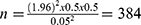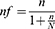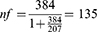Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
The Burden of Inappropriate Prescriptions and Predictors for Hospitalized Patients with Liver Cirrhosis in Ethiopia
Authors Zeleke TK , Bazezew ZA, Abebe RB
Received 22 June 2023
Accepted for publication 14 September 2023
Published 25 September 2023 Volume 2023:15 Pages 129—140
DOI https://doi.org/10.2147/HMER.S423351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Tirsit Ketsela Zeleke,1 Zegaye Agmassie Bazezew,2 Rahel Belete Abebe3
1Department of Pharmacy, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 3Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tirsit Ketsela Zeleke, Tel +251 919187613, Email [email protected]; [email protected]
Background: Pathophysiological alterations in liver cirrhosis affect how medications are metabolized and eliminated. Therefore, when prescribing medicines for patients with cirrhosis, appropriate prescription of medication is an accepted standard of practice. Since patients with cirrhosis require a complex therapy plan, it necessitates regular reviews of medication utilization. However, no research was conducted in Ethiopia. The aim of this study was to figure out the predictors of inappropriate prescriptions and the pattern of prescription in patients with cirrhosis.
Patients and methods: A cross-sectional study design was carried out at Felege-Hiwot, a specialized and comprehensive referral hospital, from June 30, 2022, to November 30, 2022, in 123 hospitalized patients with cirrhosis. Patients were recruited using a simple random sampling procedure, and data were collected using an interviewer-administered questionnaire. For the purpose of identifying determinants of inappropriate prescription, logistic regression analyses have been carried out and statistical significance was defined by a p-value of less than 0.05 and a 95% confidence range.
Results: The burden of inappropriate prescriptions among patients with cirrhosis was 35.8%. An increased number of medications prescribed (AOR = 4.88 (1.05– 22.68)), prescription by a general practitioner (AOR = 3.57 (95% CI 1.07– 11.44)), increased level of bilirubin (AOR = 3.54 (95% CI 1.95– 6.45)), and decreased level of albumin (AOR = 0.18 (95% CI 0.04– 0.72)) were predictors for an inappropriate prescription.
Conclusion: It has been found that there were inappropriate prescriptions among patients with liver cirrhosis. Prescribers should pay close attention to patients who have prescribed with higher number of medications, increased level of bilirubin and decreased level of albumin. Moreover, educational level of prescribers needs to be upgraded in order to adopt evidence-based medication prescriptions and adhere to recommended practices.
Keywords: inappropriate prescription, cirrhosis, Ethiopia
Introduction
The liver is an organ in which the majority of medications are primarily metabolized and excreted.1 Whereas Cirrhosis, which results from a variety of chronic liver illnesses, is a gradually deteriorating liver illness marked by scarring and the change of healthy liver morphology into structurally aberrant nodules.2–5 Pathophysiological alterations in liver cirrhosis impact how external agents, including medications, are eliminated, leading to drug-induced liver damage, which is defined as a short-term or ongoing liver damage brought on by medications or components of herbs.6–8 The main reasons of disability of the health-care system in developing nations are improper medication usage and inappropriate prescribing.9 Numerous medications are known to induce liver damage or perhaps have been suspected of doing so.10 More than 100 medications have been linked to aggressive hepatic failure; more than 50 medications have been shown to be associated with liver damage; and 10% of all adverse drug reactions have hepatotoxic consequences.1,11 However, it is been noticed that cirrhotic individuals frequently receive prescriptions for medications that are more likely to harm them.12 Nearly 30% of cirrhosis patients experience possibly avoidable adverse drug reactions, and 9% of all those reactions have detrimental impacts on the functioning of the liver.5,13 Likewise, improper prescription in patients with cirrhosis results in an increase in the rate of adverse drug reactions, hospitalizations and inpatient stays,14 more over due to the disruption of drug metabolism, polypharmacy is a primary cause of adverse drug reactions, which are especially harmful in hepatic diseases.15 Thus, when considering the prescription of hepatotoxic drugs to patients with cirrhosis, a safe medication prescription has more relevance,5,12,16 The existence of cirrhosis-related complications has a dual effect: first, it raises the cost of treatment, and second, it makes it more difficult to prescribe medications to cirrhotic patients so it is important to give attention to prescription of medication to treat both the underlying cause and any related complications.17,18 Additionally, it is crucial to adjust dosages in cirrhotic patients; reduced doses or fewer frequent administrations are usually recommended to avoid excessive drug deposition and active drug metabolite buildup that could result in severe adverse effects.6,19 Drug formulary references provide advice on which medications should be used cautiously, or not at all, in patients with cirrhosis.20 According to reports, the estimated inpatient mortality of patients with cirrhosis in Ethiopia is high; it causes deaths and hospital admissions in approximately 31% and 12% of cirrhotic patients.21,22 Since patients with cirrhosis require a complex therapy plan, it necessitates regular reviews of medication utilization. However, a search of the literature reveals that there is no data on medication prescription patterns among cirrhosis patients in Ethiopia. The aim of this study was to assess the burden of inappropriate medication prescriptions and its predictors among hospitalized cirrhotic patients at Felege-Hiwot Specialized and Comprehensive Referral Hospital.
Methods
Study Setting and Design
A crossectional study was carried out at Felege-Hiwot Specialized and Comprehensive Referral Hospital (FHSCRH) from June302022to November 30 2022. The hospital is situated in Bahir Dar, 562 km away from Addis Ababa, the capital city of Ethiopia, including a total bed occupancy of 400, about 15 adult ambulatory units, and 561 workers. It offers services involving medical, pediatric surgical, intensive care, orthopedic, ophthalmological, and gynecological wards. Over 7 million people in its catchment area are served, and the hospital receives about 500 patients each day.23 On average, 32 patients with cirrhosis hospitalized each month.
Study Participants and Inclusion Criteria
The source population included all patients with cirrhosis hospitalized at FHSCRH, while the study population consisted of cirrhotic patients hospitalized during the data collection period. Cirrhotic patients who were 18 years of age or above and had been given a minimum of one medication were enrolled in the study, whereas those patients who had severe mental disorders, serious health conditions such as psychiatric patients, and incomplete medical records were not included in the study.
Sample Size Determination and Sampling Techniques
The sample was determined with a single population percentage formula while taking into account the following assumptions: 5% error margin, 95% confidence range, and 50% prevalence.
●  , where Z statistics (at 95% CI = 1.96), d (margin of error), and p (population proportion)
, where Z statistics (at 95% CI = 1.96), d (margin of error), and p (population proportion)
However, according to the hospital’s monthly record of cirrhosis cases, the overall number of patients who presented during the data collection period were 207, which was fewer than 10,000. As a result, we calculated the final sample size using a population correction formula.
Where:
● nf = final sample size
● n = sample size before population correction
● N = total number of patients who could be presented
Therefore, our final sample size was 135.
Study Variables
Inappropriate prescription was the dependent variable. While, sociodemographic characteristics include residence, age, religion, sex, marital status, occupation, and educational status, clinical-related characteristics such as etiology and complications, practitioner-related variables such as specialty, and medication-related characteristics including type of medication, frequency, and dose of medications were independent variables.
Operational Definition
Inappropriate prescription: is defined as a prescription that is in noncompliance with pharmacotherapy recommended by clinical guidelines and existing literature.5,20
Data Collection Tools and Procedures
After studying a variety of literature, the authors developed an organized interviewer-administered questionnaire that was utilized for collecting data.15,20,24 The questioner had four parts, including sociodemographic variables, clinical variables, practitioner-related variables, and medication-related variables. Patients sociodemographic characteristics and practitioners’ variables were obtained by interviewing them. Clinical parameters and medication-related variables such as diagnosis, etiology, albumin level, bilirubin level, INR value, and the prescribed medications have been obtained from the patient’s medical records. The data collection process involved three trained nurses. Based on the Child-Pugh categorization, which is utilized to indicate the stage and severity of liver damage depending on the extent of hepatic impairment, the degree of illness was divided into categories A, B, and C.25 The calculation to categorize patients was calculated as follows: in the presence of encephalopathy grades 1 and 2 equal 2 points, grades 3 and 4 equal 3, and none equals 1 point. In the presence of ascites: If the answer is none, give one point; if minor, give two points; if moderate, give three points, regarding the level of bilirubin: 1 point is granted for concentrations under 2 mg/mL, 2 points for 2 to 3 mg/mL, and 3 points for 3 mg/mL or more, about the level of albumin: if 3.5 mg/mL or more = 1 point, if less than 2.8 mg/mL = 3 points, if between 2.8 and 3.5 mg/mL = 2 points and about Prothrombin Time If the time spent is less than 4 seconds = 1 point, between 4 and 6 seconds = 2 points, and over 6 seconds = 3 points. In addition, INR is utilized to replace PT, with INR under 1.7 equaling 1 point, INR between 1.7 and 2.2 equaling 2 points, and INR over 2.2 equaling 3 points. After that, patients’ levels of cirrhosis severity were listed as follows: Child-Pugh A: 5 to 6 points; Child-Pugh B: 7 to 9 points; and Child-Pugh C: 10 to 15 points.26,27
Finally, the appropriateness of prescription was assessed according to recommendations for appropriate prescribing from clinical guidelines and existing literature.5,20
Data Quality Assurance
The investigator had developed the questionnaire in English, and then English-fluent individuals translated it both forward and backward into Amharic in order to preserve uniformity. Over a period of one working day, the principal investigator instructed the supervisors and data collectors on the goals, techniques, and ethical issues of the study. In order to evaluate the questionnaire’s clarity and sociocultural suitability, a pretest was undertaken at the University of Gondar’s comprehensive and specialized hospital among 7 patients with cirrhosis (5% of the overall sample size). Additionally, the supervisors monitored on-site for the whole data collection period, and the investigators examined and verified the collected data for accuracy and consistency. Moreover, the internal consistency and reliability of the questioners were examined and resulted in a Cronbach’s alpha of 0.802.
Data Processing and Analysis
The data were examined for reliability and consistency, coded, and put into Epi-data 4.6 before being transferred to SPSS 26 for subsequent analysis. The Hosmer and Lemeshow test for goodness of fit was used to assess each variable’s fitness for consideration in the logistic regression model. To assess the impact of independent factors on the appropriateness of a prescription. Bivariate and multivariable logistic regressions were fitted. Multiple logistic regression models were applied to variables in the bivariable analysis that had a p-value lower than 0.25, and those variables were regarded to be significantly associated if their p-value was below 0.05. The adjusted odds ratio (AOR) was calculated and interpreted using a 95% confidence interval (CI). In order to find and eliminate duplicate variables that could affect our estimate, multicollinearity across the independent variables was also tested using the variance inflation factor (VIF); the VIF was in the appropriate range.28 The study’s findings were then presented using texts and tables to show the results.
Ethical Considerations
The study was carried out in line with the Helsinki Declaration. A letter of ethical clearance was received from Debre markos University College of Medicine and Health Sciences, pharmacy department, on May, 2022. An official letter was submitted to FHSCRH, and the appropriate official permission was obtained. All participants provided written consent after being fully informed about the study. Each method was applied in accordance with the laws and guidelines. No identifiable details were included in the questionnaire in order to safeguard the confidentiality of the data. Practitioners’ prescriptions identified as inappropriate received information and were urged to comply with available guidelines. Moreover, both supervisors and data collectors used protective measures against viral hepatitis infection.
Result
Sociodemographic and Clinical Characteristics of Study Participants
During the course of the research, 218 individuals with a confirmed diagnosis of cirrhosis were hospitalized; among these 123 of patients with a response rate of 91.1% were recruited in the study. Greater than fifty percent (60.2%) were male, and 51.2% were from rural areas. Regarding education level, 29.3% of participants had a higher education level, whereas almost a quarter of respondents (26.0%) were government employees, and the majority of participants (65.1%) had a monthly income below 1650 ETB (Table 1).
 |
Table 1 Frequency of Socio-Demographic Characteristics Among Patients with Cirrhosis Hospitalized at Felege-Hiwot Specialized and Comprehensive Referral Hospital (FHSCRH), Amhara Ethiopia |
Hepatitis B virus was the most typical cause of cirrhosis mentioned in 35.8% of patients. While 42.3% of the patients were presented with child Pugh score B. All the patients had complications, while 44.7% of the patients had three or more types of complications. Ascites was the most common complication, occurring in the majority of patients (82.1%), followed by portal hypertension and spontaneous bacterial peritonitis in 77.2% and 74.0%, respectively. Regarding laboratory values, the average albumin level was 2.8 ± 0.5 mg/mL (Table 2).
 |
Table 2 Frequency of Clinical Characteristics Among Patients with Cirrhosis Admitted to Medical Wards at Felege-Hiwot Specialized and Comprehensive Referral Hospital (FHSCRH), Amhara Ethiopia |
Medication Prescription Pattern Among Patients with Cirrhosis
There were a total of 483 prescriptions for 123 patients; from this furosemide was the most frequently prescribed medication (91.1%) followed by Spironolactone (78.0%) and ceftriaxone (40.7%). More than half of patients prescribed with four or above number of medications (Table 3).
 |
Table 3 Frequency of Medication Prescription Pattern Among Patients with Cirrhosis Admitted to Medical Wards at FelegeHiwot Specialized and Comprehensive Referral Hospital (FHSCRH), Amhara Ethiopia |
Appropriateness of Prescription Among Patients with Cirrhosis
There was an inappropriate prescription of medication for 44 (35.8%) patients. Out of this, inappropriate medication selection was found in 66% of patients, while inappropriate dose and duration were found in 34% of patients. Tenofovir was the most frequently inappropriately prescribed medication in 34.0% of patients, followed by metronidazole and a Spironolactone to furosemide dose ratio of 27.2% and 20.4%, respectively (Table 4).
 |
Table 4 Pattern Inappropriate Medication Prescription Among Cirrhotic Patients Hospitalized at FelegeHiwot Specialized and Comprehensive Referral Hospital (FHSCRH), Amhara Ethiopia |
Factors Associated with Medication Prescription Among Patients with Cirrhosis
Sociodemographic variables, which consist of age, residence, sex, and education level, and clinical variables, including INR value, severity of the disease, and number of complications, did not have a significant effect on the appropriateness of medication prescription. However, the number of medications prescribed, bilirubin value, albumin value, and practitioner specialty were significantly associated with inappropriate prescriptions. Increased number of medication prescriptions increases the risk of inappropriate prescription by a factor of around five (AOR = 4.88 (1.05–22.68)). Regarding the practitioner’s specialty, prescription by a general practitioner increases the risk of inappropriate prescription by 3.57 times (AOR = 3.57 (95% CI 1.07–11.44)) as compared to prescription by internists. Also, as the level of bilirubin increases, the odds of inappropriate prescription increase by 3.54 times (AOR = 3.54 (95% CI 1.95–6.45)), and as the level of albumin increases by one unit, the odds of inappropriate prescription decrease by 82% (AOR = 0.18 (95% CI 0.04–0.72)) (Table 5).
 |
Table 5 Factors Associated with Inappropriate Prescription Among Patients with Cirrhosis Hospitalized at Felege Hiwot Specialized and Comprehensive Referral Hospital (FHSCRH), Amhara Ethiopia |
Discussion
As far as we are aware, this is the first study carried out in Ethiopia to evaluate the appropriateness of prescriptions and predicators among cirrhotic patients admitted to the inpatient setting. In this study, Hepatitis B virus was found to be the main cause of liver cirrhosis, which was similar to reports from studies done in Africa and Asia.15,20,29–32 This is due to the fact that hepatitis B constitutes one of the infectious diseases that are most prevalent globally,33,34 but alcohol was the most common cause in a study conducted in the USA.24 This difference might be due to a variation in the etiology of cirrhosis in different geographic areas and sociodemographic characteristics.4,35,36 Regarding complications, the most common complications in patients with cirrhotic conditions was ascites, which was in line with findings in the USA, Europe, and Pakistan.12,15,37 This might be due to the fact that liver damage causes a decrease in protein number, which results in peritoneal accumulation of fluid in nearly all cirrhotic patients, which causes ascites.31,38 The majority of patients prescribed multiple drug therapy, which was comparable with other findings,39,40 the possible reason for this polypharmacy might be due to the need for treatment of various complications of the disease.
The overall burden of inappropriate prescriptions among patients with cirrhosis was found to be 35.8%. A large proportion of patients diagnosed with viral liver disease infections did not receive any antiviral medication, which is similar to a study done in Northern Ghana.20 The patients met the requirements for antiviral therapy because they had confirmed diagnosis of viral cirrhosis. Lack of sufficient antiviral drugs designated for liver disorders at the hospital and patients' lack of ability to afford the expensive testing required might be the causes. Patients with CHC infections were prescribed tenofovir, which was an inappropriate selection according to the first-line therapy indication for CHC. In order to treat patients who, have a long-term HCV all-genotype infection, the combined use of the NS5B polymerase and HCV NS5A inhibitors, Velpatasvir and Sofosbuvir has been approved.41,42 On the other hand, Lamivudine was prescribed to patients with CHB, which was inappropriate. Tenofovir, an effective nucleotide analogue with a high barrier to resistance, has a high likelihood of accomplishing long-term treatment targets and ought to be administered in CHB whenever feasible, while Lamivudine, a nucleotide analogue with a low barrier to resistance, can promote drug resistance and is not advised.43,44
Additionally, when the management of complications was assessed, nonconformity with guidelines recommendations was found. The spironolactone and furosemide dose proportion was inappropriate; there was a prescription with a low dose of furosemide. Furosemide and spironolactone are typically taken together as a diuretic combination at a ratio of 40 mg to 100 mg.37 Labetalol was prescribed for the primary prevention and treatment of variceal bleeding secondary to portal hypertension, however labetalol has little effect on portal venous hemodynamics.45 Instead, non-selective beta blockers such as Propranolol and nadolol and newer-generation beta-blockers such as carvedilol are first-line treatment options for primary prevention and treatment of variceal bleeding.46 Moreover, Metronidazole was prescribed for the treatment and prevention of spontaneous bacterial peritonitis (SBP). Mainly aerobic organisms, of which about 25% of gram-positive and 75% of gram-negative cause SBP.47 It is not suggested to use metronidazole for SBP given that it has a typical anaerobic antibacterial spectrum and has a limited effect on aerobic bacteria.48,49 In order to prevent SBP and treat it empirically, third-generation cephalosporins and fluoroquinolones like ciprofloxacin are recommended.50,51 Regarding analgesics, a high dose of paracetamol and tramadol exceeding the recommended daily dose were prescribed. Many of the commonly used analgesics like paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids are metabolized in the liver,52 whereas Paracetamol has impaired plasma clearance in these patients.53–55 The safety of some medications depend on the degree of cirrhosis or Child-Pugh score.26 Paracetamol therapeutic doses are frequently inappropriate among cirrhotic individuals.56 The FDA recommends, a maximum daily intake of 2 to 3 g/d.57 The deleterious effects of opioid analgesics on the central nervous system are particularly noticeable in patients with liver cirrhosis,6,58 patients with a child Pugh score of C should take 50 mg of tramadol orally once every 12 hours, and patients with cirrhosis need to use opioids cautiously.59 Omeprazole was prescribed for two patients for prophylaxis of stress-induced ulcers, which was similar to other studies.37 Proton pump inhibitors (PPI) are linked to negative side effects in cirrhosis, such as a higher likelihood of hepatic encephalopathy, SBP, and liver-related mortality. Cirrhotic patients are especially susceptible to the negative effects of PPI use.60,61 Therefore, it is advised that patients with child Pugh scores of A and B reduce their omeprazole maximum dose.62 This study also evaluated predictors of inappropriate prescriptions. Sociodemographic variables of patients which consist age, sex, educational level, residence, and occupation, Clinical parameters, which include number of complications, child Pugh score, and INR values, did not show any significant association with inappropriate prescription. Whereas an increased number of prescribed medications, levels of albumin and bilirubin, and the specialty of practitioners were significantly associated with inappropriate prescription among hospitalized liver cirrhotic patients. Patients who were receiving four or more medications had a higher risk of receiving inappropriate prescriptions. Inappropriate medicine selection and dosing may rise when patients with cirrhosis are more likely to practice polypharmacy due to cirrhosis-related problems. As indicated by previous literatures lower albumin levels and higher bilirubin increase the risk of inappropriate prescriptions; this might be due to an increase in serum bilirubin and a decrease in serum albumin are associated with the worsening and progression of the disease.63,64 As the disease progresses, medication toxicity also increases, and increased indicators of the severity of cirrhosis are often associated with inappropriate medication prescribing.12 Moreover, prescriptions by general practitioners were more inappropriate compared to prescriptions by specialists. This might be due to their relatively low education level and work experience.65 Clinical guidelines could help practitioners identify risky prescriptions and lessen the frequency of adverse effects.
Limitation
Although our study is the first we are aware of, there are some limitations to it. First, since our study design was cross-sectional, one-time laboratory measurement values were taken, which might affect severity classification. Second, practitioners might have referred to guidelines that were not the same as the ones we used for our study. We recommend further research with a prospective study design and a larger sample size. Moreover, we believe that qualitative-type studies that assess practitioners’ practice are important.
Conclusion
This study showed that cirrhotic patients frequently receive inappropriate medications, which puts patients at risk for potential adverse effects. Prescribers need to be cautious about the safety issues of using hepatotoxic drugs in the presence of underlying cirrhosis. According to this study, prescribers should pay close attention to patients who have prescribed with higher number of medications, increased level of bilirubin and decreased level of albumin. Moreover, the educational level of prescribers needs to be upgraded in order to adopt evidence-based medication prescriptions and adhere to recommended practices. Our findings will offer important insights for halting the progression of cirrhosis brought on by inappropriate prescription, which may result in unfavorable patient outcomes and increase the burden of the disease.
Abbreviations
CHB, Chronic hepatitis B; CHC, Chronic hepatitis C; FHCSRH, Felege-Hiwot comprehensive specialized referral hospital; INR, International normalized ratio; PPI, Proton pump inhibitors; SBP, Spontaneous bacterial peritonitis; USA, The United States of America.
Data Sharing Statement
There are all the necessary details in the paper. The corresponding author can provide you with additional data that has been used to support the study’s conclusions if requested.
Acknowledgments
We want to express our deepest appreciation to the medical unit workers and data collectors for their contributions to the success of this study. Additionally, we want to say sincere thanks to the study participants for their willing participation.
Funding
No funding to report.
Disclosure
The authors report no conflicts of interest related to the publication of this work.
References
1. Amarapurkar DN. Prescribing medications in patients with decompensated liver cirrhosis. Int J Hepatol. 2011;2011:1.
2. Melato M, Mucli E. Something new in liver cirrhosis epidemiology. Lancet. 1989;334(8659):395–396. doi:10.1016/S0140-6736(89)90578-3
3. Qua CS, Goh KL. Liver cirrhosis in Malaysia: peculiar epidemiology in a multiracial Asian country. J Gastroenterol Hepatol. 2011;26(8):1333–1337. doi:10.1111/j.1440-1746.2011.06732.x
4. Zhou W-C, Zhang Q-B, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20(23):7312. doi:10.3748/wjg.v20.i23.7312
5. Weersink RA, Bouma M, Burger DM, et al. Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion. BMJ Open. 2016;6(10):e012991. doi:10.1136/bmjopen-2016-012991
6. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–1161. doi:10.1007/s00228-008-0553-z
7. Gonzalez M, Goracci L, Cruciani G, Poggesi I. Some considerations on the predictions of pharmacokinetic alterations in subjects with liver disease. Expert Opin Drug Metab Toxicol. 2014;10(10):1397–1408. doi:10.1517/17425255.2014.952628
8. Francis P, Navarro VJ. Drug induced hepatotoxicity. In: StatPearls. StatPearls Publishing; 2022.
9. Holloway K, Van Dijk L. The World Medicines Situation 2011-Rational Use of Medicines. World Health Organization (WHO), Geneva WHO/EMP/MIE; 2011.
10. Maddrey WC. Drug-induced hepatotoxicity: 2005. J Clin Gastroenterol. 2005;39(4):S83–S9. doi:10.1097/01.mcg.0000155548.91524.6e
11. Lee WM. Acute liver failure. In: Yamada’s Textbook of Gastroenterology. John Wiley & Sons; 2022:1889–1905.
12. Thomson MJ, Lok AS, Tapper EB. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology. 2021;73(6):2429–2440. doi:10.1002/hep.31548
13. Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. In: Seminars in Liver Disease. Thieme Medical Publishers Inc; 2002.
14. Franz CC, Hildbrand C, Born C, Egger S, Rätz Bravo AE, Krähenbühl S. Dose adjustment in patients with liver cirrhosis: impact on adverse drug reactions and hospitalizations. Eur J Clin Pharmacol. 2013;69:1565–1573. doi:10.1007/s00228-013-1502-z
15. Bilal F, Arain MI, Dayo A, Ghoto MA, Bilal F. Evaluation of drug utilization and prevalence of cirrhotic patients by using WHO prescribing indicators at tertiary care hospital. Isra Med J. 2019;11(4):300–304.
16. Lewis JH. The rational use of potentially hepatotoxic medications in patients with underlying liver disease. Expert Opin Drug Saf. 2002;1(2):159–172. doi:10.1517/14740338.1.2.159
17. Hayward KL, Weersink RA. Improving medication‐related outcomes in chronic liver disease. Hepatol Commun. 2020;4(11):1562–1577. doi:10.1002/hep4.1612
18. Kolasani BP, Sasidharan P, Divyashanthi C, Jayabal P, Rajaseharan A. Prescribing pattern of drugs in patients with alcoholic liver disease in a tertiary care teaching hospital. Natl J Physiol Pharm Pharmacol. 2017;7(5):538.
19. Lewis J, Stine J. prescribing medications in patients with cirrhosis–a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132–1156. doi:10.1111/apt.12324
20. Mohammed BS, Aidoo M. Drug treatment of patients with liver cirrhosis in a tertiary hospital in Northern Ghana: does it comply with recommended guidelines? Int J Hepatol. 2020;2020:1–7. doi:10.1155/2020/9750194
21. Tsega E. Viral Hepatitis and CLD in Ethiopia, Epidemiological and Clinical aspects [Ph. D. thesis]. Malmo, Sweden: University of Lund; 1991.
22. Tesfaye BT, Feyissa TM, Workneh AB, Gudina EK, Yizengaw MA. Chronic liver disease in Ethiopia with a particular focus on the etiological spectrums: a systematic review and meta-analysis of observational studies. Can J Gastroenterol Hepatol. 2021;2021:1–14. doi:10.1155/2021/8740157
23. Zeleke TK, Birhan TY, Abdela OA. Medicine dose adjustment practice and associated factors among renally impaired patients in Amhara Regional State, Ethiopia. Int J Nephrol. 2021;2021:1–9. doi:10.1155/2021/8238250
24. Witkiewitz K, Litten R, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5(9):eaax4043. doi:10.1126/sciadv.aax4043
25. Kok B, Abraldes JG. Child–pugh classification: time to Abandon? In: Seminars in Liver Disease. Thieme Medical Publishers; 2019.
26. Pugh R, Murray‐Lyon I, Dawson J, Pietroni M, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
27. Tsoris A, Marlar CA. Use of the Child Pugh score in liver disease; 2019.
28. Daoud JI. Multicollinearity and regression analysis. J Phys. 2017;2017:1.
29. Nwokediuko S, Osuala P, Uduma U, Alaneme A, Onwuka C, Mesigo C. Pattern of liver disease admissions in a Nigerian tertiary hospital. Niger J Clin Pract. 2013;16(3):339–342. doi:10.4103/1119-3077.113458
30. Wang X, Lin S-X, Tao J, et al. Study of liver cirrhosis over ten consecutive years in Southern China. World J Gastroenterol. 2014;20(37):13546. doi:10.3748/wjg.v20.i37.13546
31. Fortune B, Cardenas A. Ascites, refractory ascites and hyponatremia in cirrhosis. Gastroenterol Rep. 2017;5(2):104–112. doi:10.1093/gastro/gox010
32. Merat S, Malekzadeh R, Rezvan H, Khatibian M. Hepatitis B in Iran; 2000.
33. Maddrey WC. Hepatitis B: an important public health issue. J Med Virol. 2000;61(3):362–366. doi:10.1002/1096-9071(200007)61:3<362::AID-JMV14>3.0.CO;2-I
34. Wright TL. Introduction to chronic hepatitis B infection. Official J Am Coll Gastroenterol. 2006;101:S1–S6.
35. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014–1018. doi:10.1053/he.2000.5762
36. Innes HA, Hutchinson SJ, Barclay S, et al. Quantifying the fraction of cirrhosis attributable to alcohol among chronic hepatitis C virus patients: implications for treatment cost‐effectiveness. Hepatology. 2013;57(2):451–460. doi:10.1002/hep.26051
37. Lucena IM, Andrade RJ, Tognoni G, Hidalgo R, de la Cuesta F; Spanish Collaborative Study Group o T. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440. doi:10.1007/s00228-002-0474-1
38. Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(suppl 6):vi1–vi12. doi:10.1136/gut.2006.099580
39. Elzouki A-N, Zahid M, Akbar RA, et al. Polypharmacy and drug interactions amongst cirrhotic patients discharged from a tertiary center: results from a national quality improvement audit. Arab J Gastroenterol. 2020;21(4):216–218. doi:10.1016/j.ajg.2020.09.008
40. Weersink RA, Taxis K, Drenth JP, Houben E, Metselaar HJ, Borgsteede SD. Prevalence of drug prescriptions and potential safety in patients with cirrhosis: a retrospective real-world study. Drug Saf. 2019;42:539–546. doi:10.1007/s40264-018-0744-1
41. Greig SL. Sofosbuvir/velpatasvir: a review in chronic hepatitis C. Drugs. 2016;76(16):1567–1578. doi:10.1007/s40265-016-0648-2
42. Wilton J, Wong S, Yu A, et al. Real-world effectiveness of sofosbuvir/velpatasvir for treatment of chronic hepatitis C in British Columbia, Canada: a population-based cohort study. Open Forum Infect Dis. 2020;7. doi:10.1093/ofid/ofaa055
43. World Health Organization. Guidelines for the prevention care and treatment of persons with chronic hepatitis B infection: mar-15. World Health Organization; 2015.
44. Gish R, Jia J-D, Locarnini S, Zoulim F. Selection of chronic hepatitis B therapy with high barrier to resistance. Lancet Infect Dis. 2012;12(4):341–353. doi:10.1016/S1473-3099(11)70314-0
45. Ljubičić N, Bilić A. The effects of selective and non-selective adrenoceptor blockade on the portal blood flow in patients with liver cirrhosis. Scand J Gastroenterol. 1991;26(7):751–757. doi:10.3109/00365529108998595
46. De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi:10.1016/j.jhep.2015.05.022
47. Carone L, Oxberry SG, Twycross R, Charlesworth S, Mihalyo M, Wilcock A. Spironolactone. J Pain Symptom Manage. 2017;53(2):288–292. doi:10.1016/j.jpainsymman.2016.12.320
48. Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. J Hepatol. 2000;32(1):142–153. doi:10.1016/S0168-8278(00)80201-9
49. Green T, Bandy S. Spontaneous bacterial peritonitis; 2018.
50. Runyon BA. Introduction to the revised American Association for the study of liver diseases practice guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653. doi:10.1002/hep.26359
51. Eaftsot L. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417.
52. O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–342. doi:10.1016/j.amjopharm.2012.09.004
53. Arnman R, Olsson R. Elimination of paracetamol in chronic liver disease. Acta Hepatogastroenterol. 1978;25(4):283–286.
54. Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014;14(10). doi:10.5812/hepatmon.23539
55. Toes MJ, Jones AL, Prescott L. Drug interactions with paracetamol. Am J Ther. 2005;12(1):56–66. doi:10.1097/00045391-200501000-00009
56. Benson GD, Koff RS, Tolman KG. The therapeutic use of Acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141. doi:10.1097/01.mjt.0000140216.40700.95
57. Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85:451–458. doi:10.4065/mcp.2009.0534
58. Ali HA, Afifi M, Saber TM, et al. Neurotoxic, hepatotoxic and nephrotoxic effects of tramadol administration in rats. J Mol Neurosci. 2020;70:1934–1942. doi:10.1007/s12031-020-01592-x
59. Megan W. Pain management considerations in cirrhosis; 2015.
60. Horvath A, Rainer F, Bashir M, et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep. 2019;9(1):1–9. doi:10.1038/s41598-019-48352-5
61. Janka T, Tornai T, Borbély B, et al. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol. 2020;32(2):257–264. doi:10.1097/MEG.0000000000001499
62. Weersink RA, Bouma M, Burger DM, et al. Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol. 2018;84(8):1806–1820. doi:10.1111/bcp.13615
63. Shapiro J, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20(2):137–140. doi:10.1136/gut.20.2.137
64. Samsky MD, Dunning A, DeVore AD, et al. Liver function tests in patients with acute heart failure and associated outcomes: insights from ASCEND‐HF. Eur J Heart Fail. 2016;18(4):424–432. doi:10.1002/ejhf.440
65. Sidorenkov G, Navis G. Safety of ACE inhibitor therapies in patients with chronic kidney disease. Expert Opin Drug Saf. 2014;13(10):1383–1395. doi:10.1517/14740338.2014.951328
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.