Back to Journals » Journal of Experimental Pharmacology » Volume 15
The Efficacy of Topical Formulation Containing Ciplukan (Physalis angulata Linn.) in Modulating Interleukin-17 and Interferon Gamma Expression in Mice (Mus musculus) Psoriasis Model
Authors Suwarsa O , Dharmadji HP, Rohmawaty E , Mareta S , Gunawan H , Dwiyana RF , Achdiat PA , Sutedja E , Pangastuti M
Received 26 June 2023
Accepted for publication 2 October 2023
Published 9 October 2023 Volume 2023:15 Pages 367—374
DOI https://doi.org/10.2147/JEP.S427615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Oki Suwarsa,1 Hartati Purbo Dharmadji,1 Enny Rohmawaty,2 Shela Mareta,1 Hendra Gunawan,1 Reiva Farah Dwiyana,1 Pati Aji Achdiat,1 Endang Sutedja,1 Miranti Pangastuti1
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran-Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
Correspondence: Oki Suwarsa, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +62 813 2275 0101, Email [email protected]
Background: Interleukin 17 (IL-17) and interferon gamma (IFN-γ) play a role in the pathogenesis of psoriasis vulgaris (PV). Topical corticosteroids are still utilised as first-line therapy for mild to moderate PV. However, long-term use of corticosteroid is associated with various side effects. Physalis angulata Linn. (Ciplukan) possesses anti-inflammatory properties that could serve as a potential alternative topical therapy for PV.
Objective: To assess the efficacy of topical ciplukan as an anti-inflammatory agent targeting the expression of IL-17 and IFN-γ.
Methods: Psoriasis was induced using imiquimod cream, therefore divided into five groups. Group I, the psoriasis control group, received only imiquimod cream. Groups C1 and C2 received imiquimod cream followed by a mixture of Ciplukan and vaseline in a 1:2 and 1:4 ratio, respectively. Group M, the standard therapy group, received imiquimod cream, followed by mometasone furoate cream. Lastly, group V, the vehicle group, received imiquimod cream followed by vaseline album. Expression of IL-17 and IFN-γ in mice’s skin tissue was analysed using reverse transcription polymerase chain reaction (RT-PCR) after seven days of treatment.
Results: The mean expression of IL-17 in Group C1 (22.60) was significantly lower (p = 0.012) than in the psoriasis control group (23.60), and there was no significant difference (p = 0.613) in Group M (22.41). The mean expression of IFN-γ in Group C1 (26.97) and Group C2 (27.03) was also significantly lower (p = 0.026 and p = 0.026, respectively) than Group I (28.80), and there was no significant difference (p = 0.180 and p = 0.093, respectively) than Group M (26.03).
Conclusion: Expression of IL-17 and IFN-γ in the ciplukan group is lower than in the psoriasis control group, and there is no significant difference compared to the standard therapy group.
Keywords: IFN-γ, IL-17, Physalis angulata Linn, psoriasis vulgaris
Introduction
Psoriasis, a chronic inflammatory disease characterised by epidermal hyperproliferation, is influenced by a combination of genetic and environmental factors.1–4 While the exact pathogenesis of psoriasis vulgaris (PV) remains incompletely understood,5–7 cytokines such as interleukin (IL)-17 and interferon (IFN)-γ have been implicated in its development.8–10 A decreased IL-17 level demonstrates improvement in psoriatic skin lesions and IL-17 acts synergistically with IFN-γ.8 Current first-line therapy for mild to moderate PV involves the use of topical corticosteroids, which exhibit immunosuppressive, anti-inflammatory, and antimitotic effects. However, long-term use of corticosteroids is associated with adverse effects.11,12 In contrast, Physalis angulata Linn., or ciplukan, which is a type of herb from Indonesia, is rich in phenols, physalin, withanolide, and carotenoids, which possess anti-inflammatory, antiproliferative, and immunosuppressive properties.13–16 These characteristics make it a promising candidate for alternative topical therapy for PV. Imiquimod can induce the expression of IL-17A in a mouse model of psoriasis and has been widely used in basic research for the evaluation of anti-psoriasis drugs. This is related to the mobilisation of T helper (Th)17 cells as IL-17A receptors. The expression of IL-17A also correlates with the severity of the disease in the mouse model of psoriasis.17 The severity of psoriasis was assessed using the modified Psoriasis Area and Severity Index (PASI) score, which quantitatively measured erythema, scale, and skin thickening, assigning values from 0 to 4 (0: no change, 1: mild change, 2: marked change, 3: significant change, and 4: severe change). Psoriasis in mice successfully induced with imiquimod has at least has a 3-point modified PASI score. Consequently, further investigation is necessary to assess the expression of IL-17 and IFN-γ and evaluate the efficacy of topical ciplukan (Physalis angulata Linn.) preparations as a potential alternative therapy for PV, aiming to suppress the inflammatory process and promote clinical improvement in a murine (Mus musculus) psoriasis model.
Materials and Methods
Study Design
This study was an experimental laboratory investigation employing a completely randomised design (CRD). Preliminary research was conducted to establish a suitable treatment methodology and confirm the manifestation of psoriasis lesions as anticipated. The findings from the preliminary study demonstrated that during the induction phase, the application of 5% imiquimod cream for a duration of seven days was effective. Subsequently, imiquimod cream was administered concurrently with the test substances (ciplukan, 0.1% mometasone furoate cream, and vaseline) at six-hour intervals.
Time and Place of Study
The study was conducted at the Pharmacology Division Laboratory, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, to administer treatment to the test animals. The examination of IL-17 and IFN-γ expression using reverse transcription polymerase chain reaction (RT-PCR) was performed at the Genetics and Molecular Laboratory, Faculty of Medicine, Universitas Padjadjaran. The research was conducted between September 2022 and December 2022.
Object of Study
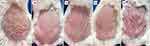
The study utilised female Mus musculus mice obtained from the Bandung Institute of Technology Laboratory, and these mice met the specified inclusion and exclusion criteria. The inclusion criteria were as follows: (1) Female Mus musculus mice aged 10 weeks; (2) body weight ranging from 20 to 25 grams; (3) active movement, responsive to running, and easily startled. Exclusion criteria encompassed: (1) the presence of any skin disorder; (2) dull and excessive hair loss. Furthermore, the drop-out criteria for experimental animals included: (1) body weight loss exceeding 20% after the adaptation period in the laboratory; (2) death of mice during the adaptation period; (3) development of secondary skin infection; (4) thickened hair growth following shaving. The experimental tests with the animals were conducted at the Laboratory of the Pharmacology Division, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran. The mice were allowed to adapt for seven days before initiating the study. To induce psoriasis, the backs of the female mice (Mus musculus) were shaved and depilated, creating a 2 cm x 3 cm area, followed by the application of imiquimod cream at a dose of 42 mg for 7 consecutive days on their backs.
Experiment Protocol
Preparation of Experimental Animals
The mice were acclimated for seven days under controlled conditions of room temperature and humidity, with a 12-hour light and 12-hour dark cycle. On the 7th day of acclimation, their body weight was measured. Throughout the study, the mice were provided with Prositera® as their food and had unrestricted access to water. For this study, five cages measuring 13 cm x 13 cm x 9.5 cm were utilized, with each cage housing five mice separated by partitions (isolated cages). The cage floors were lined with wood shavings and covered with wire mesh lids to ensure proper ventilation.
The procedure for inducing psoriasis in the mice involved shaving and depilating an area of 2 cm x 3 cm on the backs of the mice using Veet® cream. The depilated area was then cleansed with a 0.9% NaCl solution before the application of 5% imiquimod cream. The cream was applied to the shaved area of the mice’s backs at a dose of 42 mg/day for a duration of 7 days. The severity of psoriasis was assessed using the modified PASI score. Subsequently, photographs were taken, and modified PASI scores were assessed using Investigator Global Assessment, which quantitatively measured erythema, scale, and skin thickening, assigning values from 0 to 4 (0: no change, 1: mild change, 2: marked change, 3: significant change, and 4: severe change). The overall PASI score ranges from 0 to 12.
Preparation of Ciplukan Topical Preparations
To prepare the ciplukan topical formulations using Physalis angulata Linn., initially, 10 grams of vaseline album and 5 grams of ciplukan powder Lesikaf® (Kimia Farma) for Ciplukan 1, and 10 grams of vaseline album and 2.5 grams of ciplukan powder for Ciplukan 2, were accurately weighed. The vaseline album was placed in a measuring cup and heated using the water bath method until it reached a water temperature of 70°C. Once the vaseline album had melted, the ciplukan powder was added and stirred until fully dissolved. Subsequently, all ciplukan topical preparations were transferred into dry containers for storage.
Treatment
Psoriasis model mice were divided into five different treatment groups, each consisting of six mice. Group I, the control group, received 5% imiquimod cream. Group C1, or Ciplukan 1, was administered 5% imiquimod cream along with a mixture of Physalis angulata Linn. and vaseline album in a ratio of 1:2. Group C2, or Ciplukan 2, received 5% imiquimod cream along with a mixture of Physalis angulata Linn. and vaseline album in a ratio of 1:4. Group M, the standard therapy group, was given 5% imiquimod cream followed by 0.1% mometasone furoate cream. Group V, the vehicle group, was treated with 5% imiquimod cream followed by vaseline album (Figure 1).
After seven days of treatment, the experimental animals were euthanised according to their respective groups, and 50 mg of skin lesions were collected for ribonucleic acid (RNA) isolation. The isolated mouse skin RNA was then purified and mixed with RT-PCR reagents for subsequent measurement.
Statistical Analysis
The data were presented as mean ± S.E.M. For the comparison of determined values (gene expression) among multiple groups, a one-way ANOVA or Kruskal–Wallis test was performed, followed by the LSD or Mann–Whitney test. A p-value of less than 0.05 was considered statistically significant.
Results
Comparison of Mean IL-17 Expression Between Groups C1, C2, M, and V with Group I
Based on Table 1, the mean expression of IL-17 in group C1 (22.60) was lower than that in group I (23.60). Furthermore, the mean IL-17 expression in group M (22.41) was also lower compared to group I (23.60). These results showed a significant difference with a p-value < 0.05.
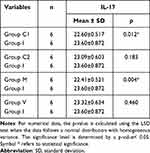 |
Table 1 Comparison of Mean IL-17 Expression Between Groups C1, C2, M, and V with Group I |
Comparison of Mean IL-17 Expression Between Groups C1 and C2 with Group M
Based on Table 2, the mean expression of IL-17 in group C1 (22.60) was observed to be higher than in group M (22.41). Similarly, the mean IL-17 expression in group C2 (23.09) was higher compared to group M (22.41). However, these results did not show a significant difference, as indicated by a p-value > 0.05.
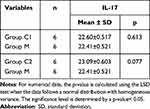 |
Table 2 Comparison of Mean IL-17 Expression Between Group C1 and C2 with Group M |
Comparison of Mean IFN-γ Expression Between Groups C1, C2, M, and V with Group I
Based on Table 3, the mean expression of IFN-γ in group C1 (26.97) was lower than that in group I (28.80). Similarly, the mean IFN-γ expression in group C2 (27.03) was lower compared to group I (28.80). Additionally, the mean IFN-γ expression in group M (26.03) was also lower than that in group I (28.80). These results showed a significant difference with a p-value < 0.05.
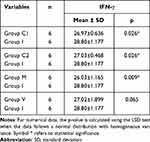 |
Table 3 Comparison of Mean IFN-γ Expression Between Groups C1, C2, M, and V with Group I |
Comparison of Mean IFN-γ Expression Between Groups C1 and C2 with Group M
Based on Table 4, the mean expression of IFN-γ in group C1 (26.97) was observed to be higher than in group M (26.03). Similarly, the mean IFN-γ expression in group C2 (27.03) was higher compared to group M (26.03). However, these results did not show a significant difference, as indicated by a p-value > 0.05.
 |
Table 4 Comparison of Mean IFN-γ Expression Between Group C1 & C2 with Group M |
Discussion
Ciplukan (Physalis angulata Linn.) is a plant from the Solanaceae family that is widely distributed in tropical regions, making it easily found in Indonesia.16 This plant contains physalins, high levels of phenols (>20 mg/g), withanolides, and carotenoids, which have antioxidant and immunosuppressive effects by inhibiting the complement system and intercellular biochemical reactions as well as macrophage activation.14,15 Physalin is a secosteroid derivative that shares a structural similarity with glucocorticoids and has anti-inflammatory effects by inhibiting the activation of the NF-κβ cascade.18
Ciplukan also has anti-inflammatory, antiproliferative, antiangiogenic, and anticancer effects due to its content of phenols, secosteroids, and saponins. There are three main pathways in ciplukan as an immunomodulator. The first pathway involves the phenolic components in ciplukan, such as flavonoids, tannins, and phenylpropanoids, which exhibit antioxidant and immunomodulatory effects. The second and third pathways are attributed to secosteroid and saponin compounds, which have anti-inflammatory and antiproliferative effects.18,19
The findings of this study revealed a significant reduction in the mean expression of IL-17 within the Ciplukan 1 group compared to the group of mice with the psoriasis model. Salgado et al20 conducted an in vitro study wherein ciplukan extract, comprising phenols (flavonoids, tannins, and phenylpropane), secosteroids (physalin and withangulin), and saponins, exhibited anti-inflammatory effects. In 2014, Mahalakshmi et al21 reported that ciplukan contains phenolic components with antioxidant and immunomodulatory effects, as observed in in vitro studies. Likewise, Soares et al19 investigated the effects of physalin B, isolated from Physalis angulata Linn., in mice intraperitoneally injected with lipopolysaccharide components from Gram-negative bacteria. The results demonstrated reductions in NO, TNF, IL-6, and IL-12 levels compared to the control group. Furthermore, Baltos et al22 conducted a study on rats in which subcutaneous administration of Physalis angulata Linn. Root extract at various doses (0.5 mg/kg, 1 mg/kg, and 5 mg/kg) was performed one hour before inducing inflammation with a 1% carrageenan injection. Notably, the 5 mg/kg dose exhibited the most significant anti-inflammatory effect, resulting in an 84% reduction in exudate volume, an 80% decrease in total inflammatory cells, a 43% decrease in adenosine deaminase activity, a 70% decrease in nitrite levels, and a 75% decrease in prostaglandin levels compared to the control group.
The mean expression of IL-17 in the group treated with 0.1% mometasone furoate cream (22.41) was found to be lower than in the other treatment groups. However, in our study, there was no statistically significant difference in the mean expression of IL-17 between the Ciplukan 1 group and the group treated with 0.1% mometasone furoate cream. It is important to note that topical corticosteroids remain the primary choice for topical treatment of PV due to their widespread use and efficacy. However, prolonged use of topical corticosteroids can lead to various side effects, including steroid acne, striae, skin atrophy, telangiectasia, perioral dermatitis, hypertrichosis, and hypopigmentation.23,24 Therefore, they were utilised as the standard therapy in this research. Li et al17 conducted a study evaluating IL-17 antagonists in a group of 24 psoriasis model mice. In their study, the psoriasis model mice were treated with a daily injection of 10 mg/kg dexamethasone as the standard therapy group. The results indicated that the psoriasis model mice treated with dexamethasone exhibited significantly lower IL-17 plasma levels compared to the control group.
These findings align with the research conducted by Ton et al18 on ciplukan, which contains physalin B and G, secosteroids structurally similar to corticosteroids. They exert an anti-inflammatory effect by inhibiting the activation of the NF-κB cascade. Furthermore, Chen et al25 explained that quercetin present in ciplukan reduced NF-κB levels in leukocyte and keratinocyte cell nuclei in a mouse model of psoriasis induced by imiquimod cream. The ability of quercetin to lower NF-κB levels resembles the anti-inflammatory effect of glucocorticoids.
In this study, the mean expression of IFN-γ was found to be significantly lower in both the Ciplukan 1 and Ciplukan 2 groups compared to the psoriasis model mice group. Ukwubile et al26 conducted a study involving mice injected subcutaneously with 0.1 mL of 0.1% carrageenan in the palms until edema and inflammation were induced. Subsequently, the mice were intraperitoneally injected with a methanol extract of Physalis angulata Linn leaves at doses of 200, 300, and 400 mg/kg BW. The results revealed that the methanol extract of Physalis angulata Linn leaves at a dose of 400 mg/kg BW reduced edema and inflammation by 62.71% compared to the control group.
Furthermore, the mean expression of IFN-γ in the group treated with 0.1% mometasone furoate cream was lower (22.41) than in the other treatment groups. However, in our study, the mean expression of IFN-γ was not significantly lower in the group treated with 0.1% mometasone furoate cream compared to the group receiving Ciplukan 1 and Ciplukan 2 topical preparations. This observation is consistent with the known anti-inflammatory effect of corticosteroids, which inhibit the release of the enzyme phospholipase A2 involved in the synthesis of prostaglandins, leukotrienes, and other arachidonic acid derivatives.27,28 Corticosteroids also suppress transcription factors such as activator protein (AP)-1 and NF-κB, while increasing the expression of the lipocortin I gene, which acts as an inhibitor of phospholipase A2.11 In addition, corticosteroids reduce the synthesis and secretion of cytokines such as IL-1, IL-2, IL-8, IL-17, GM-CSF, IFN-γ, TNF-α, and various inflammation-related proteins.11,29
Pinto et al30 investigated the topical administration of physalin E, an active component in Physalis angulata Linn., and its anti-inflammatory effect on a mouse model of dermatitis. The results demonstrated that IFN-γ levels in dermatitis lesions treated with 0.5 mg of physalin E were not statistically different from those treated with 0.05 mg of dexamethasone.
Research Limitations
This study has several limitations that should be acknowledged. Firstly, it is important to note that this study was preliminary and represents the first investigation of the effects of topical ciplukan preparations on psoriasis-model mice. Therefore, further research is needed to validate and expand upon the findings presented here. Secondly, it is worth mentioning that no specific pharmacological tests were conducted to optimize the formulation of effective topical dosage forms, determine appropriate dosages, or quantitatively assess the active ingredients in the ciplukan preparations. These aspects are essential for understanding the therapeutic potential and establishing standardised protocols for the use of ciplukan in topical treatments. Addressing these limitations in future studies will contribute to a more comprehensive understanding of the therapeutic effects of topical ciplukan preparations and provide valuable insights for developing effective and evidence-based treatment strategies for psoriasis.
Conclusion
The expression levels of IL-17 and IFN-γ in the psoriasis model mice group treated with ciplukan topical preparation are found to be lower compare to the untreated psoriasis model mice group. However, it should be noted that the expression levels are not significantly lower than those observed in the psoriasis model mice group treated with 0.1% mometasone furoate cream. These findings suggest that ciplukan topical preparations may serve as a potential alternative therapy for psoriasis vulgaris. Nevertheless, further research is warranted to compare ciplukan extract with various vehicles and to quantitatively assess the active constituents of ciplukan in topical preparations. These investigations are essential for establishing standardized protocols and determining the optimal formulation for clinical applications of ciplukan in the treatment of psoriasis vulgaris.
Ethical Approval
The guidelines for the welfare of laboratory animals by the Research Ethical Committee of Universitas Padjajaran refer to American Veterinary Medical Association (AVMA) guidelines for the Euthanasia of Animals: 2020 Edition and National Research Council of the National Academies, Guide for the Care and Use of Laboratory Animals (Eight Edition), 2011, with approval number: 933/UN6.KEP/EC/2022.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ, Wolff K. Fitzpatrick’s Dermatology.
2. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology.
3. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264–272. doi:10.1111/1346-8138.14139
4. Raychaudhuri SP, Raychaudhuri S, Bagchi D. Psoriasis and Psoriatic Arthritis Pathophysiology, Therapeutic Intervention and Complementary Medicine.
5. Dewi DAPN, Indira IG. Insiden dan profil psoriasis di poliklinik kulit dan kelamin Rumah Sakit Umum Pusat Sanglah Denpasar periode Januari 2012 sampai Desember 2014 [Incidence and profile of psoriasis in dermatovenereology clinic at Sanglah Central General Hospital Denpasar in January 2012 to December 2014]. E J Med Udayana. 2018;7(9):1–7. Indonesian.
6. Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and TH17 cytokines. Curr Rheumatol Rep. 2007;9:461–467. doi:10.1007/s11926-007-0075-1
7. Traub M, Marshall K. Psoriasis-pathophysiology, conventional, and alternative treatment approaches. Alter Med Rev. 2007;12:319–330.
8. Vecellio M, Hake VX, Davidson C, Carena MC, Wordsworth BP, Selmi C. The IL-17/IL-23 axis and its genetic contribution to psoriatic arthritis. Front Immunol. 2021;11. doi:10.3389/fimmu.2020.596086
9. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi:10.1146/annurev-immunol-032713-120225
10. Chiricozzi A, Guttman-Yassky E, Suárez-Farĩas M, et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131(3):677–687. doi:10.1038/jid.2010.340
11. Kang S, Amagai M, Bruckner AL, et al. Fitzpatrick’s Dermatology.
12. Griffiths CEM, Barker J, Bleiker TO, Chalmers R, Creamer D. Rooks’s Textbook of Dermatology.
13. Kusumaningtyas R, Laily N, Limandha P. Potential of ciplukan (Physalis Angulata L.) as source of functional ingredient. Procedia Chem. 2015;14:367–372. doi:10.1016/j.proche.2015.03.050
14. Sun CP, Qiu CY, Yuan T, et al. Antiproliferative and anti-inflammatory withanolides from physalis angulata. J Nat Prod. 2016;79(6):1586–1597. doi:10.1021/acs.jnatprod.6b00094
15. Murali Krishna T, Vadluri R, Manoj Kumar E. In vitro determination of the antioxidant activity of physalis angulata lnn. Int J Pharma Bio Sci. 2013;4(3):1.
16. Dewi S, Isbagio H, Purwaningsih EH, Kertia N, Setiabudy R, Setiati S. A double-blind, randomized controlled trial of ciplukan (Physalis angulata Linn) extract on skin fibrosis, inflammatory, immunology, and fibrosis biomarkers in scleroderma patients. Acta Med Indones. 2019;51(4):303–310.
17. Li Q, Liu W, Gao S, Mao Y, Xin Y. Application of imiquimod-induced murine psoriasis model in evaluating interleukin-17A antagonist. BMC Immunol. 2021;22(1). doi:10.1186/s12865-021-00401-3
18. Nguyen DT, Ton NL, Nguyen HP, Nguyen DC, Van Ly A, Nguyen TT. Chemical constituents of Physalis angulata L. (family solanaceae). Can Tho Univ J Sci. 2016;2:1.
19. Soares MBP, Bellintani MC, Ribeiro IM, Tomassini TCB, Ribeiro Dos Santos R. Inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis angulata L. Eur J Pharmacol. 2003;459(1):107–112. doi:10.1016/S0014-2999(02)02829-7
20. Rengifo-Salgado E, Vargas-Arana G. Physalis angulata L. (Bolsa mullaca): a review of its traditional uses, chemistry and pharmacology. Bol Latinoam Caribe Plantas Med Aromat. 2013;12(5):1.
21. Mahalakshmi A, NIdavani R. Physalis Angulata L.: an ethanopharmacological review. Indo Am J Pharm Res. 2014;4(3):1.
22. Bastos GNT, Silveira AJA, Salgado CG, Picanço-Diniz DLW, Do Nascimento JLM. Physalis angulata extract exerts anti-inflammatory effects in rats by inhibiting different pathways. J Ethnopharmacol. 2008;118(2):246–251. doi:10.1016/j.jep.2008.04.005
23. Kwatra G, Mukhopadhyay S. Topical corticosteroids: pharmacology. In: A Treatise on Topical Corticosteroids in Dermatology: Use, Misuse and Abuse. Springer; 2017.
24. Uva L, Miguel D, Pinheiro C, et al. Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. 2012;2012:1–16. doi:10.1155/2012/561018
25. Chen H, Lu C, Liu H, et al. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int Immunopharmacol. 2017;48:110–117. doi:10.1016/j.intimp.2017.04.022
26. Ukwubile CA, Oise IE. Analgesic and anti-inflammatory activity of Physalis angulata Linn. (Solanaceae) leaf methanolic extract in swiss albino mice. Int Biol Biomed J. 2016;2(4):1.
27. Di Meglio P, Duarte JH. CD8 T cells and IFN-γ emerge as critical players for psoriasis in a novel model of mouse psoriasiform skin inflammation. J Investig Dermatol. 2013;133:871–874. doi:10.1038/jid.2012.426
28. Torti DC, Feldman SR. Interleukin-12, interleukin-23, and psoriasis: current prospects. J Am Acad Dermatol. 2007;57:1059–1068. doi:10.1016/j.jaad.2007.07.016
29. Huang S. Comprehensive dermatologic drug therapy. Skin Med. 2022;20:1.
30. Pinto NB, Morais TC, Carvalho KMB, et al. Topical anti-inflammatory potential of Physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine. 2010;17(10):740–743. doi:10.1016/j.phymed.2010.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.