Back to Journals » Journal of Experimental Pharmacology » Volume 16
The Effect of Physalis angulata L. Administration on Gene Expressions Related to Lung Fibrosis Resolution in Mice-Induced Bleomycin
Authors Imaduddin UK, Berbudi A, Rohmawaty E
Received 14 September 2023
Accepted for publication 5 January 2024
Published 1 February 2024 Volume 2024:16 Pages 49—60
DOI https://doi.org/10.2147/JEP.S439932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Ummul Khair Imaduddin,1 Afiat Berbudi,2,* Enny Rohmawaty3,*
1Graduate School of Master Program in Anti Aging and Aesthetic Medicine, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Parasitology Division, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 3Pharmacology & Therapy Division, Departement of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
*These authors contributed equally to this work
Correspondence: Enny Rohmawaty, Pharmacology & Therapy Division, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang, KM.21, Hegarmanah, Kec. Jatinangor, Kabupaten Sumedang, Bandung, West Java, 45363, Indonesia, Email [email protected]
Purpose: To explore the potential therapeutic effects of Physalis angulata L. (Ciplukan) extract on lung fibrosis resolution in a Bleomycin-induced mouse model, researchers conducted a comprehensive study. The study focused on key genes associated with fibrosis progression, including Nox4, Mmp8, Klf4, and FAS, and assessed their mRNA expression levels following the administration of Ciplukan extract.
Methods: A Bleomycin-induced mice model was divided into seven groups to investigate the effects of ciplukan extract on fibrosis-related gene expressions. Mice were induced with subcutaneously injected Bleomycin to generate lung fibrosis and given different doses of the Ciplukan extract for four weeks. Lung fibrosis mRNA expression was analyzed by semi-quantitative PCR for Nox4, Klf4, Mmp8, and FAS.
Results: The administration of ciplukan extract resulted in a significant decrease in mRNA expression of Nox4 with p-value=0.000, Mmp8 with p-value =0.002, and Klf4 with p-value =0.007, indicating potential antifibrotic effects. However, FAS expression remained unchanged (p-value=0.127).
Conclusion: Ciplukan extract exhibited promising effects on fibrosis-related gene expressions, particularly Nox4, Mmp8, and Klf4. This study suggests that the extract has the potential to intervene in fibrosis progression, offering a potential avenue for therapeutic strategies.
Keywords: ciplukan, FAS, Klf4, lung fibrosis, Mmp8, Nox4, pulmonary fibrosis, Physalis angulata L
Introduction
Pulmonary Fibrosis (PF) is one of the aging diseases resulting from weakened anti-inflammatory activation and distorted resolution.1 Epithelial cell injury, chronic inflammation, and extracellular matrix protein deposition are pathological features of interstitial pneumonia, shared by idiopathic pulmonary fibrosis (IPF), another interstitial lung disease.2 Life expectancy is typically 2–3 years from diagnosis; the disease is progressive, irreversible, and incurable.3,4 An abnormal accumulation of collagen-rich ECM is a hallmark of fibrosis. Myofibroblasts are a hallmark feature of fibrotic diseases, contributing to fibrosis pathology by secreting a large amount of ECM and participating in alveolar contraction. IPF triggers lung remodeling and epithelial-to-mesenchymal transition (EMT) because of the severe damage to alveolar epithelial cells that it causes.5 Fibrogenesis in the lungs involves a balance between ECM accumulation and the removal of excess ECM.6,7 The success of fibrosis resolution includes identifying and eliminating fibroproliferative stimuli, degrading and clearing ECM, clearing altered myofibroblasts, and recovering damaged lung epithelium and alveoli.8 Pulmonary fibrosis is partially triggered by the activation of genes involved in cell proliferation, cell death, and fibroblast proliferation in response to injury.9 In vivo study of Nox4 and its subsequent products have a significant role in inducing oxidative stress.10 ROS-induced cell death in the lung epithelium may help researchers find new therapeutic targets for treating lung fibrosis.11 Nox4’s ROS production is critical in triggering the epithelial cell death that ultimately results in pulmonary fibrosis.12 Krüppel-like factor 4 (KLF4) is a transcription factor widely recognized for its capacity to either stimulate or hinder the proliferation of various cancer cells contingent upon the particular circumstances in which it operates. Lung fibrogenesis is significantly slowed by the transcription factor KLF4. The expression of this gene within fibroblasts helps prevent fibrosis from worsening and can even lead to its spontaneous resolution.13 Currently, there are two treatments for fibrosis, namely pirfenidone and nintedanib. Nonetheless, these drugs cannot cure IPF or stop the condition’s advancement.14,15 Thus, therapeutic strategies are required, such as developing nutraceutical agents that may help slow or reverse the fibrosis progression.
In Indonesia, there are many plants variety of plants rich in antioxidants. Phenolic and polyphenolic compounds, such as flavonoids, cinnamic acid derivatives, coumarins, tocopherols, and polyfunctional acids, are plants’ most common types of natural antioxidants. One of these medicinal plants is the Ciplukan (Physalis angulata L.), which was previously considered a weed.16 The extracts of Ciplukan leaves and fruits have therapeutic activities such as anti-allergic, anti-asthma, anti-malarial, anticancer, antioxidant, immunomodulatory, and anti-inflammatory activities, which result from the content of total phenolics and flavonoids.16,17 Ciplukan contains citric acid, terpenes/sterols, saponins, flavonoids, and alkaloids. Secondary metabolites like alkaloids, glycosides, flavonoids, tannins, and phenolics have been detected in the preliminary phytochemical screening of Ciplukan fruit and leaves.17 Active components found in Ciplukan include saponins, flavonoids, polyphenols, alkaloids, protein, chlorogenic acid, withangulatin A (WA), palmitic acid, acetic acid, protein, vitamin C, tannins, and malic acid.16,17 In addition to kaempferol 7-O-rhamnoside and isoquercitrin, the flavonoids Quercetin, Quercetin 3-O-methyl ether, and Ciplukan have been identified.18 Quercetin, a prominent flavonoid in the flavonol class, is part of the group of plant pigments known as flavonoids, which add color to numerous fruits, flowers, and vegetables.19
Inflammation is one of a key factor in pulmonary fibrosis, causing severe damage to alveolar epithelium and disrupting alveolar structure.20 Cytokines like TGF-β, tumor necrosis factor (TNF-α), and interleukin-1β (IL-1β) induce EMT in the mesenchymal phenotype, resembling myofibroblasts in fibrotic foci.21 NADPH oxidase contributes to cell senescence and fosters fibroblast apoptosis resistance in individuals with pulmonary fibrosis due to an imbalance in Nox4-Nrf2 signaling. Quercetin significantly suppressed TGF-β induced NOX4 mRNA expression.22 TGF-β1 triggers NOX4 expression in lung mesenchymal cells via a SMAD3-dependent mechanism, leading to hydrogen peroxide (H2O2) generation essential for myofibroblast differentiation, ECM production, and contractility. High Nox4 expression induces fibroblast differentiation into myofibroblasts, triggering significant collagen secretion and ultimately leading to PF.23 Ciplukan recognized for its anti-inflammatory effects, balances redox and reduces ROS, including through Nox4, inducing improvement of fibrosis. NOX4 plays a vital role in myofibroblast activation and lung fibroblast phenotype regulation.22 Elevated Nox4 expression induces fibroblast differentiation into myofibroblasts, resulting in substantial collagen secretion and pulmonary fibrosis. Ciplukan extract inhibits radiation-induced fibroblast differentiation into myofibroblasts by targeting the p38/MAPK/Akt/Nox4 pathway, regulating mitochondrial ROS and ATP production.24 The redox imbalance of Nox4-Nrf2 is a focus in developing PF therapies. Hence, pulmonary fibrosis may benefit from Nox4 inhibition.25 Quercetin has the potential to be involved in mitigating pulmonary fibrosis.
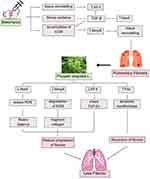
Quercetin mediates antifibrotic effects by inhibiting macrophage senescence by decreasing expression, including MMPs.26 According to the reviewed literature, Quercetin is one of the main flavonoids that helps to reduce Mmp-8 expression. When Quercetin is taken orally, Mmp-8 expression is significantly suppressed.27 Ciplukan indirectly plays an anti-fibrotic role by reducing Mmp8 and increasing IL-10 as well as Mmp9, leading to extracellular matrix (ECM) degradation and collagen fragment formation, facilitating fibrosis resolution.28 Lung fibrogenesis is significantly slowed by the transcription factor KLF4. The expression of this gene within fibroblasts helps prevent fibrosis from worsening and can even lead to its spontaneous resolution.13 KLF4 expression was decreased with Quercetin administration.29 Additionally, it directly stimulates myofibroblast apoptosis by enhancing Fas/FasL secretion, resulting in myofibroblast apoptosis and enabling fibrosis resolution.30 Quercetin increases FasL receptor expression, reducing resistance to death ligand-induced apoptosis.19 Other studies show that dasatinib and Quercetin selectively induce apoptosis in senescent cells and SASP within 48 hours in human tissue.31 Through the TGF-B/AKT/mTOR signaling pathway, Quercetin stimulates autophagy and antifibrotic activities while blocking profibrotic factors.32 The schematic diagram of the mechanism of the Physalis angulata L. on pulmonary fibrosis can be seen in Figure 1. However, research on the effect of Ciplukan extract as an adjunctive agent as antifibrotic via modulation of Nox4, Klf4, Mmp8, and FAS level expression in fibrosis lung tissue is still very limited.
Materials and Methods
Animals
Mice (Mus musculus) weighing 20–30 grams at six weeks of age were used in this study. Standard cage dimensions (54×36.5×28.5 cm3) were used to house the mice, and fine sawdust was provided as the primary nesting material. With a photoperiod of 16:8 (L:D), the room was kept at 24–26°C with humidity between 40 and 60%. The cages were washed twice weekly to ensure cleanliness and had adequate ventilation installed. The rats were provided with water ad libitum and a standardized normal chow diet (NCD) consisting of 65.5% (w/w) carbohydrates, 25% (w/w) protein, 7% (w/w) fat, and an estimated 5% (w/w) micronutrients and minerals. Seven different sets of mice were tested: Groups of mice were divided into the following categories: N (control); BLM (Bleomycin-induced); BLM + M (Bleomycin-induced + Mycophenolate mofetil); BLM + C1 (Bleomycin-induced + Ciplukan supplement at dose 1); BLM + C2 (Bleomycin-induced + Ciplukan supplement at dose 2); BLM + M + C1 (Bleomycin-induced + Mycophenolate mofetil + Ciplukan supplement at dose 1); and BLM + M + C2 (Bleomycin-induced + Mycophenolate mofetil + Ciplukan supplement at dose 2) for four weeks, 0.5 mg of Bleomycin was injected subcutaneously twice weekly into mice to create a model of pulmonary fibrosis. The mice were given Physalis angulata L. extract at 1.95 mg (dose 1) and 3.9 mg (dose 2) once daily for four weeks, beginning at week six after bleomycin induction. The mice were killed when the treatment period was over. The termination of the experimental mice was performed by intraperitoneal administration of 1 mL of ketamine (100mg/mL) and 0,1mL xylazine (20mg/mL) completed with 8.9 mL of physiological saline, for a total of 10 mL. After the sedative effects became apparent, cervical dislocation and decapitation were conducted. Figure 2a provides information on the treatment of mice during study. Tissue samples from the lungs were taken for mRNA testing. This investigation is an example of in vivo experimental research, with frozen mouse lung tissue as the research object.
Physalis angulata L. Extract
Plants of the genus P. Angulata L. (ciplukan) were gathered from all over West Java, Indonesia, except for their roots. The collected samples were identified at Universitas Padjadjaran’s Department of Biology within the Faculty of Mathematics and Natural Sciences. The specimens were then washed before proceeding with the extraction process. Ethyl acetate was then used as the solvent in a cold maceration process conducted at room temperature with repeated stirring. The entire Ciplukan plant was soaked in 70% ethanol for three separate 24-hour periods except for the roots. Afterward, the 70% ethanol solvent was separated from the specimens. The 70% ethanol solvent was then concentrated by evaporating it using a rotary evaporator. The resulting concentrated solution was further processed into a dry extract using the freeze-drying method.33
Messenger RNA (mRNA) Extraction
Lung tissue mRNA was extracted with the help of the UK-based company Bioline’s Genezole reagent. Initial sample weights ranged from 17–20 mg. Then, 200 μL of Genezole reagent was added to the sample. After incubating for 5 minutes at room temperature, the mixture was homogenized for 30 seconds. The samples were centrifuged for 10 minutes at 11,200 rpm and 4°C. The supernatant was transferred to a new 1.5 mL microcentrifuge tube that had been treated to remove any residual RNase, and then 40µL of 100% chloroform was added. The mixture was vortexed for 10 seconds and later centrifuged for 15 minutes. The 100–130 µL of the upper aqueous layer was transferred to a new RNase-free 1.5 mL microcentrifuge tube. Following a ten-minute incubation period at room temperature, 100 mL of 100% isopropanol was added, and the mixture was stirred. The incubated mixture was centrifuged at 4°Celsius for 10 minutes at 11,200 rpm to isolate the RNA. Next, 200 μL of 70% ethanol was included, and after a five-minute centrifugation, the ethanol was discarded. The RNA pellet was resuspended using RNase-free water and subjected to a 10-minute incubation at 60°C to dissolve it.
Semi-Quantitative PCR
Concentrations of mRNA were quantified by assessing absorbance at 268/280 nm using a Multimode Microplate Reader (M200 Pro, Tecan, Morrisville, NC). After electrophoresis, we used a Bioline, UK One-Step RT PCR Kit to conduct a semi-quantitative PCR. We measured Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression as a quality assurance measure. This research used the SensoQuest Labcycler, with a denaturation process occurs at a temperature of 95°C, an annealing process at 58°C for Nox4, Mmp8, and Fas primers; 57°C for Klf4 primer, and 60°C for GAPDH primer. The elongation occurred at 72°C, and 37 cycles were conducted for all primers. The BluePad Detection System and the National Institutes of Health’s Image J software were used to analyze and quantify the produced PCR bands—the PCR reaction used primers (detailed in Table 1). The electrophoresis representation can be seen in Figure 2b.
 |
Table 1 Sequences of the Primers |
Statistical Analysis
SPSS 26.0, created by the American firm IBM Corporation, was used for the statistical analysis. The mean and standard error of the mean (SEM mean) illustrate the data distribution regarding central tendency and the extent of dispersion. One-way ANOVA and the Fisher LSD post hoc test at the 95% confidence level (p<0.05) were used to test the hypothesis.
Ethics Approval and Consent to Participate
The Research Ethics Committee of Universitas Padjadjaran has granted approval for the studies under the number 453/UN6.KEP/EC/2023. This approval is based on adherence to the guidelines outlined in the 2020 Edition of the American Veterinary Medical Association (AVMA) for the Euthanasia of Animals and the Eighth Edition (2011) of the National Research Council of the National Academies’ Guide for the Care and Use of Laboratory Animals.
Results
We investigated whether Indonesian Physalis Angulata L. extract could decrease Nox4 mRNA expression, affecting the redox balance through its role as an antioxidant. After ciplukan administration, groups BLM+C1, BLM+C2, BLM+M+C1, and BLM+M+C2 showed significantly lower expression of Nox4 in lung fibrosis compared to BLM mice (p<0.05; Figure 2c). Using One-Way Anova, we see statistically significant differences (p 0.0001) in the average levels of Nox4 mRNA expression between the groups. Expression of Nox4 mRNA was found to be significantly lower in the N group compared to the BLM (p=0.000), BLM+C1-to-BLM (p=0.002), BLM+C2-to-BLM (p=0.000), BLM+M+C1-to- BLM (p=0.000), and BLM+M+C2-to- BLM (p=0.000) groups using the Fisher’s exact test. Neither BLM+M nor BLM mice showed significant differences in Nox4 expression (p>0.05).
The extract administration of Indonesian ciplukan could reduce the expression of Mmp8 mRNA, which contributes to resolving lung fibrosis by degrading ECM. The administration of ciplukan extract resulted in a significant decrease in Mmp8 expression in lung fibrosis among groups BLM+C1, BLM+C2, BLM+M+C1, and BLM+M+C2, in comparison to the BLM group of mice (p<0.05) (Figure 2d). The One-Way ANOVA analysis found a significant difference in Mmp8 mRNA expression among the groups (p = 0.002). Further testing with the Fisher LSD test showed that the N group had significantly lower MMP8 mRNA expression compared to BLM (p = 0.002), BLM+C1 to BLM (p = 0.031), BLM+C2 to BLM (p = 0.018), BLM+M+C1 to BLM (p = 0.010), and BLM+M+C2 to BLM (p = 0.000). Additionally, there was no significant difference in Mmp8 expression between BLM+M mice and BLM (p > 0.05).
We examined whether an extract of the Indonesian plant Physalis angulata L. could inhibit fibroblast activation and lung fibrosis-related Klf4 mRNA expression. Klf4 expression was significantly reduced in the BLM+C1 and BLM+M+C2 groups after ciplukan administration compared to BLM mice (p<0.05; Figure 2e). The average expression of Klf4 mRNA varied significantly (p = 0.003) between the groups, as determined by One-Way ANOVA. Klf4 mRNA expression was also significantly lower in the BLM+C1 group compared to the BLM group (p=0.029) and in the BLM+M+C2 group compared to the BLM group (P = 0.007). Klf4 expression was also comparable between BLM+M, BLM+C2, and BLM+M+C1 mice and BLM mice (p>0.05).
We investigated whether administering Physalis angulata L. extract could increase FAS mRNA expression and play a role in lung fibrosis resolution through its action in the apoptosis pathway. FAS expression in lung fibrosis was not significantly different in fibrosis-treated BLM+C2, BLM+M+C1, and BLM+M+C2 mice from BLM mice (p>0.05) (Figure 2f). The average levels of FAS mRNA were not significantly different between the groups using One-Way ANOVA (p = 0.127).
Discussion
Ciplukan, or Physalis angulata L., is an Indonesian plant that has gained popularity for its medicinal properties.34 According to a review of the available research, Ciplukan has medicinal potential for several conditions.16 First analyses of the phytochemistry of Ciplukan fruits and leaves showed that secondary metabolites like alkaloids, glycosides, flavonoids, tannins, and phenolics were present. Ciplukan has many bioactive compounds, such as saponins, flavonoids, polyphenols, alkaloids, protein, chlorogenic acid, withangulatin A (WA), palmitic acid, acetic acid, protein, vitamin C, tannins, and malic acid.33
Nox4-Nrf2 signaling is often out of whack in people with pulmonary fibrosis. NADPH oxidase makes cells resistant to apoptosis and helps cells age and grow fibroblasts. Targeting Nox4 as a way to treat pulmonary fibrosis is a good idea.25 Repairing ROS damage to the lung epithelium could open new avenues for developing therapeutic targets to treat pulmonary fibrosis.11 ROS are made by NOX4, which is a big reason why epithelial cells die, and pulmonary fibrosis happens afterward.12 Using Affymetrix, it was found that when TGF-β1 was added to human fetal lung mesenchymal cells (hFLMCs), NOX4 was one of the genes that changed the most.23 TGF-1 made both normal fibroblasts and IPF fibroblasts make more NOX4.35 Myofibroblast tissue repair and fibrogenesis are two processes in which Nox4- plays a crucial role. It is generally accepted that TGF-β stimulates fibroblast movement by triggering a Nox4-dependent generation of ROS. Fibrotic cells called myofibroblasts produce reactive oxygen species (ROS), aiding their differentiation, contraction, resistance to apoptosis, and extracellular matrix (ECM) deposition.23,36 In IPF, senescent fibroblasts/myofibroblasts have been found to have increased expression of Nox4, and the ROS produced by Nox4 promotes aging and the development of a resistance to apoptosis.25,37
These findings align with Mohammadtaghvaei et al. Quercetin led to a significant decrease in the mRNA expression of NOX4 induced by TGF-β in Hepatic Stellate Cell Line LX-.22 Quercetin inhibits the process of macrophage transition both in vivo and in vitro, consequently attenuating the MMT and the TGF-β-Smad2/3 signaling pathway. Quercetin reduces scarring and inflammation in mice with lung damage caused by Bleomycin by making the antioxidant enzyme Nrf2. However, Quercetin could not reverse the pulmonary damage caused by Bleomycin, suggesting that it cannot slow the development of IPF on its own.38 The accumulation of myofibroblasts that resist apoptosis and undergo senescence contributes to enduring fibrosis in an aging mouse model of lung fibrosis. Furthermore, reversing established lung fibrosis in aged mice is achievable by inhibiting the enzyme Nox4, which induces senescence and produces oxidative stress.25 Nox4 is triggered in fibroblasts that undergo senescence due to replication, and silencing the Nox4 gene alleviates this senescence phenotype.39 Similar to the concept of inhibiting Nox4, approaches to address the senescence of fibroblast and epithelial cells through the use of senolytic combinations are under development as potential strategies to confront challenging fibrotic disorders like IPF.40
Polymorphonuclear leukocytes (PMNs) are considered the primary cells for expressing matrix metalloproteinase-8 (MMP-8), a potentiated enzyme known as interstitial collagenase.41 In IPF patients’ lungs, MMP-8 expression is higher in leukocytes than in control lung samples. MMP-8 was found to be elevated in Bleomycin-induced mice until day 21, and in longer studies, it remained elevated until eight weeks post-Bleomycin (peak fibrosis) and then decreased during fibrosis resolution.42,43 In vivo studies, MMP-8 contributes to the fibrosis process by enhancing inflammation and inflammation-triggered fibrosis by cleaving IL-10, MIP-1a, and Cxcl10.44 In contrast, the loss of Mmp8 leads to an increase in MMP9 and IL-10 levels which possess antifibrotic abilities.45 Physalis Angulata L. contains Quercetin, a type of flavonoid. Ciplukan potentially reduces lung fibrosis by decreasing MMP-8 expression.17,18,46
Based on previous studies, the Ciplukan treatment groups in this investigation were divided into N, BLM+M, BLM, BLM+C1, BLM+C2, BLM+M+C1, and BLM+M+C2. C1 groups receiving 750mg/kg body weight and C2 receiving 1500mg/kg body weight. One-way analysis of variance (ANOVA) revealed a p=0.002 difference in MMP8 mRNA expression between the treatment groups. There was a statistically significant increase in the BLM+M group compared to the other groups tested using Fisher’s LSD (BLM+C1=0.0031, BLM+C2=0.0018, BLM+M+C1=0.0010, BLM+M+C2 =0.0000). This study showed that when BBTs were given with doses 1 and 2, with or without mycophenolate mofetil, there was a significant decrease in MMP8 mRNA expression in lung tissue. Mycophenolate mofetil combined with a higher dose produced the best results.
These findings align with Taskan et al, who reported that Quercetin reduced MMP8 at 75mg/kg body weight and 150mg/kg body weight dose, with a more pronounced reduction at the latter dose.27 Similar results were shown by Meilawaty et al, who reported that flavonoid administration reduced MMP8 in gingival fibroblasts in a rat model.47 Collagen degradation is one of the processes in fibrosis resolution.48 MMP8 is an enzyme that can cleave fibrillar collagen in extracellular collagen degradation processes.49–51 Several factors that may influence this include spontaneous fibrosis resolution, resistant fibroblasts, and oxidative stress affecting the fibrosis resolution process. From this study, Ciplukan reduces Mmp8 mRNA expression, which potentially plays a role in extracellular collagen degradation processes.
In controlling many of the most fundamental functions of cells, the zinc finger transcription factor KLF4 is essential. Its notable functions include overseeing cell fate and differentiation and potentially stimulating or restraining the growth of diverse tumor cells, depending on the specific circumstances.52,53 KLF4 is a critical regulator in suppressing lung fibrogenesis by acting as a transcription factor. Its expression in fibroblasts slows the fibrosis process and aids in its natural resolution.13 The effect of KLF4 on TGF-induced fibroblast differentiation into myofibroblasts has been reported to be both inhibiting and enhancing.54,55 Given their central role in scar tissue formation, fibroblasts, and myofibroblasts’ pro- and antifibrotic factor balance significantly impact the progression of fibrotic disorders like idiopathic pulmonary fibrosis (IPF). It is increasingly recognized that fibrosis necessitates the deactivation of intrinsic antifibrotic mechanisms, similar to tumor-suppressing molecules in tumor development. Penke et al found that KLF4 has a specific function within pulmonary mesenchymal cells, acting as a multifunctional inhibitor of fibroblast and myofibroblast functions. In vitro studies on cell activation and in vivo studies on lung fibrosis benefit from manipulating this gene’s expression.13
KLF4’s ability to increase the transcriptional activation of TGF-β1 has been linked to its role in facilitating the differentiation of cardiac myofibroblasts in mice.54 KLF4 was upregulated in mesenchymal cell subtypes during lung fibrogenesis, as in the study by Chandran et al. They show that Bleomycin-induced myofibroblast accumulation and lung fibrosis depend on KLF4 in PDGFR-β+ cells. Our results suggest that KLF4 regulates TGFβ-SMAD signaling and ECM synthesis in PDGFR-β+ lung cells in both vitro and in vivo settings, suggesting a role for KLF4 in lung fibrosis. KLF4 also regulates profibrotic factors in a lung-cell type-specific manner.56
In contrast to the study conducted by Penke et al, which revealed that the expression of KLF4 had a dual effect of inhibiting and reversing TGF-β1–induced myofibroblast differentiation in pulmonary fibrosis.13 This discovery contradicts our own findings, as the induction of fibrosis in lung tissue through bleomycin increased KLF4 mRNA expression. This study demonstrated a significant reduction in KLF4 levels in the group that received ciplukan dose one and the group that received ciplukan dose two, along with mycophenolate mofetil. The most favorable outcome was observed in the group receiving dose 2 with mycophenolate mofetil. These findings align with Xi et al, who reported that Quercetin administration decreased expression levels of KLF4.29
Fas is a cell surface transmembrane receptor that binds to its ligand before the membrane is entirely spanned. By binding to its receptor, FasL initiates a signaling cascade that can result in cell migration, differentiation, or death.57 Fas can activate apoptosis by binding to its receptor.58 Homotypic death domain (DD)-mediated interactions occur between the receptor and the adaptor protein Fas-associated protein with death domain (FADD) in the presence of the ligand CD95L. The death effector domain (DED) of FADD then binds caspase-8 and cFLIPL (the long form of the regulator of apoptosis cellular FADD-like interleukin-1-β-converting enzyme-inhibitory protein). Proteins in the Death-Inducing Signaling Complex (DISK) work together to trigger cell death.59 Excessive apoptosis of lung cells has been linked to Bleomycin-induced fibrosis in mice and human lung fibrosis. This apoptosis is mediated by the activation of the Fas/FasL pathway.60 For fibrosis to be resolved, myofibroblasts must undergo apoptosis, after which macrophages and dendritic cells will eliminate any remaining cells.61,62 The upregulation of anti-apoptotic and prosurvival proteins is a common mechanism by which myofibroblasts in fibrotic tissues become resistant to apoptosis. As an essential transcription factor that promotes cell survival by turning on anti-apoptotic genes, Inflammatory cytokines and growth factors can potentially induce NF-B activation.63 TGF-β1 and endothelin-1 (ET-1) are potent activators of myofibroblast differentiation, and they both activate FAK and PI3K/AKT to promote resistance to apoptosis.64
On the contrary, Hohmann et al discovered that Quercetin directly diminishes pulmonary fibrosis by increasing ligand-induced apoptosis in fibroblasts from older people with idiopathic pulmonary fibrosis65 and a study by Ryu et al demonstrated that in canine osteosarcoma cells, Quercetin triggered apoptosis via DNA fragmentation, alternations of the cell cycle, and ROS.66 Both studies used Quercetin, whereas our research utilized Quercetin from ciplukan, which has a different percentage composition. Other studies by Schafer et al showed that effectively combining dasatinib and Quercetin eliminates senescent fibroblasts.67 During this study, significant variations were not detected in the tissue FAS mRNA expression across all groups. The administration of Quercetin with or without mycophenolate mofetil does not affect the apoptosis process. It could be attributed to the lungs having a less efficient healing rate and the potential occurrence of apoptosis-resistant cells in those tissues. Thus, it might necessitate a longer time to determine whether there is an influence on the apoptosis process.
Research Limitations
The limitation of this study is that no other supporting examinations were not conducted to confirm the results of this research. Therefore, further research is needed to validate and expand upon the findings presented here. Addressing these limitations in future studies be achieved by conducting examinations using other methods such as qPCR, Western blot, and immunofluorescence.
Conclusion
The extract of Ciplukan exhibited promising effects on fibrosis-related gene expressions, particularly Nox4, Mmp8, and KlfF4. This study suggests that the extract has the potential to intervene in fibrosis progression, offering a potential possibility for therapeutic strategies. Further investigations are necessary to fully explain the mechanisms underlying the antifibrotic effects of Ciplukan and its potential as a therapeutic agent by examining the expression of other genes related to inhibiting the fibrosis process, oxidative processes such as Nrf2, and gene expressions related to the fibrosis resolution process, such as ECM degradation besides Mmp8, or gene expressions related to apoptosis processes like FasL, DR4, DR5, and Caspases.
Abbreviations
BLM, Bleomycin; C, Ciplukan; cFLIPL, cellular FLICE-inhibitory protein; DD, Death Domain; DED, Death Effector Domain; DISK, Death-Inducing Signaling Complex; DNA, Deoxyribonucleic acid; DR, Death Receptors; ET-1, Endothelin-1; FAK, Focal Adhesion Kinase; FADD, Fas-Associated Protein with Death Domain; FasL, Fas Ligand; GAPDH, Glyceraldehyde 3-Phosphate Dehydrogenase; IL, interleukin; Klf4, Krüppel-like factor 4; LSD, Least Significant Difference; Mmp, matrix metalloproteinase; M, Mycophenolate mofetil; mRNA, Messenger RNA; mTOR, Mammalian Target of Rapamycin; Nrf2, Nuclear factor-erythroid-2 Related Factor 2; N, control; Nox4, NADPH oxidase 4 protein; PDGFR-β+, Platelet-derived growth factor receptor beta; PI3K, PhosphoInositide 3-Kinase; PMNs, Polymorphonuclear leukocytes; ROS, Reactive Oxygen Species; SASP, Senescence Associated Secretory Phenotype; TGF-β, Transforming Growth Factor Beta; UK, United Kingdom; WA, Withangulatin A.
Data Sharing Statement
Upon request, students can acquire the data underpinning this study’s findings by contacting the corresponding author.
Acknowledgment
The authors thank Dr. Aziiz Mardanarian Rosdianto, S.Kep., Ners. for kindly contributing during the animal process until its termination in the Faculty of Medicine, Universitas Padjadjaran laboratory.
Disclosure
The authors state no conflict of interest.
References
1. Venosa A. Senescence in pulmonary fibrosis: between aging and exposure. Front Med. 2020;7:1–22.
2. Tao N, Li K, Liu J, Fan G, Sun T. Liproxstatin-1 alleviates bleomycin-induced alveolar epithelial cells injury and mice pulmonary fibrosis via attenuating inflammation, reshaping redox equilibrium, and suppressing ROS/p53/α-SMA pathway. Biochem Biophys Res Commun. 2021;551:133–139. doi:10.1016/j.bbrc.2021.02.127
3. Du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 2010;9(2):129–140. doi:10.1038/nrd2958
4. Luppi F, Spagnolo P, Cerri S, Richeldi L. The big clinical trials in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18(5):428–432. doi:10.1097/MCP.0b013e3283567ff9
5. Shaikh SB, Prabhu A, Bhandary YP. Targeting anti-aging protein sirtuin (Sirt) in the diagnosis of idiopathic pulmonary fibrosis. J Cell Biochem. 2019;120(5):6878–6885. doi:10.1002/jcb.28033
6. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi:10.1056/NEJMoa1402584
7. King TE, Bradford WZ, Castro-Bernardini S, et al. A Phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi:10.1056/NEJMoa1402582
8. Glasser SW, Hagood JS, Wong S, Taype CA, Madala SK, Hardie WD. Mechanisms of lung fibrosis resolution. Am J Pathol. 2016;186(5):1066–1077. doi:10.1016/j.ajpath.2016.01.018
9. Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172(4):417–422. doi:10.1164/rccm.200501-017PP
10. Ma MW, Wang J, Dhandapani KM, Brann DW. Deletion of NADPH oxidase 4 reduces severity of traumatic brain injury. Free Radic Biol Med. 2018;117(December 2017):66–75. doi:10.1016/j.freeradbiomed.2018.01.031
11. Cheresh P, Kim S, Tulasiram S, Kamp D. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):1028–1040. doi:10.1016/j.bbadis.2012.11.021
12. Carnesecchi S, Deffert C, Donati Y, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15(3):607–619. doi:10.1089/ars.2010.3829
13. Penke LR, Speth JM, Huang SK, Fortier SM, Baas J, Peters-Golden M. KLF4 is a therapeutically tractable brake on fibroblast activation that promotes resolution of pulmonary fibrosis. JCI Insight. 2022;7(16). doi:10.1172/jci.insight.160688
14. Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med. 2018;7(8):1–21. doi:10.3390/jcm7080201
15. Van Manen MJG, Geelhoed JJM, Tak NC, Wijsenbeek MS. Optimizing quality of life in patients with idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2017;11(3):157–169. doi:10.1177/1753465816686743
16. Pillai JR, Wali AF, Menezes GA, et al. Chemical composition analysis, cytotoxic, antimicrobial and antioxidant activities of physalis angulata l.: a comparative study of leaves and fruit. Molecules. 2022;27(5):1.
17. Anh H Le T, Le Ba V, Do TT, et al. Bioactive compounds from physalis angulata and their anti-inflammatory and cytotoxic activities. J Asian Nat Prod Res. 2021;23(8):809–817. doi:10.1080/10286020.2020.1825390
18. Augustine AA, Ufuoma O. Flavonoids from the leaves of physalis angulata Linn. Planta Med. 2013;79(13):PJ5.
19. Kobayashi K, Araya J, Minagawa S, et al. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J Immunol. 2016;197(2):504–516. doi:10.4049/jimmunol.1600265
20. Fathimath Muneesa M, Shaikh SB, Jeena TM, Bhandary YP. Inflammatory mediators in various molecular pathways involved in the development of pulmonary fibrosis. Int Immunopharmacol. 2021;96(March):107608. doi:10.1016/j.intimp.2021.107608
21. Rieder F, Kessler SP, West GA, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011;179(5):2660–2673. doi:10.1016/j.ajpath.2011.07.042
22. Mohammadtaghvaei N, Afarin R, Mavalizadeh F, Shakerian E, Salehipour Bavarsad S, Mohammadzadeh G. Effect of quercetin on the expression of NOXs and P-Smad3C in TGF-Β-activated hepatic stellate cell line LX-2. Hepat Mon. 2021;21(6):e116875. doi:10.5812/hepatmon.116875
23. Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15(9):1077–1081. doi:10.1038/nm.2005
24. Wang MC. Natural plant resource flavonoids as potential therapeutic drugs for pulmonary fibrosis. Heliyon. 2023;9(8):e19308. doi:10.1016/j.heliyon.2023.e19308
25. Hecker L, Logsdon NJ, Kurundkar D, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231ra47. doi:10.1126/scitranslmed.3008182
26. Geng F, Xu M, Zhao L, et al. Quercetin alleviates pulmonary fibrosis in mice exposed to silica by inhibiting macrophage senescence. Front Pharmacol. 2022;13(July):1–11. doi:10.3389/fphar.2022.912029
27. Taskan MM, Gevrek F. Quercetin decreased alveolar bone loss and apoptosis in experimentally induced periodontitis model in Wistar rats. Antiinflamm Antiallergy Agents Med Chem. 2020;19(4):436–448. doi:10.2174/1871523019666200124114503
28. Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani SG. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care. 2020;9(4):184–198. doi:10.1089/wound.2019.1032
29. Xi J, Zhang B, Luo F, Liu J, Yang T. Quercetin protects neuroblastoma SH-SY5Y cells against oxidative stress by inhibiting expression of krüppel-like factor 4. Neurosci Lett. 2012;527(2):115–120. doi:10.1016/j.neulet.2012.08.082
30. Redente EF, Chakraborty S, Sajuthi S, et al. Loss of fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis. JCI Insight. 2021;6(1):1–20. doi:10.1172/jci.insight.141618
31. Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi:10.1016/j.ebiom.2018.12.052
32. Phenotype TS associated S, Diseases A related. The senescence-associated secretory phenotype and age-related diseases. Biology. 2020;2020:1–16.
33. Rohmawaty E, Rosdianto AM, Usman HA, et al. Antifibrotic effect of the ethyl acetate fraction of ciplukan (Physalis angulata Linn.) in rat liver fibrosis induced by CCI4. J Appl Pharm Sci. 2021;11(12):175–182.
34. Ozaslan C, Farooq S, Onen H, Ozcan S, Bukun B, Gunal H. Germination biology of two invasive physalis species and implications for their management in arid and semi-arid regions. Sci Rep. 2017;7(1):1–12. doi:10.1038/s41598-017-17169-5
35. Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFb1-induced fibroblast differentiation into myofibroblast. Thorax. 2010;65(8):733–738. doi:10.1136/thx.2009.113456
36. Cameli P, Carleo A, Bergantini L, Landi C, Prasse A, Bargagli E. Oxidant / antioxidant disequilibrium in idiopathic pulmonary fibrosis pathogenesis. Inflammation. 2019. doi:10.1007/s10753-018-00955-2
37. Jarman ER, Khambata VS, Cope C, et al. An inhibitor ofNADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol. 2014;50:158–169. doi:10.1165/rcmb.2013-0174OC
38. Boots AW, Veith C, Albrecht C, et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm Med. 2020;20(1):1–16. doi:10.1186/s12890-020-1142-x
39. Sanders YY, Liu H, Liu G, Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med. 2015;79:197–205. doi:10.1016/j.freeradbiomed.2014.12.008
40. Horowitz JC, Thannickal VJ. Mechanisms for the resolution of organ fibrosis. Physiology. 2019;34(1):43–55. doi:10.1152/physiol.00033.2018
41. Anacker J, Segerer SE, Hagemann C, et al. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod. 2011;17(10):637–652. doi:10.1093/molehr/gar033
42. Craig VJ, Quintero PA, Fyfe SE, et al. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J Immunol. 2013;190(8):4283–4296. doi:10.4049/jimmunol.1201043
43. Cabrera S, Selman M, Lonzano-Bolaños A, et al. Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am J Physiol. 2013;304(9):L593–L601. doi:10.1152/ajplung.00320.2012
44. García-Prieto E, González-López A, Cabrera S, et al. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PLoS One. 2010;5(10):e13242. doi:10.1371/journal.pone.0013242
45. García-de-alba C, Becerril C, Ruiz V, et al. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med. 2010;182(9):1144–1152. doi:10.1164/rccm.201001-0028OC
46. Lakhanpal P, Rai DK. Quercetin: a Versatile Flavonoid. Int J Med Update. 2007;2(2):22–37. doi:10.4314/ijmu.v2i2.39851
47. Meilawaty Z, Shita ADP, Kuncaraningtyas PL, Dharmayanti AWS, Hamzah Z. Potensi ekstrak daun singkong (Manihot esculenta Crantz) terhadap ekspresi MMP-8 fibroblas gingiva pada model tikus dengan disfungsi ovarium dan periodontitis. Potential of cassava (Manihot esculenta Crantz) leaf extract on the MMP-8 expression of. J Kedokt Gigi Univ Padjadjaran. 2020;32(2):105. doi:10.24198/jkg.v32i2.27466
48. McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol. 2013;304(11):L709–L721. doi:10.1152/ajplung.00418.2012
49. Song F, Wisithphrom K, Zhou J, Windsor LJ. Matrix metalloproteinase dependent and independent collagen degradation. Front Biosci. 2006;11:3100–3120. doi:10.2741/2036
50. Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolym. 2002;66(1):19–32. doi:10.1002/bip.10201
51. Stultz CM. Localized unfolding of collagen explains collagenase cleavage near imino-poor sites. J Mol Biol. 2002;319(5):997–1003. doi:10.1016/S0022-2836(02)00421-7
52. Ghaleb AM, Yang VW, Brook S, Brook S. Krüppel-like factor 4 (KLF4): what we currently know. Cancer Res. 2017;611:27–37.
53. Farrugia MK, Vanderbilt DB, Salkeni MA, et al. Kruppellike pluripotency factors as modulators of cancer cell therapeutic responses. Cancer Res. 2016;76(7):1677–1682. doi:10.1158/0008-5472.CAN-15-1806
54. Zhang Y, Wang Y, Liu Y, Wang N, Qi Y, Du J. Krüppel-like factor 4 transcriptionally regulates TGF-β1 and contributes to cardiac myofibroblast differentiation. PLoS One. 2013;8:1.
55. Hu B, Wu Z, Liu T, Ullenbruch MR, Jin H, Phan SH. Gut-enriched kru¨ppel-like factor interaction with Smad3. Am J Respir Cell Mol Biol. 2006;36:78–84. doi:10.1165/rcmb.2006-0043OC
56. Chandran RR, Xie Y, Gallardo-Vara E, et al. Distinct roles of KLF4 in mesenchymal cell subtypes during lung fibrogenesis. Nat Commun. 2021;12(1):1–17. doi:10.1038/s41467-021-27499-8
57. Levoin N, Jean M, Legembre P. CD95 structure, aggregation and cell signaling. Front Cell Dev Biol. 2020;8(May):1–13. doi:10.3389/fcell.2020.00314
58. Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nat Genet. 2001;28(2):113–118. doi:10.1038/88815
59. Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14(22):5579–5588. doi:10.1002/j.1460-2075.1995.tb00245.x
60. Golan-Gerstl R, Wallach-Dayan SB, Amir G, Breuer R. Epithelial cell apoptosis by fas ligand-positive myofibroblasts in lung fibrosis. Am J Respir Cell Mol Biol. 2007;36(3):270–275. doi:10.1165/rcmb.2006-0133OC
61. Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66.
62. Iredale JP, Benyon C, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102(3):538–549. doi:10.1172/JCI1018
63. Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-κB signaling. J Clin Immunol. 2005;25:541–550. doi:10.1007/s10875-005-8217-6
64. Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-β1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41(4):484–493. doi:10.1165/rcmb.2008-0447OC
65. Hohmann MS, Habiel DM, Coelho AL, Verri WA, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60(1):28–40. doi:10.1165/rcmb.2017-0289OC
66. Ryu S, Park S, Lim W, Song G. Quercetin augments apoptosis of canine osteosarcoma cells by disrupting mitochondria membrane potential and regulating PKB and MAPK signal transduction. J Cell Biochem. 2019;120(10):17449–17458. doi:10.1002/jcb.29009
67. Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi:10.1038/ncomms14532
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.