Back to Journals » International Journal of Nanomedicine » Volume 14
The influence of female mice age on biodistribution and biocompatibility of citrate-coated magnetic nanoparticles
Authors Pinheiro WO , Fascineli ML, Farias GR , Horst FH , de Andrade LR , Corrêa LH , Magalhães KG , Sousa M.H. , de Almeida MC , Azevedo RB , Lacava ZGM
Received 12 December 2018
Accepted for publication 1 March 2019
Published 8 May 2019 Volume 2019:14 Pages 3375—3388
DOI https://doi.org/10.2147/IJN.S197888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Willie O Pinheiro,1,2 Maria L Fascineli,1 Gabriel R Farias,1 Frederico H Horst,1 Laise Rodrigues de Andrade,1 Luis Henrique Corrêa,3 Kelly Grace Magalhães,3 Marcelo Henrique Sousa,4 Marcos C de Almeida,1 Ricardo B Azevedo,1 Zulmira GM Lacava1,2
1Department of Genetics and Morphology, Institute of Biological Sciences, CNANO, University of Brasilia, Brasilia, DF 70910-900, Brazil; 2Post-graduation Program in Molecular Pathology, Faculty of Medicine, University of Brasilia, Brasília, DF 70910-900, Brazil; 3Laboratory of Immunology and Inflammation, Department of Cell Biology, University of Brasilia, Brasilia, DF 70910-900, Brazil; 4Green Nanotechnology Group, Faculty of Ceilandia, University of Brasilia, Brasília, DF 72220-900, Brazil
Background: Magnetic nanoparticles (MNPs) have been successfully tested for several purposes in medical applications. However, knowledge concerning the effects of nanostructures on elderly organisms is remarkably scarce.
Purpose: To fill part of this gap, this work aimed to investigate biocompatibility and biodistribution aspects of magnetic nanoparticles coated with citrate (NpCit) in both elderly and young healthy mice.
Methods: NpCit (2.4 mg iron) was administered intraperitoneally, and its toxicity was evaluated for 28 days through clinical, biochemical, hematological, and histopathological examinations. In addition, its biodistribution was evaluated by spectrometric (inductively coupled plasma optical emission spectrometry) and histological methods.
Results: NpCit presented age-dependent effects, inducing very slight and temporary biochemical and hematological changes in young animals. These changes were even weaker than the effects of the aging process, especially those related to the hematological data, tumor necrosis factor alpha, and nitric oxide levels. On the other hand, NpCit showed a distinct set of results in the elderly group, sometimes reinforcing (decrease of lymphocytes and increase of monocytes) and sometimes opposing (erythrocyte parameters and cytokine levels) the aging changes. Leukocyte changes were still observed on the 28th day after treatment in the elderly group. Slight evidence of a decrease in liver and immune functions was detected in elderly mice treated or not treated with NpCit. It was noted that tissue damage or clinical changes related to aging or to the NpCit treatment were not observed. As detected for aging, the pattern of iron biodistribution was significantly different after NpCit administration: extra iron was detected until the 28th day, but in different organs of elderly (liver and kidneys) and young (spleen, liver, and lungs) mice.
Conclusion: Taken together, the data show NpCit to be a stable and reasonably biocompatible sample, especially for young mice, and thus appropriate for biomedical applications. The data showed important differences after NpCit treatment related to the animals’ age, and this emphasizes the need for further studies in older animals to appropriately extend the benefits of nanotechnology to the elderly population.
Keywords: elderly, nanotoxicity, maghemite nanoparticles, superparamagnetic iron oxide nanoparticles, SPIO, nano-safety, inductively coupled plasma optical emission spectrometry, ICP-OES
Introduction
Advances in nanotechnology strongly affect biomedical applications, enabling new approaches to be developed for diagnosis and therapy.1 Several nanostructured materials are potentially useful in this area, and among these, platforms based on magnetic nanoparticles (MNPs) may be highlighted.2–4 MNPs based on iron oxides, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3), have been widely investigated for in vivo applications because of their potential biocompatibility. To achieve the indispensable colloidal and chemical stability,5 MNPs are dispersed in a carrier solvent and coated with a stabilizing molecular layer6 provided by several different molecules, such as dextran,7 dimercaptosuccinic acid,8 polyaspartic acid,9 or citrate.10
Several MNP-based systems have succeeded in pre-clinical and clinical tests/purposes, such as delivery and controlled release of drugs to specific sites, enhancement of contrast in magnetic resonance imaging, and magnetic hyperthermia for tumor treatment.11–13 However, despite the increasing number of studies and publications related to the biomedical use of MNPs, little is known about their use in elderly organisms.
Although aging is not a disease, it is the main risk factor for chronic diseases.14 Age-related physiological, immunological, and genetic changes, associated with the concomitant presence of different diseases and possible drug interactions, make the treatment of elderly patients particularly challenging.15,16 In this context, new therapeutic strategies using MNPs may represent powerful alternatives in the treatment of age-associated diseases, like neoplasms. However, the lack of studies makes it difficult to assess nanoparticle treatments in elderly organisms.
Li et al17 emphasized that understanding the effects of nanostructure exposure on the elderly is critical, and therapies developed that are based on nanoparticle use should involve suitable animal models with special attention to nanotoxicity. In fact, the possible toxicity of MNPs is a priority in nanomedicine.18 Kaur et al19 highlighted that although the use of MNPs for therapeutic purposes, such as hyperthermia, has been announced as an important biomedical advancement, concerns about their biocompatibility need to be addressed individually for each formulation and administration, especially in elderly organisms. Besides that, some studies show under representation of elderly patients in clinical trials and, consequently, a lack of standardized treatments and clear management guidelines for these patients.20 In this context, the present work aimed to evaluate the effects of age on biocompatibility and biodistribution aspects of maghemite nanoparticles coated with citrate (NpCit) administered to elderly and young mice.
Materials and methods
Synthesis and characterization of magnetic nanoparticles coated with citrate
The NpCit sample was synthesized and kindly supplied by Dr Emilia CO Lima (UFG, Goiania, GO, Brazil). Maghemite (γ-Fe2O3) nanoparticles were obtained via the oxidation of magnetite nanoparticles synthesized by the alkaline hydrolysis of ions Fe(II) and Fe(III).21 γ-Fe2O3 nanoparticles were functionalized with citrate ions as described previously.22 The functionalized nanoparticles were dispersed in water, yielding stable colloidal suspension at neutral pH. The total iron concentration in the colloidal suspension and the Fe(III)/Fe(II) ratio were determined by atomic absorption and colorimetric analysis, respectively. Diffraction pattern was analyzed using a Shimadzu XRD-600 diffractometer. The nanoparticle shape and size dispersity were determined by transmission electron microscopy (TEM; Jeol JEM-1011). The stability of NpCit colloidal suspension was verified through several characteristics, namely the polydispersity index (PDI), zeta potential, pH, and hydrodynamic size, using a Zetasizer NanoZS (Malvern Instruments). The measurements were performed in triplicate, at 25°C, with a fixed detection angle of 173°.
Animals, NpCit treatment, and experimental design
Elderly (E) non-isogenic Swiss mice aged 14–16 months (weighing 40±5 g) and young (Y) mice aged 03–04 months (weighing 30±5 g) were used. All the procedures of housing and handling of animals were carried out according to the international practices for animal use and care, after being approved by the Animal Ethics Committee of the Institute of Biological Sciences at the University of Brasilia/Brazil (CEUA, reference number 17672/2016).
A single injection of NpCit containing 2.4 mg iron (150 μL) was administered intraperitoneally into the animals.23 PBS was used in elderly (EC) and young (YC) control groups (n=09).
Biological material was collected at 1, 7, and 28 days after the NpCit injection. Elderly treated groups (n=07) were accordingly named E1, E7, and E28, while young treated groups (n=05) were named Y1, Y7, and Y28. The biodistribution and biocompatibility aspects were evaluated. Comparisons of data obtained from the treated groups and the corresponding controls, as well as from the elderly and the corresponding young groups, were performed.
Biocompatibility tests
Biocompatibility evaluations of NpCit sample were performed through clinical observations (loss of weight, diarrhea, alopecia, inappetence, motor disorders, and salivary gland secretions).24 Changes in biochemical and hemogram indexes, production levels of inflammatory cytokines tumor necrosis factor alpha (TNF-α) and nitric oxide (NO), and histological analyses were examined.
All animals (elderly and young) were monitored from 2 weeks before the beginning of the experiments, and twice a week during the experimental period. The animals were weighed at the time of NpCit or PBS administration, immediately before euthanasia, and weekly in the case of groups euthanized 28 days after treatment (E28/Y28).
Biochemical analyses were performed specifically to assess hepatic, renal, tissue damage, and nutritional status due to administration of NpCit. After obtaining a total of 700 μL of blood by cardiac puncture, the serum was separated by centrifugation and used for evaluations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatinine K, urea, and albumin, using a ChemWell-T automatic biochemical analyzer (Labtest, Brazil).
Blood samples (300 μL) were used for evaluating the possible effects of NpCit on the inflammatory, allergic, and anemia processes through cell count and cell type identification (leukogram, platelet, and erythrogram), using a multiple automated hematology analyzer for veterinary use, the Sysmex pocH-100iV Diff (Curitiba/Paraná, Brazil).
Effects of NpCit injection on the induction of inflammatory process were also investigated through quantification of levels of TNF-α in the serum of mice by the ELISA, according to the manufacturer’s instructions (eBioscience and R&D System). Plates were read at 450 nm using a SpectraMax M3 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). In addition, measurement of NO production was detected by using Griess reagent. After incubating the plate with this reagent at room temperature, the OD was determined in a SpectraMax M3 (Molecular Devices) at a test wavelength of 540 nm.
For analyses of morphology and acute toxicity of NpCit, the brain, spleen, liver, lungs, and kidneys were removed from elderly and young animals. Fragments of these organs were fixed in 4% buffered paraformaldehyde, dehydrated in alcohol solutions, diaphanized in xylol, and embedded in paraffin. Tissue sections (3–5 μm) were stained with H&E and analyzed under a Zeiss Axiophot light microscope.
NpCit distribution analyses
Biodistribution aspects of NpCit nanoparticles were evaluated in two different ways: quantification of iron in the organs and histological analysis.25 After euthanasia, part of the organs (liver, spleen, lung, kidneys, and brain) and the blood were processed for determination of the amount of iron through inductively coupled plasma optical emission spectrometry (ICP-OES) using an Optima 8000 ICP-OES Spectrometer.26 For histological analysis, slides were submitted to Perls’ staining methods with nuclear flash red. In 3–5 μm sections, the presence of iron clusters in the tissues was detected with a Zeiss Axiophot light microscope.
Statistical analyses
Statistical analyses were performed using SPSS software version 18 and Prisma version 6.01. The normality of the continuous variables was evaluated by the Shapiro–Wilk test. Differences between the analyzed groups were investigated through ANOVA (for data following normal distribution) or Kruskal–Wallis (in the case of non-normal distribution) test. For significant results of ANOVA, pairwise comparisons were performed by Student’s t-test or Mann–Whitney U test according to the distribution of normality.
Results
Nanoparticle characterization
The characterization data of the NpCit sample are summarized in Table 1. The visual observation showed the NpCit sample to be in red-brown color, characteristic of maghemite, and absence of detectable nanoparticle precipitation, even in the presence of a magnetic field, despite the high concentration of nanoparticles (18×1018 particles/mL). Transmission electron micrographs showed NpCit nanoparticles with spherical-like shapes (Figure 1A). The random counting of 441 MNPs in TEM revealed a mean diameter of 10.8±2.7 nm, as obtained by the log normal curve (Figure 1B). Measurements, especially zeta potential (−40 mV) and PDI (0.290), indicated the high colloidal stability of the sample at pH =7.21
  | Figure 1 (A) TEM micrograph (scale bar: 200 nm) and (B) histogram of MNP size distribution. |
Biocompatibility evaluation
The intraperitoneal treatment of mice with NpCit sample (2.4 mg iron) did not induce obvious clinical or behavioral changes such as hair loss, diarrhea, inappetence, salivary gland secretions, and motor dysfunction throughout the entire experimental period in all elderly and young animals investigated. Further, NpCit did not induce significant variation in the animal weight in elderly and young treated groups when compared to their corresponding controls.
The biochemical analyses (Table 2) showed through comparisons with non-treated controls that elderly animals (EC) presented lesser amounts of ALT and albumin than young animals (YC). However, the administration of NpCit induced different effects in elderly and young animals. In the elderly group, a temporary significant decrease in the urea and an increase in the albumin levels were observed at day 1 after NpCit treatment (E1) in comparison to the control (EC). Levels of the enzymes AST, creatinine K, ALT, and LDH were not significantly different in all the treated elderly groups (E1, E7, and E28) compared to EC, at any point of time.
In the young animals, NpCit administration caused significant decreases in urea, albumin, and LDH levels at day 7 after NpCit injection (Y7) compared to the YC group. In this group, NpCit did not cause alterations in the ALT, AST, and creatinine K levels in all treated young groups (Y1, Y7, and Y28) compared to the control (YC).
The leukogram analysis (Table 3) showed, through comparisons between the elderly groups and their corresponding young groups, that the elderly animal groups EC, E1, E7, and E28 presented significant decreases in the rate of lymphocytes (small cell rate [lymphocytes], W-SCR) and in the absolute counting of lymphocytes (absolute small cell count [lymphocytes], W-SCC) when compared to YC, Y1, Y7, and Y28, respectively. In addition, the same elderly groups showed significant increases in the mean cell rates of basophils, eosinophils, and monocytes (W-MCR), when compared to the young groups.
In the elderly groups, the NpCit treatment induced significant decreases in the rate of lymphocytes (W-SCR) observed in the day 1 group (E1) and in the day 28 group (E28) compared to the EC group. In contrast, significant increases in the mean cell rate (basophils, eosinophils, and monocytes) were also observed in groups E1 and E28, compared to EC.
In the young groups (Y1, Y7, and Y28), different from the elderly groups, NpCit did not induce any significant alterations in the leukocyte parameters as compared to the control young group (YC).
Regarding platelets, significant differences were not found either in comparison between the control elderly and young groups, or in comparison of NpCit-treated elderly animals with the control elderly mice (Table 3). The only significant alterations in platelet count were observed in the young group at day 7 (Y7) in comparison with both the control young animals (YC) and the treated elderly animals, also at day 7 (E7).
The erythrogram analysis (Table 4) revealed that the aging process caused significant alterations in most of the parameters investigated. In fact, the elderly groups in comparison to their corresponding young groups showed significant decreases in the total erythrocyte counts (RBC) for E1, E7, and E28 groups compared to their corresponding young groups Y1, Y7, and Y28. Besides, significant decreases in the hemoglobin (HGB) concentration, mean corpuscular volume, and hematocrit values were observed for EC, E1, E7, and E28 compared to YC, Y1, Y7, and Y28 groups, respectively. On the other hand, mean corpuscular hemoglobin was significantly higher in EC group than in YC group, while the red cell distribution width was higher in EC, E1, and E28 than in YC, Y1, and Y28 groups, respectively (Table 4).
Concerning the pro-inflammatory cytokines, NpCit administration in elderly mice triggered a decrease in TNF-α levels at day 7 (E7) compared to the untreated mice (EC). There was also a significant decrease of TNF-α levels in young mice submitted to NpCit administration at day 7 (Y7) compared to the corresponding elderly mice (E7), as shown in Figure 2A. Moreover, there was a decrease in NO levels in the serum of untreated control elderly mice (EC) and treated young mice at day 1 (Y1), when both groups were compared to control young mice (YC) (Figure 2B).
The histological analysis of tissues after H&E staining showed no abnormalities in all tissues analyzed for both young and elderly animals, treated or not treated with NpCit sample (data not shown). Briefly, the brains had no pathologies in the white and gray matter; in the spleens, both red and white pulps were easily distinguishable and there were no large numbers of white cells; the observed livers presented hepatocytes in polyhedral shape, radiating from a central vein; in the lungs, there were no signs of fibrosis; and in the kidneys, cells from glomerulus, proximal, and distal convoluted tubules presented normal patterns.
NpCit biodistribution
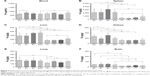
The NpCit biodistribution assays in the organs and blood performed by ICP-OES are shown in Figure 3. Comparisons between EC and YC animals showed that although the amount of iron in the blood was similar in both groups (Figure 3A), the spleen, liver, kidney, lung, and brain presented a significantly higher concentration of iron in the elderly group (Figure 3B–F) than in the young group.
In fact, the distribution of iron in both animal groups was also different; while the EC group presented the iron in descending order, spleen > blood > liver > kidney > lung > brain, in the YC mice, the order was blood > spleen > liver > lung > kidney > brain.
Concerning the effects induced by NpCit in the elderly groups, there were significant changes in comparison with the control untreated mice: an increase of iron levels in the blood and decrease in the kidneys at day 1 and an increase in the liver at day 28. The significant effects of NpCit on the young treated groups when compared to the YC group were different from the elderly groups and characterized by iron level increases in the lungs at day 1, in the brain at day 7, and in the spleen, liver, and lungs at day 28.
Due to the initial differences in the amount of iron between elderly and young groups, the comparison of elderly treated animals (E1, E7, and E28) with the corresponding young treated groups (Y1, Y7, and Y28) led to significant differences, particularly in the spleen, liver, and brain, as shown in Figure 3.
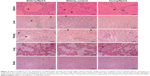
Through microscopic observation after Perls’ staining (Figure 4), it was possible to observe iron aggregates in all the analyzed organs, except in the brain. In general, the concentration was higher in the elderly animals than in the young animals, in both NpCit treated and control groups. The highest iron concentration was observed in the spleen of elderly mice of the day 7 group. In this group, the iron concentration was higher than in the EC group or the corresponding young group (Y7). In the young groups, highest concentrations in the spleen were observed at day 28.
To facilitate the comparison between the effects related to aging and/or the NpCit treatment, a table containing a summary of the main effects of aging and/or NpCit treatment in mice is presented (Table S1).
Discussion
This work was performed to compare the effects of citrate-coated MNPs on healthy elderly and young mice, focusing on biocompatibility and biodistribution aspects. Citrate is a biocompatible small molecule that has been widely accepted as the capping agent to stabilize water-dispersible MNPs for biomedical applications, and it is even commercially available. This is attributed to the fact that citrate is capable of adsorption onto the surface of MNPs (especially iron oxides) by coordinating via one or two carboxylate groups, leaving at least one carboxylate group exposed to the bulk medium. This makes the nanoparticle surface hydrophilic, negatively charged in a wide range of pH (including the physiological one), and cytocompatible, preventing particle agglomeration and providing functional groups to be used for further surface modification. Moreover, citrate detachment from the surface of MNPs under physiological conditions is negligible, and the physical–chemical characteristics of citrate-capped MNPs can be preserved over years.21,27 Indeed, previous in vivo investigations have shown the biocompatibility and non-mutagenic effects of citrate coatings,28–30 in spite of adverse effects described in in vitro tests.31
However, citrate-coated MNPs can be colloidally unstable in a biological system when dispersed in a medium with high ionic strength, such as PBS buffer. Sharma et al32 reported coagulation of citrate-stabilized iron oxide particles in PBS, a high increase of the hydrodynamic diameter (>15-fold), and formation of precipitates. To avoid this undesirable effect, in our work, dilution/dispersion of citrate-capped MNPs was performed in water (ie, low ionic strength medium). Therefore, zeta potential (−40 mV), hydrodynamic size (~78 nm), and PDI (0.290) indicated the high colloidal stability of diluted NpCit samples at pH =7.
Szigeti et al27 showed that citric acid as a surface-capping agent to control size and biocompatibility and to avoid agglomeration of the synthesized particles is able to chelate Ca2+ ions. However, physiological Ca2+ concentration does not affect in vivo colloidal properties. The authors affirm that at the physiological pH of 7.4, the zeta potential of citrate-coated MNPs and the steric stabilization effect of citrate coating further enhance the colloidal stability. Thus, even if there are other ions in addition to Ca2+ and other complexing molecules, the citrate-coated samples present considerable biological stability and thus suitability for use in in vivo tests.27,33,34
Concerning the in vivo biocompatibility tests, differences related to the aging process and some significant changes induced by the NpCit sample were observed. Liver and renal functions represent important predictors of toxicity and aging. Dysfunctions in the hepatobiliary system represent more than 50% of toxicity events occasioned by chemical agents.35 These are traditionally assessed by measuring the serum levels of AST and ALT.36,37 AST is widely distributed in the organism38 and was not altered by mice aging or after NpCit treatment, suggesting no damage in the cytoplasmic and mitochondrial membranes.36 In accordance with this, the administration of NpCit did not induce changes in the ALT enzyme levels of young or elderly animals, despite the presence of higher levels of iron in the liver. However, ALT which is usually produced in high concentrations in the liver presented a twofold reduction in the EC animals, thus indicating possible liver cell lesions in this group38–41 with a subsequent decrease in the mitochondrial number,14 an aging characteristic not detected in this study.
Kidney function was evaluated by serum creatinine and urea levels.37 The absence of creatinine level changes that are related to aging or NpCit treatment is indicative of preserved kidney function.42,43 Indeed, no increase was observed in serum urea, which may derive from a decreased glomerular filtration37 related to aging or nephrotoxicity. On the other hand, a decrease in serum urea may be associated with liver impairment, since this organ is responsible for the production of urea during protein catabolism.42 NpCit led to a reduction of urea levels in both elderly and young animals, but this effect was temporary and not observed at the 28th day after treatment, even if the iron concentration was still higher than usual at this time.
Other biochemical changes were investigated through albumin and LDH levels. Albumin is an abundant serum protein produced in the liver.44 In this study, the albumin level in elderly animals appeared lower than in young animals, another sign of possible kidney or liver dysfunction consistent with the aging process. However, no significant differences in the LDH enzyme levels were observed between elderly and young groups, nor were they seen after NpCit treatment, indicating once again that there was no damage to the body’s tissues or any production of excessive free radicals related to the NpCit treatment or even to aging.
The hemogram analysis showed the predominance of myeloid cells in the elderly group and higher lymphoid cells in the young group, considered a hallmark difference between elderly and young mice.45 Lymphocytes are crucial for the immune response,42 and the decrease in lymphocyte-dependent immunity could increase oxidative stress and foment diseases related to aging.46 Remarkably, these differences were even more pronounced and durable after NpCit treatment of elderly mice. At the same time, NpCit did not affect leukocyte populations in young groups, evidencing one important aspect of nanoparticle applications to be taken into account in older organisms. On the other hand, myeloid cells are responsible for the phagocytosis of foreign particles, and their increase in the elderly group could explain why the increase of iron in the blood was observed only on the first day after the NpCit injection. Interestingly, erythrogram differences were essentially only related to the aging process.
The pro-inflammatory cytokine TNF-α may be associated with the triggering mechanisms of typical age-related diseases and inflammatory processes.47 Accordingly, a tendency to having a higher level of TNF-α was observed in the EC group. Nevertheless, significant TNF-α decreases were temporarily induced 7 days after NpCit treatment. The other cytokine investigated, NO, was also modulated after the NpCit injection. NO is involved in many physiological and pathological processes and can have opposite actions such as damage or protection of cells during inflammatory response.48 In this study, NO levels in the elderly mice were lower than in the young mice, despite a higher number of phagocytes observed in the elderly animals, whether they were treated or not with NpCit.48 Low levels of NO production are typically involved in liver damage, and they can enhance the risk of several diseases associated with older individuals, which was not evidenced by biochemical data. In addition, although NpCit did not affect NO production in the elderly mice, it induced a temporary significant reduction of NO levels in young mice 1 day after the treatment. In a previous study, the NP-induced neurotoxicity was correlated with the increase of NO production,34 indicating that different NP samples may have diverse actions in organisms.
The findings that the effects induced by NpCit in the young group were slight and no longer observed after the seventh day after treatment suggest the biocompatibility of the sample. This supposition is strongly supported by both the absence of histological alterations in all the organs investigated and absence of clinical changes usually associated with aging or toxicity.24 It is reasonable to extend this conclusion to the elderly mice, but since they still presented some leukocyte alterations at day 28 after NpCit treatment, longer investigations should be carried out before finally reaching conclusions about the sample’s biocompatibility and safeness in biomedical applications for the elderly.
The presence of excessive iron was observed until the 28th day of NpCit treatment in both the elderly group (liver and kidneys) and the young group (spleen, liver, and lungs) as revealed by ICP-OES and histology studies. Grotto49 has pointed out that an elevated amount of iron in tissues can damage lipid structures, proteins, and DNA, causing severe tissue damage or mutations. However, as mentioned earlier, signs of structural damage, including necrosis and even inflammation, were never observed throughout the whole study period, despite obvious high concentration of MNPs in some organs. Accordingly, young mice exposed to a bolus dose of dextran-coated magnetite NPs did not show morphological or ultramicroscopic alterations, even when the MNPs were retained for long term (6 months) in the liver and spleen.50
It is well established that excessive iron, such as iron coming from metabolized NPs, is gradually deposited in various organs,51,52 especially in the spleen and liver,32,53,54 corroborating the findings of the present study in both elderly and young animals. However, some differences related to age were found. Although the amount of iron detected in the blood of EC animals was slightly lower than that of YC groups, all the organs (spleen, liver, kidney, lung, and brain) of older mice presented a significantly higher concentration of iron than those of young animals, highlighting an important difference in iron biodistribution related to age.
Furthermore, the elderly and young animals presented a completely different iron biodistribution profile after NpCit injection. The significantly higher iron concentration in the liver at the 28th day after the injection, relative to their respective controls, was the only similar result between the two animal groups. The difference in iron distribution is another important aspect, because several diseases are associated with alterations in iron metabolism55 and should be considered when investigating the biodegradation of iron oxide nanoparticles designed as a diagnostic or therapeutic agent for those diseases, especially in older animals.34
In agreement with our results, some literature data have shown that older animals are more susceptible to the adverse effects of nanoparticles than young animals.17 Inhalation of SiO2 nanoparticles under identical conditions caused more pulmonary and cardiovascular damage in elderly rats than in young animals.56 Aluminum or copper nanoparticles induced higher neurotoxicity in older adult rats than in young adult rats, suggesting that the elderly animals are more vulnerable to brain damage induced by nanoparticles.34 Furthermore, aged mice present more intestinal permeability and consequently more NP absorption, and a higher liver deposition and hence more liver injuries, which can culminate in increased oxidative stress and inflammation levels after NP administration.57 In spite of the relevance of these data, an extensive search in the literature (Table 5) concerning the administration of nanomaterials in older organisms and its effects reveals a huge gap in the area. Investigations have been performed in classical aging models such as Caenorhabditis elegans58,59 and Drosophila melanogaster,60 and rats61 which showed opposite effects of NPs, and in animals deficient for antioxidant enzymes,62 due to their similarities with aged organisms. Certainly, more information concerning nanotoxicity and biodistribution may come from the data obtained from aged patients treated with nanomaterials.63–65 In a recent review of patients with lung cancer treated with nanoparticles, Herrera et al66 included data related to the safety and tolerability of nanomaterials, while highlighting the underrepresentation of elderly patients in the clinical trials.
  | Table 5 Nanoparticle investigations in aged organisms |
Despite all the age-dependent effects observed in the present study, it is important to remember that the investigated elderly animals did not present any histological alterations or the clinical characteristics of old mice, thus evidencing they were in good health. Certainly, studies focusing on the safety of iron-based MNPs for animals bearing chronic diseases are mandatory for a better understanding of the effects of nanostructured materials on older organisms.
Conclusion
The NpCit sample induced age-dependent effects on mice. In the young animals, it caused very slight and temporary biochemical and hematological changes, such as changes in albumin and urea levels and HGB parameters. The changes associated with the natural aging process were more numerous and profound in the elderly than those induced by NpCit in the young animals. These aging changes made the elderly mice susceptible to NpCit effects that were either synergistic (leukocytes) or opposite (erythrocytes, albumin, TNF-a, and NO levels) to the aging changes and sometimes more durable (leukocytes). Iron distribution was also age dependent and showed different patterns until the 28th day of treatment. Remarkably, no significant changes were seen in the clinical studies or in the histological features after the treatment, indicating the biocompatibility of NpCit and its potential use in biomedical applications. However, the differences in data concerning the biodistribution and biocompatibility of nanoparticles between elderly and young animals emphasize the need for more studies performed for a longer time window to appropriately extend the benefits of nanotechnology to the elderly population.
Acknowledgments
We acknowledge Dr Emilia CO Lima from the Federal University of Goiás, Goiânia, GO, Brazil, for providing the NpCit sample, the Coordination for Further Training of Graduate Staff (CAPES), the Brazilian National Council for Technological and Scientific Development (CNPq), the Foundation to Support Research in the Federal District (FAPDF), the Network of Nanobiotechnology CON-NANO (CAPES), the National Institute of Science and Technology in Nanobiotechnology (INCT-Nanobiotecnologia), the Center for Nanoscience and Nanobiotechnology of the University of Brasília (CNANO-UnB), and the Dean of Research and Post-Graduation of the University of Brasília (DPP-UnB) for financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Mohammadi Z, Zack MS, Seidi K, Barati M, Akbarzadeh A, Zarghami N. The effect of chrysin loaded PLGA-PEG on metalloproteinase gene expression in mouse 4T1 tumor model. Drug Res (Stuttg). 2017;67(4):211–216. doi:10.1055/s-0042-122136 | ||
Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol Semin Orig Investig. 2008;26:57–64. doi:10.1016/j.urolonc.2007.03.015 | ||
Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi:10.1016/j.ejps.2012.12.006 | ||
Kossatz S, Grandke J, Couleaud P, et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015;17(1):1–17. doi:10.1186/s13058-014-0509-4 | ||
Cassim SM, Giustini AJ, Baker I, et al. Development of novel magnetic nanoparticles for hyperthermia cancer therapy. Proc SPIE Int Soc Opt Eng. 2011;7901:790115. | ||
Buske N, Sonntag H, Götze T. Magnetic fluids – their preparation, stabilization and applications in colloid science. Colloids and Surfaces. 1984;12:195–202. doi:10.1016/0166-6622(84)80099-2 | ||
Lacava LM, Lacava ZGM, Azevedo RB, et al. Use of magnetic resonance to study biodistribution of dextran-coated magnetic fluid intravenously administered in mice. J Magn Magn Mater. 2002;252:367–369. doi:10.1016/S0304-8853(02)00654-6 | ||
Chaves SB, Lacava LM, Lacava ZGM, et al. Light microscopy and magnetic resonance characterization of a DMSA-coated magnetic fluid in mice. IEEE Trans Magn. 2002;38(5):3231–3233. doi:10.1109/TMAG.2002.802495 | ||
Sadeghiani N, Barbosa LS, Guedes MHA, et al. Magnetic resonance of polyaspartic acid-coated magnetite nanoparticles administered in mice. IEEE Trans Magn. 2005;41(10):4108–4110. doi:10.1109/TMAG.2005.855334 | ||
Lacava ZGM, Azevedo RB, Martins EV, et al. Biological effects of magnetic fluids: toxicity studies. J Magn Magn Mater. 1999;201:431–434. doi:10.1016/S0304-8853(99)00002-5 | ||
Ivkov R. Magnetic nanoparticle hyperthermia: A new frontier in biology and medicine? Int J Hyperthermia. 2013;6736(8):703–705. doi:10.3109/02656736.2013.857434 | ||
Candido NM, Calmon MF, Taboga SR, et al. High efficacy in hyperthermia-associated with polyphosphate magnetic nanoparticles for oral cancer treatment. J Nanomed Nanotechnol. 2014;05(03):1–11. | ||
Wu W, Jiang CZ, Roy VAL. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale. 2016;8(47):19421–19474. doi:10.1039/c6nr07542h | ||
Sheedfar F, Biase SD, Koonen D, et al. Liver diseases and aging: friends or foes? Aging Cell. 2013;12(6):950–954. doi:10.1111/acel.12128 | ||
Hasty P, Vijg J. Genomic priorities in aging. Science. 2002;296(5571):1250–1251. doi:10.1126/science.1071808 | ||
Jenkins EO, Deal AM, Anders CK, et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist. 2014;19(10):1076–1083. doi:10.1634/theoncologist.2014-0184 | ||
Li Y, Zhang Y, Yan B. Nanotoxicity overview: Nano-threat to susceptible populations. Int J Mol Sci. 2014;15(3):3671–3697. doi:10.3390/ijms15033671 | ||
Calero M, Chiappi M, Lazaro-Carrillo A, et al. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J Nanobiotechnology. 2015;13(1):1–15. doi:10.1186/s12951-014-0062-4 | ||
Kaur P, Aliru ML, Chadha AS, et al. Hyperthermia using nanoparticles – promises and pitfalls punit. Int J Hyperthermia. 2016;32(1):76–88. doi:10.3109/02656736.2015.1120889 | ||
Orucevic A. Breast cancer in elderly caucasian women – an institution-based study of correlation between breast cancer prognostic markers, tnm stage, and overall survival. Cancers (Basel). 2015;7(3):1472–1483. doi:10.3390/cancers7030846 | ||
Shrestha S, Jiang P, Sousa MH, et al. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J Mater Chem B. 2016;4(2):245–256. doi:10.1039/C5TB02007G | ||
Morais PC, Santos RL, Pimenta ACM, et al. Preparation and characterization of ultra-stable biocompatible magnetic fluids using citrate-coated cobalt ferrite nanoparticles. Thin Solid Films. 2006;515(1):266–270. doi:10.1016/j.tsf.2005.12.079 | ||
Silva AC, Oliveira TR, Mamani JB, et al. Magnetohyperthermia for treatment of gliomas: experimental and clinical studies. Einstein. 2010;8(3):361–367. doi:10.1590/S1679-45082010RW1757 | ||
Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69(6):621–632. doi:10.1093/gerona/glt136 | ||
Kückelhaus S, Tedesco AC, Oliveira DM, et al. Optical emission spectroscopy as a tool for the biodistribution investigation of cobalt-ferrite nanoparticles in mice. J Appl Phys. 2005;97(10):2003–2006. doi:10.1063/1.1852311 | ||
Sousa MH, Geraldo J, Depeyrot J, et al. Chemical analysis of size-tailored magnetic colloids using slurry nebulization in ICP-OES. Microchem J. 2011;97(2):182–187. doi:10.1016/j.microc.2010.09.002 | ||
Szigeti K, Hegedus N, Racz K, et al. Thallium labeled citrate-coated prussian blue nanoparticles as potential imaging agent. Contrast Media Mol Imaging. 2018;(4):1–10. doi:10.1155/2018/2023604 | ||
Kückelhaus S, Garcia VAP, Lacava LM, et al. Biological Investigation of a citrate-coated cobalt-ferrite-based magnetic fluid. J Appl Phys. 2003;93(10):6707–6708. doi:10.1063/1.1558665 | ||
Bonadio RS, Arcanjo AC, Lima ECD, et al. DNA methylation alterations induced by transient exposure of MCF-7 cells to maghemite nanoparticles. Nanomedicine. 2017;12(23):2637–2649. doi:10.2217/nnm-2017-0241 | ||
Neves WP, Sousa CRS, Miranda-Vilela AL, et al. Comparative efficacy of a biocompatible citrate-functionalized magnetic fluid mediating radiofrequency hhyperthermia and magnetohyperthermia to treat ectopic ehrlich-solid-tumor-bearing elderly mice. J Cancer Sci Ther. 2017;9:392–393. | ||
Pernodet N, Fang X, Sun Y, et al. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. 2006;2(6):766–773. doi:10.1002/smll.200500492 | ||
Sharma A, Cornejo C, Mihalic J, et al. Physical characterization and in vivo organ distribution of coated iron oxide nanoparticle. Sci Rep. 2018;8(1):4916. doi:10.1038/s41598-018-23317-2 | ||
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8(1):1–13. doi:10.1038/s41598-017-17765-5 | ||
Sharma A, Muresanu DF, Patnaik R, et al. Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats. Mol Neurobiol. 2013;48(2):386–396. doi:10.1007/s12035-013-8500-0 | ||
Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23(3):217–226. doi:10.1055/s-2003-42641 | ||
Sutherland RJ. Biochemical evaluation of the hepatobiliary system in dogs and cats. Vet Clin North Am Small Anim Pract. 1989;19(5):899–927. | ||
Quimby FW, Luong HR. Clinical chemistry of the laboratory mouse. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. California: Elsevier; 2007:171–216. | ||
Dewar HA, Rowell NR, Smith AJ. Serum glutamic oxalacetic transaminase in acute myocardial infarction. Br Med J. 1958;2(5105):1121–1125. | ||
Bruce R, Todd JK, Ledune L. Serum transaminase: its clinical use in diagnosis and prognosis. Br Med J. 1958;2(5105):1125–1128. | ||
Almiersjo O, Bengnmark S, Engevik L, et al. Serum enzyme changes after hepatic dearterialization in man. Ann Surg. 1968;167:9–17. doi:10.1097/00000658-196801000-00002 | ||
Chalifoux A, Lagacé A. Enzymes sériques pour le diagnostic de la nécrose hépatique aigue expérimentale. Can J Comp Med. 1969;33(3):178–186. | ||
Thrall MA, Baker DC, Campbell TW, et al. Hematologia e Bioquímica Clínica Veterinária. Roca, São Paulo. 2007:259–260. | ||
Pulchinelli Junior A, Junior AJC, Gimenes AC. Clinical laboratory findings in the elderly. J Bras Patol Med Lab. 2012;48(3):169–174. doi:10.1590/S1676-24442012000300004 | ||
Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev. 2010;24(1):53–63. doi:10.1016/j.tmrv.2009.09.005 | ||
Li J, Gárcia CC, Riedt T, et al. Murine hematopoietic stem cell reconstitution potential is maintained by osteopontin during aging. Sci Rep. 2018;8(1):1–9. | ||
Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90(17):7915–7922. | ||
Ewers I, Rizzo LV, Filho JK. Imunologia e envelhecimento. Einstein. 2008;6(Supl 1):13–20. | ||
Habib S, Ali A. Biochemistry of nitric oxide. Indian J Clin Biochem. 2011;26(1):3–17. doi:10.1007/s12291-011-0108-4 | ||
Grotto HZW. Fisiologia e metabolismo do ferro. Rev Bras Hematol Hemoter. 2010;32(2):8–17. doi:10.1590/S1516-84842010005000050 | ||
Estevanato L, Cintra D, Baldini N, et al. Preliminary biocompatibility investigation of magnetic albumin nanosphere designed as a potential versatile drug delivery system. Int J Nanomedicine. 2011;6:1709–1717. doi:10.2147/IJN.S21323 | ||
Nallathamby PD, Mortensen NP, Palko HA, et al. New surface radiolabeling schemes of super paramagnetic iron oxide nanoparticles (SPIONs) for biodistribution studies. Nanoscale. 2015;7(15):6545–6555. doi:10.1039/C4NR06441K | ||
Han X, Yao P, Cheng C, et al. Preparation and in vivo biodistribution of ultra-small superparamagnetic iron oxide nanoparticles with high magnetic targeting response. J Nanosci Nanotechnol. 2018;18(2):879–886. doi:10.1166/jnn.2018.14110 | ||
Cançado RD. Sobrecarga e quelação de ferro na anemia falciforme. Rev Bras Hematol Hemoter. 2007;29(3):316–326. doi:10.1590/S1516-84842007000300025 | ||
Rodrigues D, Freitas M, Costa VM, et al. Quantitative histochemistry for macrophage biodistribution on mice liver and spleen after the administration of a pharmacological-relevant dose of polyacrylic acid-coated iron oxide nanoparticles. Nanotoxicology. 2017;11(2):256–266. doi:10.1080/17435390.2017.1291865 | ||
Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov. 2017;16(6):400–423. doi:10.1038/nrd.2016.248 | ||
Chen Z, Meng H, Xing G, et al. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible population. Environ Sci Technol. 2008;42(23):8985–8992. | ||
Wei Y, Li Y, Jia J, et al. Aggravated hepatotoxicity occurs in aged mice but not in young mice after oral exposure to zinc oxide nanoparticles. NanoImpact. 2016;3–4:1–11. doi:10.1016/j.impact.2016.09.003 | ||
Kim J, Takahashi M, Shimizu T, et al. Effects of potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Ageing Dev. 2008;129(6):322–331. | ||
Scharf A, Piechulek A, von Mikecz A. Effect of nanoparticles on the biochemical and behavioral aging phenotype of the nematode caenorhabditis elegans. ACS Nano. 2013;7(12):10695–10703. doi:10.1021/nn403443r | ||
Zhang Y, Wang Z, Li X, et al. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv Mater. 2016;28(7):1387–1393. doi:10.1002/adma.201503893 | ||
Gaté L, Disdier C, Cosnier F, et al. Biopersistence and translocation to extrapulmonary organs of titanium dioxide nanoparticles after subacute inhalation exposure to aerosol in adult and elderly rats. Toxicol. 2017;265:61–69. | ||
Shibuya S, Ozawa Y, Watanabe K, et al. Palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in mice. PLoS One. 2014;9(10):1–9. doi:10.1371/journal.pone.0109288 | ||
Zheng Q, Yao Y, Nan K. Weekly intravenous nanoparticle albumin-bound paclitaxel for elderly patients with stage IV non-small-cell lung cancer: a series of 20 cases. J Biomed Res. 2012;26(3):159–164. doi:10.7555/JBR.26.20110106 | ||
Okuma Y, Hosomi Y, Takahashi S, et al. A phase II study of nanoparticle albumin-bound paclitaxel plus carboplatin as the first-line therapy in elderly patients with previously untreated advanced non small cell lung cancer. Cancer Chemother Pharmacol. 2016;78(2):383–388. doi:10.1007/s00280-016-3092-9 | ||
Valerio MR, Ancona C, Marchese A, et al. Impressive objective response to nab-paclitaxel plus trastuzumab as fifth line therapy in an elderly HER-2 positive breast cancer patient. J Cancer Ther. 2017;08(11):933–994. | ||
Herrera DA, Ashai N, Perez-Soler R, et al. Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: an evaluation of the clinical evidence. Expert Opin Pharmacother. 2019;20(1):95–102. doi:10.1080/14656566.2018.1546290 |
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.