflammatory cytokines in human mesangial cells.180 Recently, Zhao and co-workers showed that BSP intervention enhanced DWH in mouse models by reducing macrophage penetration, promoting angiogenesis, suppressing NLRP3 inflammasome overactivation, lowering IL-1β release and improving insulin sensitivity.140 Therefore, BSP can be an effective therapeutic drug candidate for accelerating diabetic wounds via suppressing pyroptosis-mediated cell death. However, further research is required to explore the pharmacological target of BSP in GSDMD/GSDME-dependent pyroptosis as a potential treatment for DFUs.
Dynamic interactions between extracellular matrix (ECM) and growth factors have been associated with the process of wound healing.189 Heparin sulfate (HS) glycosaminoglycan is essential to maintain the extracellular matrix (ECM), which consists of scaffold proteins. A series of studies have revealed that HS modulates the function of proteolytic enzymes, morphogens, chemokines and growth factors produced in the tissues.190,191 However, Bame and co-workers have shown that local matrix metalloproteases and serine proteases eradicate the actions of HS.192 In the early period of DWH, the normal balance of HS is disrupted. The research by Wang et al suggests that HS enhances wound healing in diabetic rats by mitigating inflammation.141 The inflammasome represents the complex of multiple proteins that activate the immune system of the host in response to harmful stimuli. Mirza and colleagues have suggested that the NLRP3 inflammasome plays a crucial role in regulating the inflammatory response in wounds of diabetic mice.11 Menini et al report that the accumulation of DAMPs stimulates the NLRP3 inflammasome, resulting in insulin resistance and organ dysfunction.193 Glucotoxicity and chronic inflammation in diabetes are linked to the overactivation of the NLRP3 inflammasome axis.193 Therefore, impeding the NLRP3 inflammasome accelerates DWH in glucotoxicity. Intriguingly, Wang et al found that HS mitigates inflammation and promotes tissue wound healing in diabetic rats by inhibiting the activation of NLRP3 inflammasome and IL-1β cleavage.141 Therefore, HS could be a promising anti-pyroptotic drug for accelerating DWH.
Sanguisorba officinalis L., a traditional Chinese medicinal plant, is commonly applied for the treatment of burns and scalds.194,195 Current research suggests that S. officinalis L. possesses promising anti-oxidant, anti-inflammatory, anti-infectious and anti-allergic effects.196–198 Cheng et al indicated that oral supplementation of Sanguisorba officinalis extract resulted in faster wound contraction, reduced epithelialization timeline, increased hydroxyproline substances and elevated IL-1β and VEGF levels, promoting collagen synthesis and angiogenesis in experimental burn wounds.199 Song and co-workers report that Sanguisorba officinalis L. facilitates diabetic wounds in rats by modulating the NLRP3/Caspase-1 canonical pyroptotic signaling pathways.142 Intriguingly, the researchers found that ESO could markedly mitigate inflammation and enhance diabetic wound closure via modulating the NF-κB/NLRP3 signaling axis, obstructing M1-like polarization and increasing M2-like polarization of macrophages. Therefore, inflammasome-dependent pyroptosis and homeostasis in the immune system can be targeted with ESO in diabetic wounds to provide new insights into DWH processes.
Genistein (GST), a legume isoflavone, is recognized for its estrogen-like actions.200 The administration of GST regulates the anti-oxidant defense system and pro-inflammatory factors to enhance the process of wound healing.201 Current evidence suggests that NLRP3 accelerates wound healing in barrier tissues including skin and epithelial cells.202,203 Further research revealed that GST enhances wound healing in hyperglycemic mice by regulating the ASC/NLRP3/Caspase-1 inflammasome, nuclear factor erythroid 2-related factor and associated indicators.143 In-vitro analysis showed that ESO administration significantly suppressed the formation of NLRP3, Caspase-1 and IL-1β in RAW264.7 cells treated with LPS. Therefore, the administration of GST may be effective in preventing and treating delayed wound healing through the alleviation of inflammation and Caspase-1-dependent pyroptosis during the inflammatory phase.
Human umbilical cord mesenchymal stem cells (UCMSCs) are multipotent stem cells obtained and cultured from the umbilical cord.204 Multiple studies suggest that UCMSCs exert significant proliferation efficiency compared to BM-derived MSCs, which may accelerate wound healing and tissue regeneration.205–208 A study by Wang et al demonstrated that ApoEVs derived from UCMSCs improved wound healing in db/db mice by suppressing the level of pyroptosis-associated proteins such as NLRP3, cleaved caspase-1, GSDMD, IL-1 and IL-18.144 The authors showed that NLRP3, Caspase-1, GSDMD and F4/80 co-localized in pyroptotic BMDMs and ApoEV treatment significantly reduced the number of positive cells and fluorescence intensity in LPS/ATP-induced macrophages. Research investigations have shown that higher blood sugar levels may result in macrophage oxidative stress through the generation of excessive ROS in oxidative phosphorylation.209 Studies on humans and animals have revealed that oxidative stress-related enzymes and metabolites significantly impede wound healing.210–214 Growing evidence indicates that excessive ROS formation may activate NLRP3 inflammasome axis, resulting in pyroptosis.215 ApoEVs derived from UCMSCs have been shown to effectively inhibit the accumulation of ROS, mitigate the generation of OS in macrophages and alleviate the occurrence of pyroptosis.144 It has been demonstrated that the administration of ApoEVs is effective in modulating different types of cell death in a variety of cell types. Therefore, UCMSCs may provide a novel therapy for treating T2D-mediated wound healing and extend our understanding of the regulation of cell death. Regrettably, the existing evidence does not establish a definitive relationship between apoptosis and the prevention of pyroptosis. Several studies have indicated that apoptotic products can potentially affect pyroptosis, thereby providing information regarding the regulation of cell death.
Rapamycin (RAP), an mTOR inhibitor, is commonly used as an immunosuppressive drug following renal, liver and heart transplantation.216 Xiao et al showed that RAP increased epidermal autophagy in a rat model of severe second-degree burn lesions.217 Previous studies indicate that RAP impairs wound healing by inhibiting the activation of γδ T cells in skin tissues.218 The mammalian target of rapamycin (mTOR) inhibits autophagy, which is necessary for the activation of NLRP3 and the release of IL-1β pro-inflammatory cytokine.219,220 NF-κB is a significant nuclear transcription factor that regulates multiple cellular processes in the inflammatory response. Recent research suggests that RAP mitigates inflammation by modulating the activation of NF-κB pathway.221 Chai and co-workers have reported that RAP suppresses HG-induced NLRP3 inflammasome activation by restricting the mTOR/NF-κB pathway in macrophages.145 Elevated glucose levels promote the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the production of ROS, leading to the activation of redox-sensitive NF-κB. RAP blocks the NF-κB pathway by activating mTOR, resulting in the inhibition of the endogenous IKK. In the previous work, the authors also showed that a deficit of mTOR hinders the activation of NF-κB pathway and NLRP3 inflammasome.145 Therefore, RAP may treat diabetes-induced inflammatory response in wound healing. The therapeutic significance of recent discoveries could be confirmed by in vivo studies exploring the regulation of inflammasome for the treatment of DWH.
Paeoniflorin (PAF) is a monoterpene glycoside that originated from the dried and peeled root of Paeoniae albaradix.146 PAF is an anti-oxidant and anti-inflammatory compound found in Paeonia alba radix.222 Shao et al discovered that PAF treatment efficaciously suppressed the secretion level of inflammatory factors and chemokines (specifically TNF-α, IL-1β and MCP-1) by blocking TLR2, resulting in the alleviation of diabetic nephropathy.222 Past research has indicated that PAF mitigates LPS-induced inflammation in mice by inhibiting the activation of NLRP3 inflammasome.223 It has been demonstrated that PAF accelerates wound healing in diabetic rats through the activation of the Nrf2 pathway.224 Chemokines including chemokine (C-X-C) ligands 1 and 2 trigger C-X-C motif chemokine receptor 2 (CXCR2) in neutrophils. IL-1β activates the NF-κB signaling pathway, leading to the primary expression of these chemokines. TNF-α modulates the networks of chemokine in inflammation-induced disorders by activating the NF-κB signaling pathway. Previous studies have revealed that restricting CXCR2 mitigates and reverses the progression of T1DM in mouse models.225 Moreover, inhibition or knockdown of CXCR2 improved islet transplant survival and function. Recently, PAF has been found to exert significant suppressory effects on the NLRP3 inflammasome and NF-κB-mediated inflammatory responses in DFUs through the inhibition of the chemokine receptor CXCR2.146 Therefore, PAF may be a potential NLRP3-inflammasome-mediated pyroptotic inhibitor in alleviating the pathogenesis and progression of DWH.
Bacillus subtilis, a probiotic, modulates the immune response, remodels gut microbiota, extends fermentation and boosts the economy.226 Past studies identified a new cyclic lipopeptide from Bacillus subtilis CAU21 and bacteriocin (BAC-IB17) from Bacillus subtilis KIB17.227,228 B. subtilis (WB800N) can also modulate immunological responses.229 Current research has shown that amoxicillin decreases microbiota alpha and beta diversities, disrupting gut microbiota and relieving diabetic wounds in mice.230 Bacillus subtilis is an oral microbe that regulates gut diversity and composition (WB800N and DSM 32315).231,232 A pioneering study performed by Li and co-workers showed that Bacillus subtilis WB800N accelerates wound healing in diabetic mice by regulating the function of TLR2.147 TLR2 is the primary factor implicated in the innate immune response. Stimulating the immune response proved advantageous for the healing of wounds in patients with diabetes.233 The TLR2/NLRP3/Caspase-1 axis plays a regulatory role in immune response.234 The previous work showed that Bacillus subtilis WB800N upregulated the protein expression levels of TLR2, NLRP3, ASC and Caspase-1 in diabetic mice.147 TLR2 is essential for pro-IL-1β induction, whereas NLRP3/ASC is indispensable for Caspase-1 activation and subsequent cleavage of pro-IL-1β, resulting in the production of mature IL-1β. IL-1β is a pro-inflammatory cytokine that plays a fundamental role in driving infection-mediated inflammation.235 The combination of Bacillus subtilis and TLR2 antagonist abolished the suppressory effects of SsnB on serum IL-1β, IL-37 and skin wound cell apoptosis.147 Bacillus subtilis WB800N was found to induce inflammation and cell apoptosis through the activation of TLR2, leading to an expedited healing process for diabetic wounds. Thereofre, these findings imply that WB800N might be a canonical pyroptotic inhibitor in the pharmacological treatment and management of DWH.
Glyburide (GLD) is a popular sulfonylurea medication for T2DM, which can inhibit NLRP-3 inflammasome overactivation and enhance insulin production from pancreatic β-cells through a distinct mechanism.236 A recent work by He et al suggests that GLD exhibits diverse anti-inflammatory actions including the inhibition of inflammasome assembly.237 It has been reported that GLD inhibits the activation of inflammasomes by blocking ATP-sensitive potassium channels (KATP).238 Previous research showed that GLD accelerates wound healing by anti-inflammation and RIP140 degradation.239 Diabetic wounds promote the activation of inflammasomes in cultured macrophages through pathways mediated by ROS and IL-1β, contributing to a pro-inflammatory positive-feedback loop that perpetuates the inflammatory response.85 To ascertain the potential enhancement of wound healing in db/db mice by the inhibition of inflammasome activity, Koh et al administered the NLRP-3 inflammasome blocker GLD and the irreversible Caspase-1 blocker YVAD directly into the wounds of the animals.11 The application of pharmacological inhibitors or bone marrow transfer from mice lacking NLRP3 or Caspase-1 to db/db mice can inhibit the activation of inflammasomes and facilitate early healing in diabetic wounds. This is achieved by reducing the pro-inflammatory characteristics of macrophages and promoting factors that aid in wound healing. The application of GLD remarkably improved tissue healing in db/db mice via suppressing NLRP3/Caspase-1-dependent pyroptosis. Therefore, GLD could be a promising pyroptotic inhibitor to accelerate wound repair in patients with DM. However, more clinical research is highly required to clarify the potential therapeutic advantages of GLD in suppressing pyroptosis in various aspects of DWH.
NLRP3 inflammasomes are crucial pro-inflammatory mediators regulating host responses to diverse stresses.240 Ac-YVAD-cmk is a selective, irreversible inhibitor of Caspase-1 that may inhibit the activation of pyroptosis and mitigate the release of the pro-inflammatory cytokines IL-1β and IL-18. In the prior study, the administration of YVAD was shown to downregulate the concentrations of IL-1β and IL-18 in the dermal wounds of diabetic db/db mice.11 This finding aligns with the inhibitory effects of Ac-YVAD on the NLRP3 inflammasome, which ultimately resulted in enhanced reepithelialization and augmented collagen accumulation. Thus, Ac-YVAD-cmk could potentially serve as a promising therapeutic agent for improving DWH. Further research is required to examine the possible therapeutic benefits of Ac-YVAD-cmk in DWH.
The development of effective inhibitors of NLRP3 inflammasomes is currently in progress. BAY 11–7082 is an inhibitor of I-B kinase-I, which suppresses the ATPase activity required to activate NLRP3 and directly targets its inflammasome.241 A work published by Sung and colleagues indicates that Bay11-7082 promotes wound healing by suppressing the expression of matrix metalloproteinases (MMPs) induced by mechanical injury and TNF-α in the posterior cruciate ligament.242 Mechanistically, BAY 11–7082 impedes the activation of NLRP3 inflammasome-dependent inflammatory pathways and enhances angiogenesis. NF-kB and inflammasome blockage are difficult to distinguish in complicated biological processes including wound healing. The previous study by Bitto et al showed that the application of Bay 11–7082 to skin wounds in db/db mice resulted in an expedited healing process and increased reepithelialization.9 Additionally, there was a reduction in the presence of NLRP3 inflammasome components and associated factors such as Caspase-1, IL-1β and IL-18. Current investigations have revealed that NLRP3 agonists promote the production of ROS, which further induces the activation of NLRP3 inflammasome through the ROS-sensitive NLRP3 ligand TXNIP.243 Mitochondria and NADPH oxidase are the primary sources of ROS triggered by external stimuli. The mitochondrial respiratory chain primarily produces ROS in cells. ROS overproduction induces DNA damage, lipid peroxidation and protein oxidation in oxidative stress.244 Dai et al showed that the application of Bay 11–7082 further accelerates wound healing in diabetic rats by attenuating the formation of ROS.67 More importantly, Bay 11–7082 enhanced wound closure and suppressed the activation of NLRP3-inflammasome components including ASC, caspase-1 and IL-1β in STZ-induced diabetic rats.67 Therefore, Bay 11–7082 may have potential therapeutic benefits in accelerating wound healing in diabetic patients.
A diaryl sulfonylurea-containing molecule MCC950, is a potent and specific NLRP3 inflammasome inhibitor.245 Accumulating studies have indicated that MCC950 ameliorates diabetic complications including diabetic renal injury by suppressing NLRP3/Caspase-1-dependent pyroptosis.246,247 It has also been reported that MCC950 obstructs the activation of NLRP3 but not AIM2, NLRC4 or NLRP1 inflammasomes.248 Previous studies indicated that MCC950 inhibits the activation of canonical and non-canonical inflammasome signaling pathways and alleviates the production of pro-inflammatory mediator IL-1β by hindering the oligomerization of ASC.249 In addition, Wang and co-workers showed that MCC950 intervention promoted diabetic corneal wound healing and reinnervation by inhibiting the activation of the NLPR3/Caspase-1/IL-1β-dependent pyroptotic signaling axis.137 The authors clearly showed that the application of MCC95O downregulated the pyroptosis-dependent protein and mRNA expression levels, resulting in the alleviation of diabetic wounds in mice. Therefore, these findings suggest that MCC950 could be a promising inflammasome-mediated pyroptotic inhibitor in accelerating diabetic wounds.
Conclusions and Perspectives
Specifically, the NLRP3-Caspase-1-GSDMD signaling axis has been identified as the principal mechanism underlying pyroptosis in the pathogeneiss and progression of DWH. Furthermore, long non-coding RNAs (lncRNAs) play a crucial role in regulating cell pyroptosis in DWH. Natural compounds and traditional medicines have been found to suppress pyroptosis-regulated cell death by inhibiting the activation of NLRP3 inflammasome. However, the current evidence is limited and does not establish strong directions of the contributory roles of pyroptosis in the progression of DWH. The majority of studies have been conducted using animal models. Therefore, further investigations are required to investigate the specific mechanisms and potential functions of pyroptosis-mediated cell death in the progression of DWH. Inflammatory pyroptosis may be able to serve as a biological marker for predicting the pathogenesis and progression of DWH. There is a limited understanding of effective strategies to modulate pyroptosis in order to prevent or treat DWH. The first and most important approach in treating diabetic complications including DWH is lowering blood glucose levels. Therefore, further investigations are highly required to explore the complex relationship between pyroptosis, diabetes and DWH. Increasing evidence indicates that some natural compounds/agents can be employed as therapeutic targets in inhibiting pyroptosis and alleviating inflammation for the treatment and management of DWH. In conclusion, research efforts to address these questions and other essential concerns would offer an innovative perspective for the effective treatment and management of DWH in the near future.
Funding
This review work was partially supported by the grants of the Zhejiang Provincial Natural Science Foundation of China (LQ21H090001), the National Natural Science Foundation of China (Grants 82172428 and 81972150) and the Post-Doctoral Research Start-up Fund of Lishui People’s Hospital, Zhejiang, China (2023bsh001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Translational Res. 2021;236:109–116. doi:10.1016/j.trsl.2021.05.006
2. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199(1):17–24. doi:10.4049/jimmunol.1700223
3. Akkus G, Sert M. Diabetic foot ulcers: a devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World j Diabetes. 2022;13(12):1106–1121. doi:10.4239/wjd.v13.i12.1106
4. Gosak L, Svensek A, Lorber M, Stiglic G. Artificial Intelligence Based Prediction of Diabetic Foot Risk in Patients with Diabetes: a Literature Review. Appl Sci. 2023;13(5):2823. doi:10.3390/app13052823
5. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vascular Surg. 2016;63(2 Suppl):3s–21s. doi:10.1016/j.jvs.2015.10.003
6. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann Vasc Surg. 2016;33:149–158. doi:10.1016/j.avsg.2015.11.025
7. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi:10.1007/s00125-008-0940-0
8. Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204. doi:10.2337/db12-0420
9. Bitto A, Altavilla D, Pizzino G, et al. Inhibition of inflammasome activation improves the impaired pattern of healing in genetically diabetic mice. Br J Pharmacol. 2014;171(9):2300–2307. doi:10.1111/bph.12557
10. Ding S, Xu S, Ma Y, Liu G. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules. 2019;9(12).
11. Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63(3):1103–1114. doi:10.2337/db13-0927
12. Feng H, Gu J, Gou F, et al. High Glucose and Lipopolysaccharide Prime NLRP3 Inflammasome via ROS/TXNIP Pathway in Mesangial Cells. J Diabetes Res. 2016;2016:6973175. doi:10.1155/2016/6973175
13. Qiu YY, Tang LQ. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res. 2016;114:251–264. doi:10.1016/j.phrs.2016.11.004
14. Qiu Z, He Y, Ming H, Lei S. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J Diabetes Res. 2019;2019:8151836. doi:10.1155/2019/8151836
15. Liu D, Yang P, Gao M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci. 2019;133(4):565–582. doi:10.1042/cs20180600
16. Huang C, Ogawa R. Role of Inflammasomes in Keloids and Hypertrophic Scars-Lessons Learned from Chronic Diabetic Wounds and Skin Fibrosis. Biomolecules. 2022;23(12).
17. Brokatzky D, Mostowy S. Pyroptosis in host defence against bacterial infection. Dis Models Mech. 2022;15(7). doi:10.1242/dmm.049414
18. Hu Y, Wang B, Li S, Yang S. Pyroptosis, and its Role in Central Nervous System Disease. J Mol Biol. 2022;434(4):167379. doi:10.1016/j.jmb.2021.167379
19. Hu X, Chen H, Xu H, et al. Role of Pyroptosis in Traumatic Brain and Spinal Cord Injuries. Int J Bio Sci. 2020;16(12):2042–2050. doi:10.7150/ijbs.45467
20. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Mar. 2019;52(2):e12563. doi:10.1111/cpr.12563
21. Rao Z, Zhu Y, Yang P, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12(9):4310–4329. doi:10.7150/thno.71086
22. Li X, Xiao GY, Guo T, Song YJ, Li QM. Potential therapeutic role of pyroptosis mediated by the NLRP3 inflammasome in type 2 diabetes and its complications. Front Endocrinol. 2022;13:986565. doi:10.3389/fendo.2022.986565
23. Yang S, Feng Y, Chen L, et al. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Translational res. 2023;254:115–127. doi:10.1016/j.trsl.2022.10.008
24. Yang H, Zhang Y, Du Z, Wu T, Yang C. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging. 2023;15(3):791–809. doi:10.18632/aging.204513
25. Verma V, Dhanda RS, Møller NF, Yadav M. Inflammasomes and Their Role in Innate Immunity of Sexually Transmitted Infections. Front Immunol. 2016;7:540. doi:10.3389/fimmu.2016.00540
26. Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Critical Rev Immunol. 2010;30(5):463–487. doi:10.1615/critrevimmunol.v30.i5.50
27. Rathinam VA, Fitzgerald KA. Inflammasome Complexes: emerging Mechanisms and Effector Functions. Cell. 2016;165(4):792–800. doi:10.1016/j.cell.2016.03.046
28. Fu J, Wu H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Ann Rev Immunol. 2023;41(1):301–316. doi:10.1146/annurev-immunol-081022-021207
29. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
30. Wang K, Sun Q, Zhong X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180(5):941–955.e20. doi:10.1016/j.cell.2020.02.002
31. Sun Q, Scott MJ. Caspase-1 as a multifunctional inflammatory mediator: noncytokine maturation roles. J Leukocyte Biol. 2016;100(5):961–967. doi:10.1189/jlb.3MR0516-224R
32. Liu X, Xia S. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discovery. 2021;20(5):384–405. doi:10.1038/s41573-021-00154-z
33. Li H, Wang Z, Fang X, et al. Poroptosis: a form of cell death depending on plasma membrane nanopores formation. iScience. 2022;25(6):104481. doi:10.1016/j.isci.2022.104481
34. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
35. Xu Z, Chen ZM, Wu X, Zhang L, Cao Y, Zhou P. Distinct Molecular Mechanisms Underlying Potassium Efflux for NLRP3 Inflammasome Activation. Front Immunol. 2020;11:609441. doi:10.3389/fimmu.2020.609441
36. Li Q, Shi N, Cai C, et al. The Role of Mitochondria in Pyroptosis. Front Cell Develop Biol. 2020;8:630771. doi:10.3389/fcell.2020.630771
37. Wang Y, Shi P, Chen Q, et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11(12):1069–1082. doi:10.1093/jmcb/mjz020
38. De torre-minguela C, Gómez AI, Couillin I, Pelegrín P. Gasdermins mediate cellular release of mitochondrial DNA during pyroptosis and apoptosis. Biomolecules. 2021;35(8):e21757. doi:10.1096/fj.202100085R
39. Alu A, Han X, Ma X, Wu M, Wei Y, Wei X. The role of lysosome in regulated necrosis. Acta pharmaceutica Sinica B. 2020;10(10):1880–1903. doi:10.1016/j.apsb.2020.07.003
40. Green JP, Yu S, Martín-Sánchez F, Pelegrin P, Lopez-Castejon G. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Biomolecules. 2018;115(40):E9371–e9380. doi:10.1073/pnas.1812744115
41. Loomis WP, Bergsbaken T. Monitoring Calcium Fluxes and Lysosome Exocytosis During Pyroptosis. Methods Mol Biol. 2023;2641:171–178. doi:10.1007/978-1-0716-3040-2_14
42. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: an Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20(13):3328. doi:10.3390/ijms20133328
43. Pelegrin P, Surprenant A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signalling. 2009;5(2):129–137. doi:10.1007/s11302-009-9141-7
44. Dahl G. ATP release through pannexon channels. Philos Trans R Soc London. 2015;370(1672). doi:10.1098/rstb.2014.0191
45. Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi:10.1038/nature10558
46. Mazgaeen L, Gurung P. Recent Advances in Lipopolysaccharide Recognition Systems. Int J Mol Sci. 2020;21(2):379. doi:10.3390/ijms21020379
47. Yi YS. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152(2):207–217. doi:10.1111/imm.12787
48. Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta pharmaceutica Sinica B. 2021;11(9):2768–2782. doi:10.1016/j.apsb.2021.02.006
49. Aglietti RA, Estevez A, Gupta A, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113(28):7858–7863. doi:10.1073/pnas.1607769113
50. Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43(5):923–932. doi:10.1016/j.immuni.2015.10.009
51. Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Iimmunol. 2015;194(8):3937–3952. doi:10.4049/jimmunol.1402658
52. Inoue S, Browne G, Melino G, Cohen GM. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ 2009;16(7):1053–1061. doi:10.1038/cdd.2009.29
53. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
54. Hou J, Zhao R, Xia W, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nature Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
55. Zhang JY, Zhou B, Sun RY, et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31(9):980–997. doi:10.1038/s41422-021-00506-9
56. van Daalen KR, Reijneveld JF, Bovenschen N. Modulation of Inflammation by Extracellular Granzyme A. Front Immunol. 2020;11:931. doi:10.3389/fimmu.2020.00931
57. Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Ann Rev Immunol. 2008;26(1):389–420. doi:10.1146/annurev.immunol.26.021607.090404
58. Zhang Z, Zhang Y, Xia S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415–420. doi:10.1038/s41586-020-2071-9
59. Zhou Z, He H. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Biomolecules. 2020;368(6494). doi:10.1126/science.aaz7548
60. Feng Z, Zang C, Zhang L, Yin S, Zhuang Q, Wang X. STING activation promotes inflammatory response and delays skin wound healing in diabetic mice. Biochem Biophys Res Commun. 2022;611:126–131. doi:10.1016/j.bbrc.2022.04.085
61. Balderas-Cordero D, Canales-Alvarez O, Sánchez-Sánchez R, Cabrera-Wrooman A, Canales-Martinez MM, Rodriguez-Monroy MA. Anti-Inflammatory and Histological Analysis of Skin Wound Healing through Topical Application of Mexican Propolis. Int J Mol Sci. 2023;24(14). doi:10.3390/ijms241411831
62. Mori R, Tanaka K, de Kerckhove M, et al. Reduced FOXO1 expression accelerates skin wound healing and attenuates scarring. Am J Pathol. 2014;184(9):2465–2479. doi:10.1016/j.ajpath.2014.05.012
63. Zhao P, Cai Z, Zhang X, Liu M, Xie F, Liu Z. Hydrogen Attenuates Inflammation by Inducing Early M2 Macrophage Polarization in Skin Wound Healing. Biomolecules. 2023;16(6).
64. Yang P, Wang X. Topical insulin application accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization. Int J Med. 2020;133(19).
65. Ketelut-Carneiro N, Fitzgerald KA. The Many Ways a Cell Can Die. J Mol Biol. 2022;434(4):167378. doi:10.1016/j.jmb.2021.167378
66. Ito H, Kanbe A, Sakai H, Seishima M. Activation of NLRP3 signalling accelerates skin wound healing. Exp dermatol. 2018;27(1):80–86. doi:10.1111/exd.13441
67. Dai J, Zhang X, Wang Y, Chen H, Chai Y. ROS-activated NLRP3 inflammasome initiates inflammation in delayed wound healing in diabetic rats. Int J Clin Exp Pathol. 2017;10(9):9902–9909.
68. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in Chronic Wounds. Int J Mol Sci. 2016;17(12):2085. doi:10.3390/ijms17122085
69. Ao X, Yan H, Huang M, et al. Lavender essential oil accelerates lipopolysaccharide-induced chronic wound healing by inhibiting caspase-11-mediated macrophage pyroptosis. Kaohsiung J Med Sci. 2023;39(5):511–521. doi:10.1002/kjm2.12654
70. Ou ZL, Wang J, Shi R, Deng J, Liu Y, Luo GX. Influence of reactive oxygen species responsive self-assembled nanomicelle loaded with pyroptosis inhibitor on full-thickness skin defects in diabetic rats. Zhonghua shao shang za zhi. 2023;39(1):35–44. doi:10.3760/cma.j.cn501225-20221109-00483
71. Chen C, Wang Q, Li D, Qi Z, Chen Y, Wang S. MALAT1 participates in the role of platelet-rich plasma exosomes in promoting wound healing of diabetic foot ulcer. Int J Biol Macromol. 2023;238:124170. doi:10.1016/j.ijbiomac.2023.124170
72. He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. doi:10.1038/nature16959
73. Sharif H, Wang L, Wang WL, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–343. doi:10.1038/s41586-019-1295-z
74. Song Y, Yang L, Guo R, Lu N, Shi Y, Wang X. Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22. Biochem Biophys Res Commun. 2019;509(2):359–366. doi:10.1016/j.bbrc.2018.12.139
75. Wu A, Sun W, Mou F. lncRNA‑MALAT1 promotes high glucose‑induced H9C2 cardiomyocyte pyroptosis by downregulating miR‑141‑3p expression. Mol Med Rep. 2021;23(4).
76. Zuo Y, Chen L, He X, et al. Atorvastatin Regulates MALAT1/miR-200c/NRF2 Activity to Protect Against Podocyte Pyroptosis Induced by High Glucose. Diabetes Metabolic Syndrome Obesity. 2021;14:1631–1645. doi:10.2147/dmso.s298950
77. Boal-Carvalho I, Mazel-Sanchez B, Silva F, Garnier L, Yildiz S, Bonifacio JP. Influenza A viruses limit NLRP3-NEK7-complex formation and pyroptosis in human macrophages. EMBO Reports. 2020;21(12):e50421. doi:10.15252/embr.202050421
78. He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
79. Lara-Reyna S, Caseley EA. Inflammasome activation: from molecular mechanisms to autoinflammation. Clinical Translational immunol. 2022;11(7):e1404. doi:10.1002/cti2.1404
80. Yu ZW, Zhang J, Li X, Wang Y, Fu YH, Gao XY. A new research hot spot: the role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. doi:10.1016/j.lfs.2019.117138
81. Xu YF, Wu YX, Wang HM, Gao CH, Xu YY, Yan Y. Bone marrow-derived mesenchymal stem cell-conditioned medium ameliorates diabetic foot ulcers in rats. Clinics. 2023;78:100181. doi:10.1016/j.clinsp.2023.100181
82. Kambara H, Liu F, Zhang X, et al. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep. 2018;22(11):2924–2936. doi:10.1016/j.celrep.2018.02.067
83. Karnam K, Sedmaki K, Sharma P, Venuganti VVK, Kulkarni OP. Selective inhibition of PKR by C16 accelerates diabetic wound healing by inhibiting NALP3 expression in mice. Feb. 2023;72(2):221–236. doi:10.1007/s00011-022-01667-y
84. Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One. 2015;10(3):e0119106. doi:10.1371/journal.pone.0119106
85. Ding Y, Ding X. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxidative Med Cell Longevity. 2022;2022:9687925. doi:10.1155/2022/9687925
86. Geng K, Ma X, Jiang Z, et al. Innate Immunity in Diabetic Wound Healing: focus on the Mastermind Hidden in Chronic Inflammatory. Front Pharmacol. 2021;12:653940. doi:10.3389/fphar.2021.653940
87. Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Biomolecules. 2018;50(4):1–14. doi:10.1038/s12276-018-0058-5
88. Zhang X, Dai J, Li L, Chen H. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J Diabetes Res. 2017;2017:5281358. doi:10.1155/2017/5281358
89. Wan L, Bai X, Zhou Q, et al. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int J Bio Sci. 2022;18(2):809–825. doi:10.7150/ijbs.63219
90. Lee MKS, Sreejit G, Nagareddy PR, Murphy AJ. Attack of the NETs! NETosis primes IL-1β-mediated inflammation in diabetic foot ulcers. Clin Sci. 2020;134(12):1399–1401. doi:10.1042/cs20200240
91. Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–1145. doi:10.1016/j.cub.2007.05.074
92. Wang Z, Zhang S. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med Cell Longevity. 2020;2020:4063562. doi:10.1155/2020/4063562
93. Luo Z. Targeting the NOD-Like Receptor Pyrin Domain Containing 3 Inflammasome to Improve Healing of Diabetic Wounds. Adv Wound Care. 2023;12(11):644–656. doi:10.1089/wound.2021.0148
94. González P, Lozano P. Hyperglycemia and Oxidative Stress: an Integral, Updated and Critical Overview of Their Metabolic Interconnections. Biomolecules. 2023;24(11).
95. Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi:10.1016/j.freeradbiomed.2013.07.036
96. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi:10.1038/ni.1831
97. Jiang L, Fei D, Gong R, et al. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflammation Res. 2016;65(11):905–915. doi:10.1007/s00011-016-0973-7
98. Zhan Y, Xu D, Tian Y, et al. Novel role of macrophage TXNIP-mediated CYLD-NRF2-OASL1 axis in stress-induced liver inflammation and cell death. JHEP Rep. 2022;4(9):100532. doi:10.1016/j.jhepr.2022.100532
99. Jia Y, Cui R, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020;32:101534. doi:10.1016/j.redox.2020.101534
100. Jung H, Kim MJ, Kim DO, et al. TXNIP maintains the hematopoietic cell pool by switching the function of p53 under oxidative stress. Cell Metab. 2013;18(1):75–85. doi:10.1016/j.cmet.2013.06.002
101. Masutani H. Thioredoxin-Interacting Protein in Cancer and Diabetes. Antioxid Redox Signaling. 2022;36(13–15):1001–1022. doi:10.1089/ars.2021.0038
102. Yoshihara E. TXNIP/TBP-2: a Master Regulator for Glucose Homeostasis. Antioxidants. 2020;9(8):765. doi:10.3390/antiox9080765
103. Joshi S, Wang W, Peck AB, Khan SR. Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol. 2015;193(5):1684–1691. doi:10.1016/j.juro.2014.11.093
104. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327(5963):296–300. doi:10.1126/science.1184003
105. Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187(11):6143–6156. doi:10.4049/jimmunol.1101284
106. Deng Y, Han X, Yao Z, et al. PPARα Agonist Stimulated Angiogenesis by Improving Endothelial Precursor Cell Function Via a NLRP3 Inflammasome Pathway. Cell Phys Biochem. 2017;42(6):2255–2266. doi:10.1159/000479999
107. Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2(12):1117–1133. doi:10.1177/1947601911423654
108. Luo Z, Tian M, Yang G, et al. Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduction Targeted Therapy. 2022;7(1):218. doi:10.1038/s41392-022-01080-1
109. Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in Cell Death, Inflammation, and Pyroptosis. Ann Rev Immunol. 2020;38(1):567–595. doi:10.1146/annurev-immunol-073119-095439
110. Eguchi R, Suzuki A, Miyakaze S, Kaji K, Ohta T. Hypoxia induces apoptosis of HUVECs in an in vitro capillary model by activating proapoptotic signal p38 through suppression of ERK1/2. Cell. Signalling. 2007;19(6):1121–1131. doi:10.1016/j.cellsig.2006.12.004
111. Zhang M, Chen Y, Qiu Y, et al. PCSK9 Promotes Hypoxia-Induced EC Pyroptosis by Regulating Smac Mitochondrion-Cytoplasm Translocation in Critical Limb Ischemia. JACC. 2023;8(9):1060–1077. doi:10.1016/j.jacbts.2023.05.016
112. Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64(3):520–539. doi:10.1124/pr.111.005538
113. Hu JJ, Liu X. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature Immunol. 2020;21(7):736–745. doi:10.1038/s41590-020-0669-6
114. Zhang Y, Zhang R, Han X. Disulfiram inhibits inflammation and fibrosis in a rat unilateral ureteral obstruction model by inhibiting gasdermin D cleavage and pyroptosis. Int J Med. 2021;70(5):543–552. doi:10.1007/s00011-021-01457-y
115. Hu S, Wang L, Xu Y, Li F, Wang T. Disulfiram attenuates hypoxia-induced pulmonary hypertension by inhibiting GSDMD cleavage and pyroptosis in HPASMCs. Respir Res. 2022;23(1):353. doi:10.1186/s12931-022-02279-0
116. Zhuang L, Luo X, Wu S, et al. Disulfiram alleviates pristane-induced lupus via inhibiting GSDMD-mediated pyroptosis. Cell Death Discovery. 2022;8(1):379. doi:10.1038/s41420-022-01167-2
117. Cai Q, Sun Z, Xu S, et al. Disulfiram ameliorates ischemia/reperfusion-induced acute kidney injury by suppressing the caspase-11-GSDMD pathway. Renal Failure. 2022;44(1):1169–1181. doi:10.1080/0886022x.2022.2098764
118. Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: an Update. Cell Transplantation. 2016;25(5):829–848. doi:10.3727/096368915x689622
119. Lee BH, Park JN, Lee EJ, Moon YW, Wang JH. Therapeutic Efficacy of Spherical Aggregated Human Bone Marrow-Derived Mesenchymal Stem Cells Cultured for Osteochondral Defects of Rabbit Knee Joints. Am j Sports Med. 2018;46(9):2242–2252. doi:10.1177/0363546518780991
120. Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomaterials Res. 2018;22:36. doi:10.1186/s40824-018-0148-4
121. Wong VW, Sorkin M, Gurtner GC. Enabling stem cell therapies for tissue repair: current and future challenges. Biotechnol. Adv. 2013;31(5):744–751. doi:10.1016/j.biotechadv.2012.11.006
122. Jing H, He X, Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Translational Res. 2018;196:1–16. doi:10.1016/j.trsl.2018.01.005
123. Sufianov A, Kostin A, Begliarzade S, et al. Exosomal non coding RNAs as a novel target for diabetes mellitus and its complications. Non-Coding RNA Res. 2023;8(2):192–204. doi:10.1016/j.ncrna.2023.02.001
124. Chang W, Wang M, Zhang Y, et al. Roles of long noncoding RNAs and small extracellular vesicle-long noncoding RNAs in type 2 diabetes. Traffic. 2022;23(11):526–537. doi:10.1111/tra.12868
125. Li B, Luan S, Chen J, et al. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–826. doi:10.1016/j.omtn.2019.11.034
126. Glenn JD, Whartenby KA. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World j Stem Cells. 2014;6(5):526–539. doi:10.4252/wjsc.v6.i5.526
127. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am Vol. 2010;92(10):1927–1937. doi:10.2106/jbjs.i.01284
128. Zhou Y, Yuan J, Zhou B, et al. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology. 2011;133(1):133–140. doi:10.1111/j.1365-2567.2011.03421.x
129. Li M, Luan F, Zhao Y, et al. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int Wound J. 2017;14(1):64–73. doi:10.1111/iwj.12551
130. Jo H, Brito S, Kwak BM. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int J Med. 2021;22(5).
131. Bian D, Wu Y, Song G. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Jan. 2022;13(1):24. doi:10.1186/s13287-021-02697-9
132. Hu CH, Tseng YW, Chiou CY, et al. Bone marrow concentrate-induced mesenchymal stem cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther. 2019;10(1):275. doi:10.1186/s13287-019-1383-x
133. Sun B, Guo S, Xu F, et al. Concentrated Hypoxia-Preconditioned Adipose Mesenchymal Stem Cell-Conditioned Medium Improves Wounds Healing in Full-Thickness Skin Defect Model. Int Scholarly Res Notices. 2014;2014:652713. doi:10.1155/2014/652713
134. Li M, Zhao Y, Hao H, et al. Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int j Lower Extremity Wounds. 2015;14(1):73–86. doi:10.1177/1534734615569053
135. Laksmitawati DR, Noor SU, Sumiyati Y, Hartanto A, Widowati W, Pratami DK. The effect of mesenchymal stem cell-conditioned medium gel on burn wound healing in rat. Vet World. 2022;15(4):841–847. doi:10.14202/vetworld.2022.841-847
136. Karnam K, Sedmaki K, Sharma P, et al. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1β secretion in diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2020;1866(11):165903. doi:10.1016/j.bbadis.2020.165903
137. Wang Y, Wan L, Zhang Z, Li J, Qu M, Zhou Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp. Eye Res. 2021;209:108668. doi:10.1016/j.exer.2021.108668
138. Li X, Wang T, Tao Y, Wang X, Li L, Liu J. MF-094, a potent and selective USP30 inhibitor, accelerates diabetic wound healing by inhibiting the NLRP3 inflammasome. Exp. Cell. Res. 2022;410(2):112967. doi:10.1016/j.yexcr.2021.112967
139. Tombulturk FK, Todurga-Seven ZG, Huseyinbas O, Ozyazgan S, Ulutin T, Kanigur-Sultuybek G. Topical application of metformin accelerates cutaneous wound healing in streptozotocin-induced diabetic rats. Jan. 2022;49(1):73–83. doi:10.1007/s11033-021-06843-7
140. Zhao Y, Wang Q, Yan S, et al. Bletilla striata Polysaccharide Promotes Diabetic Wound Healing Through Inhibition of the NLRP3 Inflammasome. Front Pharmacol. 2021;12:659215. doi:10.3389/fphar.2021.659215
141. Wang T, Zhao J, Zhang J, et al. Heparan sulfate inhibits inflammation and improves wound healing by downregulating the NLR family pyrin domain containing 3 (NLRP3) inflammasome in diabetic rats. J Diabetes. 2018;10(7):556–563. doi:10.1111/1753-0407.12630
142. Song J, Zeng J, Zheng S, et al. Sanguisorba officinalis L. promotes diabetic wound healing in rats through inflammation response mediated by macrophage. Phytotherapy Res. 2023;37(9):4265.
143. Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophysical Res Commun. 2016;478(3):1021.
144. Wang Y, Jing L, Lei X, et al. Umbilical cord mesenchymal stem cell-derived apoptotic extracellular vesicles ameliorate cutaneous wound healing in type 2 diabetic mice via macrophage pyroptosis inhibition. Analytical Cell Pathol. 2022;2022.
145. Dai J, Jiang C, Chen H, Chai Y. Rapamycin Attenuates High Glucose-Induced Inflammation Through Modulation of mTOR/NF-κB Pathways in Macrophages. Front Pharmacol. 2019;10:1292. doi:10.3389/fphar.2019.01292
146. Sun X, Wang X, Zhao Z, Chen J, Li C, Zhao G. Paeoniflorin inhibited nod-like receptor protein-3 inflammasome and NF-κB-mediated inflammatory reactions in diabetic foot ulcer by inhibiting the chemokine receptor CXCR2. Drug Dev Res 2021;82(3):404–411. doi:10.1002/ddr.21763
147. Mi J, Xie C, Zeng L, et al. Bacillus subtilis WB800N alleviates diabetic wounds in mice by regulating gut microbiota homeostasis and TLR2. J Appl Microbiol. 2022;133(2):436–447. doi:10.1111/jam.15547
148. Bassett SA, Barnett MP. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients. 2014;6(10):4273–4301. doi:10.3390/nu6104273
149. Xu J, Zhao X, Jiang X, et al. Tubastatin A Improves Post-Resuscitation Myocardial Dysfunction by Inhibiting NLRP3-Mediated Pyroptosis Through Enhancing Transcription Factor EB Signaling. J Am Heart Assoc. 2022;11(7):e024205. doi:10.1161/jaha.121.024205
150. Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–2587. doi:10.2337/db12-1450
151. Udumula MP, Mangali S, Kalra J, et al. High fructose and streptozotocin induced diabetic impairments are mitigated by Indirubin-3-hydrazone via downregulation of PKR pathway in Wistar rats. Sci Rep. 2021;11(1):12924. doi:10.1038/s41598-021-92345-2
152. Nakamura T, Furuhashi M, Li P, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140(3):338–348. doi:10.1016/j.cell.2010.01.001
153. Nakamura T, Arduini A, Baccaro B, Furuhashi M, Hotamisligil GS. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 2014;63(2):526–534. doi:10.2337/db13-1019
154. Carvalho-Filho MA, Carvalho BM, Oliveira AG, et al. Double-stranded RNA-activated protein kinase is a key modulator of insulin sensitivity in physiological conditions and in obesity in mice. Endocrinology. 2012;153(11):5261–5274. doi:10.1210/en.2012-1400
155. Mangali S, Bhat A, Jadhav K, et al. Upregulation of PKR pathway mediates glucolipotoxicity induced diabetic cardiomyopathy in vivo in Wistar rats and in vitro in cultured cardiomyocytes. Biochem Pharmacol 2020;177:113948. doi:10.1016/j.bcp.2020.113948
156. Yim HC, Wang D, Yu L, et al. The kinase activity of PKR represses inflammasome activity. Cell Res 2016;26(3):367–379. doi:10.1038/cr.2016.11
157. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr.Opin Pharmacol 2010;10(4):482–496. doi:10.1016/j.coph.2010.04.001
158. Grammatiki M, Rapti E, Karras S, Ajjan RA, Kotsa K. Vitamin D and diabetes mellitus: causal or casual association? Jun. 2017;18(2):227–241. doi:10.1007/s11154-016-9403-y
159. Maddaloni E, Cavallari I, Napoli N, Conte C. Vitamin D and Diabetes Mellitus. Front Hormone Res. 2018;50:161–176. doi:10.1159/000486083
160. White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–3843. doi:10.1128/iai.00353-08
161. Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of Topically Applied Vitamin D during Corneal Wound Healing. PLoS One. 2016;11(4):e0152889. doi:10.1371/journal.pone.0152889
162. Lu X, Vick S, Chen Z, Chen J, Watsky MA. Effects of Vitamin D Receptor Knockout and Vitamin D Deficiency on Corneal Epithelial Wound Healing and Nerve Density in Diabetic Mice. Diabetes. 2020;69(5):1042–1051. doi:10.2337/db19-1051
163. Lv D, Cao X, Zhong L, et al. Targeting phenylpyruvate restrains excessive NLRP3 inflammasome activation and pathological inflammation in diabetic wound healing. Cell Rep Med. 2023;4(8):101129. doi:10.1016/j.xcrm.2023.101129
164. Huang W, Jiao J, Liu J, et al. MFG-E8 accelerates wound healing in diabetes by regulating ”NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discovery. 2020;6(1):84. doi:10.1038/s41420-020-00318-7
165. Bian F, Xiao Y, Zaheer M, et al. Inhibition of NLRP3 Inflammasome Pathway by Butyrate Improves Corneal Wound Healing in Corneal Alkali Burn. Int J Mol Sci. 2017;18(3):562. doi:10.3390/ijms18030562
166. Xiao M, Li L, Li C, Liu L, Yu Y, Ma L. 3,4-Methylenedioxy-β-Nitrostyrene Ameliorates Experimental Burn Wound Progression by Inhibiting the NLRP3 Inflammasome Activation. Plastic Reconstructive Surg. 2016;137(3):566e–575e. doi:10.1097/01.prs.0000479972.06934.83
167. Chiu HW, Chen CH, Chang JN, Chen CH, Hsu YH. Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy. J Mol Med. 2016;94(7):809–819. doi:10.1007/s00109-016-1389-0
168. Li M, Hou Q, Zhong L, Zhao Y, Fu X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front Immunol. 2021;12:681710. doi:10.3389/fimmu.2021.681710
169. Ochoa-Gonzalez F, Cervantes-Villagrana AR, Fernandez-Ruiz JC, et al. Metformin Induces Cell Cycle Arrest, Reduced Proliferation, Wound Healing Impairment In Vivo and Is Associated to Clinical Outcomes in Diabetic Foot Ulcer Patients. PLoS One. 2016;11(3):e0150900. doi:10.1371/journal.pone.0150900
170. Han X, Tao Y, Deng Y, Yu J, Sun Y, Jiang G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep. 2017;16(6):8691–8698. doi:10.3892/mmr.2017.7707
171. Zhao P, Sui BD, Liu N, et al. Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 2017;16(5):1083–1093. doi:10.1111/acel.12635
172. Yang F, Qin Y, Wang Y, et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int J Bio Sci. 2019;15(5):1010–1019. doi:10.7150/ijbs.29680
173. Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089–1104. doi:10.1080/15384101.2020.1743911
174. Zhang J, Huang L, Shi X, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging. 2020;12(23):24270–24287. doi:10.18632/aging.202143
175. Zhou X, Wang Q, Nie L, et al. Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Arch Oral Biol. 2020;116:104763. doi:10.1016/j.archoralbio.2020.104763
176. Yuan Y, Fan X. Metformin Protects against Spinal Cord Injury and Cell Pyroptosis via AMPK/NLRP3. Inflammasome Pathway. 2022;2022:3634908. doi:10.1155/2022/3634908
177. Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11(2):655–668.
178. He X, Wang X, Fang J, et al. Bletilla striata: medicinal uses, phytochemistry and pharmacological activities. J Ethnopharmacol. 2017;195:20–38. doi:10.1016/j.jep.2016.11.026
179. Ji X, Yin M, Nie H. A Review of Isolation, Chemical Properties, and Bioactivities of Polysaccharides from Bletilla striata. BioMed Research International. 2020;2020:5391379. doi:10.1155/2020/5391379
180. Yue L, Wang W, Wang Y, et al. Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways. Int J Biol Macromol. 2016;89:376–388. doi:10.1016/j.ijbiomac.2016.05.002
181. Chen Z, Zhao Y, Zhang M, et al. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydr Polym. 2020;227:115362. doi:10.1016/j.carbpol.2019.115362
182. Zhang C, He Y, Chen Z, Shi J, Qu Y. Effect of Polysaccharides from Bletilla striata on the Healing of Dermal Wounds in Mice. EvidComplementary Alternative Med. 2019;2019:9212314. doi:10.1155/2019/9212314
183. Zhang Y, Lv T, Li M, et al. Anti-aging effect of polysaccharide from Bletilla striata on nematode Caenorhabditis elegans. Pharmacogn Mag. 2015;11(43):449–454. doi:10.4103/0973-1296.160447
184. Chen Z, Cheng L, He Y, Wei X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: a review. Int J Biol Macromol. 2018;120(Pt B):2076–2085. doi:10.1016/j.ijbiomac.2018.09.028
185. Ding L, Shan X, Zhao X, et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr Polym. 2017;10(157):1538–1547. doi:10.1016/j.carbpol.2016.11.040
186. Luo Y, Diao H, Xia S, Dong L, Chen J, Zhang J. A physiologically active polysaccharide hydrogel promotes wound healing. J Biomed Mater Res Part A. 2010;94(1):193–204. doi:10.1002/jbm.a.32711
187. Wu XG, Xin M, Chen H, Yang LN, Jiang HR. Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. J Pharm Pharmacol. 2010;62(9):1152–1157. doi:10.1111/j.2042-7158.2010.01137.x
188. Yu L, Nie X, Pan H, Ling S, Zhang D, Bian K. Diabetes mellitus ulcers treatment with Bletilla striata polysaccharide]. China J Chinese Materia Medica. 2011;36(11):1487–1491.
189. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regeneratio. 2009;17(2):153–162. doi:10.1111/j.1524-475X.2009.00466.x
190. Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200(4):423–428. doi:10.1002/path.1437
191. Chang Z, Meyer K, Rapraeger AC, Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB j. 2000;14(1):137–144. doi:10.1096/fasebj.14.1.137
192. Bame KJ. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology. 2001;11(6):91r–98r. doi:10.1093/glycob/11.6.91r
193. Menini S, Iacobini C, Vitale M. The Inflammasome in Chronic Complications of Diabetes and Related Metabolic Disorders. Cells. 2020;9(8). doi:10.3390/cells9081812
194. Gawron-Gzella A, Witkowska-Banaszczak E, Bylka W, Dudek-Makuch M, Odwrot A, Skrodzka N. Chemical Composition, Antioxidant and Antimicrobial Activities of Sanguisorba officinalis L. Extracts. Pharm Chem J. 2016;50(4):244–249. doi:10.1007/s11094-016-1431-0
195. Cheng D-L, Cao X-P. Pomolic acid derivatives from the root of Sanguisorba officinalis. Phytochemistry. 1992;31(4):1317–1320. doi:10.1016/0031-9422(92)80499-5
196. Chen JF, Tan L, Ju F, et al. Phenolic glycosides from Sanguisorba officinalis and their anti-inflammatory effects. Nat Product Res. 2022;36(8):2097–2104. doi:10.1080/14786419.2020.1849202
197. Yasueda A, Kayama H, Murohashi M, et al. Sanguisorba officinalis L. derived from herbal medicine prevents intestinal inflammation by inducing autophagy in macrophages. Sci Rep. 2020;10(1):9972. doi:10.1038/s41598-020-65306-4
198. Zhou P, Li J, Chen Q, et al. A Comprehensive Review of Genus Sanguisorba: traditional Uses, Chemical Constituents and Medical Applications. Front Pharmacol. 2021;12:750165. doi:10.3389/fphar.2021.750165
199. Zhang H, Chen J, Cen Y. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int J Biol Macromol. 2018;112:862–867. doi:10.1016/j.ijbiomac.2018.01.214
200. Wang TT. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17(2):271.
201. Park E, Lee Sm Fau - Jung I-K, Jung Ik Fau - Lim Y, Lim Y, Fau - Kim J-H, Kim JH. Effects of genistein on early-stage cutaneous wound healing. Biochem Biophysical Res Commun. 2011;410(3):514.,
202. Hirota SA, Ng J, Fau - lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflammatory Bowel Dis. 2011;17(6):1359.
203. Zaki MH. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379.
204. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World j Stem Cells. 2014;6(2):195–202. doi:10.4252/wjsc.v6.i2.195
205. Zhang B, Wang M, Gong A, et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33(7):2158.
206. Panepucci RA, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22(7):1263.
207. Chen W, Liu J. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials. 2013;34(38):9917.
208. Zhao J, Yu G, Cai M, et al. Bibliometric analysis of global scientific activity on umbilical cord mesenchymal stem cells: a swiftly expanding and shifting focus. Stem Cell Res Therapy. 2018;9(1):1.
209. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242.
210. Banerjee M, Vats P. Reactive metabolites and antioxidant gene polymorphisms in Type 2 diabetes mellitus. Redox Bbiol. 2014;2:170.
211. Bandeira Sde M, Guedes Gda S, da Fonseca LJS, et al. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxidative Med Cell Longevity. 2012;2012. doi:10.1155/2012/819310
212. Bacanlı M, Anlar HG, Aydın S, et al. d-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2017;110:434.
213. Jamal Gilani S, Bin-Jumah M N, Al-Abbasi FA, et al. Fustin ameliorates hyperglycemia in streptozotocin induced type-2 diabetes via modulating glutathione/Superoxide dismutase/Catalase expressions, suppress lipid peroxidation and regulates histopathological changes. Saudi J Biol Sci. 2021;28(12):6963.
214. Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed icine Pharmacother. 2017;90:500.
215. Abais JM, Xia M, Fau - Zhang Y, et al. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxidants Redox Signaling. 2015;22(13):1111.
216. Jia X, Cao B, An Y, Zhang X, Wang C. Rapamycin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting IL-1β and IL-18 production. Int Immunopharmacol. 2019;67:211.
217. Xiao M, Li L, Fau - Hu Q, et al. Rapamycin reduces burn wound progression by enhancing autophagy in deep second-degree burn in rats. Regeneration. 2013;21(6):852.
218. Mills RE, Taylor KR, Podshivalova K, McKay DB, Jameson JM. Defects in Skin γδ T Cell Function Contribute to Delayed Wound Repair in Rapamycin-Treated Mice1. J Immunol. 2008;181(6):3974–3983. doi:10.4049/jimmunol.181.6.3974
219. Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. ?mol Biol Cell. 2009;20(7):1992–2003. doi:10.1091/mbc.e08-12-1249
220. Dai J, Zhang X, Li L, Chen H, Chai Y. Autophagy Inhibition Contributes to ROS-Producing NLRP3-Dependent Inflammasome Activation and Cytokine Secretion in High Glucose-Induced Macrophages. Cell Phys Biochem. 2017;43(1):247–256. doi:10.1159/000480367
221. Liu YC, Gao XX, Chen L, You XQ. Rapamycin suppresses Aβ(25-35)- or LPS-induced neuronal inflammation via modulation of NF-κB signaling. Neuroscience. 2017;355:188–199. doi:10.1016/j.neuroscience.2017.05.005
222. Wen J, Xu B, Sun Y, et al. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res. 2019;146:104308. doi:10.1016/j.phrs.2019.104308
223. Yin N, Gao Q, Tao W, et al. Paeoniflorin relieves LPS-induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. Journal of Leukocyte Biology. 2020;108(1):229–241. doi:10.1002/jlb.3ma0220-355r
224. Sun X, Wang X, Zhao Z, Chen J, Li C, Zhao G. Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochem. 2020;122(8):151649. doi:10.1016/j.acthis.2020.151649
225. Citro A, Valle A. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes. 2015;64(4):1329–1340. doi:10.2337/db14-0443
226. Zou XY, Zhang M, Tu WJ, et al. Bacillus subtilis inhibits intestinal inflammation and oxidative stress by regulating gut flora and related metabolites in laying hens. Animal. 2022;16(3):100474. doi:10.1016/j.animal.2022.100474
227. Liu Y, Ding S, Dietrich R, Märtlbauer E, Zhu K. A Biosurfactant-Inspired Heptapeptide with Improved Specificity to Kill MRSA. Angewandte Chemie. 2017;56(6):1486–1490. doi:10.1002/anie.201609277
228. Ansari A, Zohra RR, Tarar OM, Qader SAU, Aman A. Screening, purification and characterization of thermostable, protease resistant Bacteriocin active against methicillin resistant Staphylococcus aureus (MRSA). BMC Microbiol 2018;18(1):192. doi:10.1186/s12866-018-1337-y
229. Tan F, Limbu SM, Qian Y, Qiao F, Du ZY, Zhang M. The Responses of Germ-Free Zebrafish (Danio rerio) to Varying Bacterial Concentrations, Colonization Time Points, and Exposure Duration. Front Microbiol. 2019;10:2156. doi:10.3389/fmicb.2019.02156
230. Huon JF, Montassier E, Leroy AG, Grégoire M. Phages versus Antibiotics To Treat Infected Diabetic Wounds in a Mouse Model: a Microbiological and Microbiotic Evaluation. mSystems. 2020;5(6). doi:10.1128/mSystems.00542-20
231. Bortoluzzi C, Serpa Vieira B, de Paula Dorigam JC, et al. Bacillus subtilis DSM 32315 Supplementation Attenuates the Effects of Clostridium perfringens Challenge on the Growth Performance and Intestinal Microbiota of Broiler Chickens. Microorganisms. 2019;7(3):71. doi:10.3390/microorganisms7030071
232. Li Y, Xu Q, Huang Z, et al. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J Appl Microbiol. 2016;120(1):195–204. doi:10.1111/jam.12972
233. Shofler D, Rai V, Mansager S, Cramer K, Agrawal DK. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Jun. 2021;17(6):681–690. doi:10.1080/1744666x.2021.1912598
234. Koch KN, Hartung ML, Urban S, et al. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest. 2015;125(8):3297–3302. doi:10.1172/jci79337
235. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184(10):5743–5754. doi:10.4049/jimmunol.0903937
236. Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187(1):61–70. doi:10.1083/jcb.200903124
237. He Y, Chang Y, Peng Y, et al. Glibenclamide Directly Prevents Neuroinflammation by Targeting SUR1-TRPM4-Mediated NLRP3 Inflammasome Activation In Microglia. Mol Neurobiol. 2022;59(10):6590–6607. doi:10.1007/s12035-022-02998-x
238. Arita Y, Yoshinaga Y. Glyburide inhibits the bone resorption induced by traumatic occlusion in rats. Jun. 2020;55(3):464–471. doi:10.1111/jre.12731
239. Lin YW, Liu PS, Pook KA, Wei LN. Glyburide and retinoic acid synergize to promote wound healing by anti-inflammation and RIP140 degradation. Sci Rep. 2018;8(1):834. doi:10.1038/s41598-017-18785-x
240. Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–247. doi:10.1038/ni.1703
241. Chiazza F, Couturier-Maillard A, Benetti E, et al. Targeting the NLRP3 Inflammasome to Reduce Diet-Induced Metabolic Abnormalities in Mice. Mol Med. 2016;21(1):1025–1037. doi:10.2119/molmed.2015.00104
242. Xu C, Zhang Y, Sutrisno L, Yang L, Chen R, Sung KLP. Bay11-7082 facilitates wound healing by antagonizing mechanical injury- and TNF-α-induced expression of MMPs in posterior cruciate ligament. Connective Tissue Res. 2019;60(4):311–322. doi:10.1080/03008207.2018.1512978
243. Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–441. doi:10.1016/j.immuni.2013.08.037
244. Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi:10.1016/j.immuni.2012.01.009
245. Wu D, Chen Y, Sun Y, et al. Target of MCC950 in Inhibition of NLRP3 Inflammasome Activation: a Literature Review. Inflammation. 2020;43(1):17–23. doi:10.1007/s10753-019-01098-8
246. Zhang C, Zhu X, Li L, et al. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes, metabolic syndrome and obesity: targets and therapy. Diabetes Metabolic Syndrome Obesity. 2019;12:1297–1309. doi:10.2147/dmso.s199802
247. Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules. 2018;23(3):522. doi:10.3390/molecules23030522
248. Zeng W, Wu D, Sun Y, et al. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci Rep. 2021;11(1):19305. doi:10.1038/s41598-021-98437-3
249. Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Mar. 2015;21(3):248–255. doi:10.1038/nm.3806
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


