Back to Journals » International Journal of Women's Health » Volume 16
The Association Between Exercise During Pregnancy and the Risk of Preterm Birth
Authors Zhang J, Xiao Y, Bai S, Lin S, Du S, Wang Z
Received 11 November 2023
Accepted for publication 22 January 2024
Published 5 February 2024 Volume 2024:16 Pages 219—228
DOI https://doi.org/10.2147/IJWH.S447270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Jin Zhang,1,* Yang Xiao,2,* Shuoxin Bai,3 Shaoqian Lin,2 Shuang Du,1 Zhiping Wang4
1Department of Occupational and Environmental Health, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Center for Disease Control and Prevention, Jinan, Shandong, 250021, People’s Republic of China; 3Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 4School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiping Wang, School of Public Health, Cheeloo College of Medicine, Shandong University, 44 Wenhuaxi Road, Jinan, Shandong, 250012, People’s Republic of China, Email [email protected]
Purpose: We aimed to examine the association between exercise during pregnancy and preterm birth (PTB).
Methods: This study was a baseline survey of the Jinan birth cohort. The study subjects were the mothers one month after giving birth, which were investigated by questionnaires retrospectively containing physical exercise (frequency, time, and primary exercise patterns) during pregnancy and each trimester. Individual time spent on exercise and energy expenditure were assessed based on the questionnaires. PTB was clinically defined as a newborn born on or before the last day of the 37th week. Adjusted odds ratios (OR) were estimated using logistic regression to assess the relationship between exercise during pregnancy and the risk of PTB. Variable selection for the multivariate models was guided by the directed acyclic graph. The median effect was analyzed by the sequential test.
Results: The prevalence of PTB in this study was 4.38% (285/6501). The adjusted OR (95% CI) for the risk of PTB related to exercise during pregnancy was 0.74 (0.58– 0.95). During the 1st and 2nd trimesters, the ORs (95% CI) for 2.5 to 7 hours of exercise per week were 0.77 (0.59– 0.99) and 0.74 (0.57– 0.96). During the 3rd trimester, the ORs (95% CI) for 2.5 to 7 hours and more than 7 hours of exercise per week were 0.74 (0.56– 0.96) and 0.65 (0.44– 0.94). After stratifying the subjects, the association was only found among subjects without pregnancy complications. Pregnancy complications partially mediated (52.40%) the relationship between exercise during pregnancy and PTB.
Conclusion: Exercise during pregnancy can reduce the risk of PTB for women without pregnancy complications. 2.5 to 7 hours of exercise (like walking) per week may be appropriate in three trimesters of pregnancy, and the time could be extended in the 3rd trimester.
Keywords: pregnant women, exercise, trimester, mediation analysis
Introduction
The World Health Organization defined preterm birth (PTB) as less than 37 weeks gestation.1 PTB has posed significant risks to the health of both the children and the mothers. Complications of PTB were the main cause of death in children younger than 5 years of age globally in 2016, accounting for approximately 35% of deaths among newborn babies.2 China had the second-highest rate of PTB in the world in 2014,3 which suggested that we should pay attention to the health of premature children. At the same time, women with preterm babies have a higher risk of developing cardiovascular disease prematurely and experiencing related mortality in the future.4 Since PTB leads a huge burden of disease, it is important to focus on women’s lifestyles during pregnancy to prevent PTB.
Physical exercise was a kind of planned, organized, and repetitive exercise designed to promote a healthy body. Exercise during pregnancy referred to any physical activity during pregnancy.5 Some studies have shown that both sedentary behavior and high-intensity exercise during pregnancy can increase the risk of premature rupture of membranes, and moderate-intensity exercise has been found to improve adverse pregnancy outcomes.6–8
Although there have been many studies on the effect of exercise during pregnancy on PTB, of which the conclusions have been inconsistent. Studies from different regions have not found any correlation between exercise during pregnancy and PTB.9,10 However, a birth cohort in Japan showed that compared with mothers with moderate levels of exercise, lower levels increased the risk of preterm delivery.6 Since people in different regions have different exercise habits, more research needs to be done. Furthermore, few previous studies have examined each trimester separately. As the internal physiological environment of a woman is a constantly changing process during pregnancy, further research is needed to examine the relationship between exercise during each trimester and PTB.
Some countries have set standards for exercise during pregnancy. For example, the American Congress of Obstetricians and Gynecologists, the Society of Obstetricians and Gynecologists of Canada, and the Canadian Society of Exercise Physiology had some detailed recommendations for pregnant women.11–13 It is recommended that women with no medical contraindications get at least 20 to 30 minutes of aerobic exercise and strength conditioning exercises every day during pregnancy. These areas have high economic levels and belong to developed countries. Most of them have developed exercise health guidelines for pregnant women.14 However, in developing countries, although there have been studies on exercise during pregnancy and maternal health, they still lack appropriate guidelines for their own guidance.15 The latest physical activity guidelines for Chinese do not include a part specially for pregnant woman. It is necessary to make some appropriate guidelines for women in developing countries to have scientific physical exercise during pregnancy.
Therefore, based on the data from a birth cohort in Jinan, China, we aimed to examine the association between exercise during pregnancy and PTB, which could provide a basis for the establishment of exercise standards during pregnancy in China.
Materials and Methods
Source of Population
The source population was the women who gave their babies a second dose of the vaccine a month after giving birth, which constituted the baseline population of the Jinan birth cohort. The cohort was conducted in the capital city of Shandong Province in China, from December 2018 to December 2019. Data were collected in 15 community vaccination clinics which were selected from 116 community vaccination clinics in the study area. The details of the Jinan birth cohort have been described in the previous literature.16,17
The study was approved by the Ethics Committee of Preventive Medicine of Shandong University (Approved number: 20170315) with the informed consent of all participants.
Study Subjects
The study subjects were the mothers of the Jinan birth cohort, who received a face-to-face questionnaire survey one month after giving birth. The exclusion criteria of this study were as follows: (1) the infant was delivered with congenital disease; (2) leaving Jinan during pregnancy; (3) having mental diseases or other diseases that affect normal communication; (4) multiple births or stillbirths.
Outcome Assessment
The outcome was PTB, which was defined as a newborn born on or before the end of the last day of the 37th week after the mother’s last menstrual period.18 This is the most extensively used and accepted definition of preterm birth.19 Gestational age information was calculated from the date of last menstrual period and birth recorded in the questionnaire. The date of the last menstruation was confirmed according to the B-ultrasound diagnosis of the hospital.
Exposure Assessment
A self-designed questionnaire for basic information of infants and mothers was used for a one-to-one face-to-face questionnaire survey. The information about infants included birth outcomes, newborn birth weight, and so on, the maternal information including her general demographic characteristics, family economic conditions, last menstrual period and gestational age, physical exercise during pregnancy (including the frequency, time, and primary exercise patterns), and others included maternal tobacco exposure, and work during pregnancy.
The interview questionnaire contained items about physical exercise during pregnancy and each trimester, refers to the 1st trimester (0 to 12 weeks of gestation), the 2nd trimester (13 to 28 weeks of gestation), and the 3rd trimester (after 28 weeks of gestation). There were some questions about exercise on the questionnaire: 1) “Have you exercised during pregnancy? The exercise means any planned, organized, and repetitive physical activity”. 2) “What is your primary exercise patterns (walking, brisk walking, yoga/Pilates/maternity gymnastics, swimming, squat and others) during pregnancy?” For each trimester, take the 1st trimester as an example, 3) “How many times do you exercise per week during the 1st trimester?” 4) “How many hours do you exercise at a time?”.
Since Chinese pregnancy health guidelines do not involve exercise during pregnancy, the subjects’ activity was grouped according to the previous studies and existing guidelines.11,12 Time spent on exercise were grouped as none and low (0–2.49h/week), moderate (2.5–7h/week), and high (>7h/week).20 One study defined “moderate exercise” as that requiring 4.5 METs (Metabolic Equivalent of Energy).20 So, energy expenditures were grouped as none and low (0–11.24 MET.h/week), moderate (11.25–31.5 MET.h/week), and high (>31.5 MET.h/week). The calculation of energy expenditure was based on the previous criterion.21
Based on the questionnaires completed, the amount of time the women spent exercising during the entire pregnancy and three trimesters was calculated, and energy expenditure at different times was assessed.
Covariates
Four types of factors were considered to be covariates. Mother-related characteristics included maternal age (less than 25, 25–30, and more than 30 years old), occupation (housewife/others), and education (less than or equal to 9, 10–12, and more than 12 years).22 Pregnancy-related characteristics consisted of parity (primiparous/multiparous), delivery mode (natural birth/cesarean), pregnancy hypertension (yes/no), delivery complications (yes/no), work during pregnancy (yes/no), and tobacco exposure during pregnancy (yes/no).22,23 Pregnancy complications included gestational hypertension (HDP), amniotic fluid embolism, abnormal umbilical cord, and so on. Work during pregnancy (no) meant pregnant women did not work during the whole pregnancy. Both active and passive smoking were classified as maternal tobacco exposure due to the low number of active smokers, passive smoking is defined as more than 30 minutes of tobacco exposure per week. In addition, we also selected infant gender (male/female) and family income (less than 6000 yuan/more than or equal to 6000 yuan).22
Statistical Analyses
Categorical variables were compared by using the Chi-square test. For continuous variables, normality was tested by the Kolmogorov–Smirnov test. The independent sample t-test was used for normal data, the Wilcoxon-Mann–Whitney test was used for nonnormal data.
To assess the relationship between exercise during pregnancy and the risk of PTB, multiple logistic regression was used to calculate odds ratios (ORs) and 95% of the corresponding confidence intervals (95% CIs).
Variable selection for the multiple models was guided by the directed acyclic graph (DAG). The DAG was plotted to recognize potential causality, mediation, or confounding factors.24 Furthermore, women’s exercise activity during pregnancy was grouped by time spent and energy expenditure, and the none and low group was used as a reference. The effect of each trimester was observed separately. For subgroup analysis, the subjects were stratified by whether they had pregnancy complications or not.
The mediating effect was analyzed by sequential test. Sequential test can effectively reduce the required sample size and is widely used in the study of median effect.25
Statistical analyses were performed using the statistical computing environment R 4.0.5. P-value of <0.05 was considered statistically significant.
Results
The Characteristics of the Study Population
The final sample size for the study was 6501 women with singleton live births, included 285 PTBs and 6216 full-term births, the preterm rate was 4.38%.
Compared to women with full-term birth, mothers who experienced PTB tended to be older, be housewife, have more than one parity, be cesarean, have pregnancy complications, and lower family income (Table 1).
 |
Table 1 Characteristics of the Study Population by PTB Status |
The Characteristics of Exercise During Each Trimester
During the 3rd trimester and throughout pregnancy, the distribution of time spent was statistically different between the preterm and full-term women (P=0.005; P=0.045). The distribution of energy expenditure was statistically different during the 3rd trimester (P=0.007). More details were provided in Supplementary Table 1. Besides, for the patterns of exercise, most subjects (90.64%) chose the option of walking.
DAG
DAG (Figure 1) was plotted according to our data analysis and previous researches.26,27 As demonstrated, when examining the relationship between PTB and exercise during pregnancy, the potential confounding factors may include age, parity, occupation, and family income. Pregnancy complications was a potential mediator, and it was not adjusted in multivariate analysis.28
 |
Figure 1 Directed acyclic graph of the association between exercise during pregnancy and PTB. |
The Effect of Exercise During Pregnancy on PTB
Among women who did not exercise during pregnancy, the PTB rate was 5.37%. Among women who exercise during pregnancy, the PTB rate was 3.97%. Exercise during pregnancy was associated with 27% (OR = 0.73, 95% CI: 0.57–0.94) lower odds of PTB. After adjusting for the covariates, exercise during pregnancy was associated with 26% (OR = 0.74, 95% CI: 0.58–0.95) lower odds of PTB. For time spent on exercise per week, the moderate group (2.5–7h/week) was less likely to have PTB, the adjusted OR (95% CI) was 0.72 (0.55–0.94). For energy expenditure per week, the moderate group (11.25–31.5 MET.h/week) was less likely to have PTB, the adjusted OR (95% CI) was 0.75 (0.58–0.96) (Table 2).
 |
Table 2 Association Between PTB and Exercise During Pregnancy |
For each trimester (Figure 2), according to the time spent on exercise, using the none and low group (0–2.4h/week) as a reference, different trimesters had different results. During the 1st trimester, the moderate group (2.5–7h/week) was less likely to have PTB, the adjusted OR (95% CI) was 0.77 (0.59–0.99). During the 2nd trimester, the moderate group was less likely to have PTB, the adjusted ORs (95% CI) were 0.74 (0.57–0.96). During the 3rd trimester, the moderate group and the high group (>7h/week) were less likely to have PTB, the adjusted ORs (95% CI) were 0.74 (0.56–0.96) and 0.65 (0.44–0.94). According to the energy expenditure (Supplementary Table 2), during the 3rd trimester, the moderate group (>7h/week) was less likely to have PTB, the adjusted ORs (95% CI) were 0.74 (0.57–0.95). More details were provided in Supplementary Tables 2 and 3.
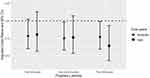 |
Figure 2 Association between PTB and exercise during each trimester. |
The Results of Subgroup Analysis
Among subjects with pregnancy complications, PTB rates were high regardless of whether they exercised or not, and the adjusted OR (95% CI) for the risk of PTB related to exercise during pregnancy was 0.87 (0.45–1.74), which was not significant. Among subjects without pregnancy complications, exercise during pregnancy was associated with PTB, and the adjusted OR (95% CI) was 0.72 (0.55–0.95) (Table 3). However, after interaction analysis, no statistically significant interaction terms were found.
 |
Table 3 Association Between PTB and Exercise During Pregnancy in Subgroup Analysis |
The Mediating Effect of Pregnancy Complications on the Relationship Between PTB and Exercise During Pregnancy
Pregnancy complications partially mediated the relationship between exercise and PTB, and the mediating effect accounted for 52.40% of the total effect. More details for the results of mediating analysis were provided in Supplementary Table 4.
Discussion
In our study, the PTB rates were 3.97% and 5.37% among women who did or did not exercise during pregnancy. We found that exercise during pregnancy was associated with 26% (OR = 0.74, 95% CI: 0.58–0.95) lower odds of PTB.
We further divided pregnancy into three trimesters, to observe the relationship between exercise during each trimester and PTB. The results suggested that, during the 1st and 2nd trimesters, 2.5 to 7 hours of exercise per week was associated with lower odds of PTB. During the 3rd trimester, both 2.5 to 7 hours and more than 7 hours of exercise per week were associated with lower odds of PTB. Compared to the 1st and 2nd trimesters, the 3rd trimester may require a longer period of exercise.
For the association between exercise during pregnancy and PTB, most relevant studies agree with us. One study in Denmark showed a reduced risk of PTB among the women who exercised during pregnancy (OR = 0.82, 95% CI: 0.76–0.88).29 A study in Southern California reported that both moderate exercise (OR = 0.90, 95% CI: 0.84–0.96) and vigorous exercise (OR = 0.67, 95% CI: 0.46–0.98) during pregnancy were associated with lower risk of PTB.30 A meta-analysis included only randomized controlled trials (RCTs) of overweight or obese pregnant women, showed that women who had an aerobic exercise for about 30–60 min three to seven times per week had a lower percentage of PTB (RR = 0.62, 95% CI: 0.41–0.95) compared with controls.31 And two studies in China also had similar results. Huang did analyses about the relationship between maternal exercise frequency and duration during pregnancy and PTB, and the adjusted ORs ranged from 0.43 to 0.65.32 Cai reported that women who participated in physical exercise 1–2 times, 3–4 times, and over five times per week had 20% (OR =0.80, 95% CI: 0.68–0.92), 30% (OR = 0.70, 95% CI: 0.60–0.82), and 32% (OR = 0.68, 95% CI: 0.59–0.78) lower odds of PTB, respectively.22 The reason why the third trimester requires a longer period of exercise may be that pregnant women gained more weight during the 3rd trimester than during the 1st and 2nd trimesters, and proper exercise helps pregnant women maintain a reasonable weight.33 Obesity alters levels of related inflammatory cytokines, and elevated levels of inflammatory cytokines can stimulate increased levels of oxytocin and lead to PTB.34 Also, a longer time of relaxed exercise activities in the 3rd trimester may help pregnant women relieve tension, promote blood circulation, increase pelvic floor muscle strength, which can reduce the risk of PTB.35
However, there were studies with negative results. For example, a cohort study in Brazil found no link between high or moderate physical activity and PTB,9 which may be due to racial differences and more research is needed.
Until now, the mechanism between exercise and PTB has several hypotheses. Firstly, placental hypoplasia is one of the important causes of PTB, and there is much evidence that exercise during pregnancy promotes placenta development.36,37 For one thing, exercise leads to a significant increase in placenta volume during the second trimester.36 For another, exercise during pregnancy promotes placenta angiogenesis.37 Additionally, exercise during pregnancy may confer a protective effect against PTB through IL-10 mediated pathways.38
After stratifying the subjects by whether they had pregnancy complications or not, the association between PTB and exercise during pregnancy only was found among subjects without pregnancy complications. People with pregnancy complications always are considered to be at high risk for PTB,26 this may account for the effect of exercise on PTB was not significant in these people. We did not adjust pregnancy complications in multivariate analysis, because it was a potential mediator in the causal pathway between exposure and outcome, the adjustment of it in the model may affect the estimation of the results based on previous studies.28 And our study indicated that pregnancy complications had a partial mediating effect, which was consistent with previous studies. A meta-analysis showed that exercise during pregnancy reduces the risk of HDP,10 which is considered a risk factor for PTB.26
There are some strengths of this study. Firstly, this study specifically analyzes the 1st, 2nd, and 3rd trimester exercise and PTB. Secondly, this study provides relevant evidence for the formulation of relevant standards in China. Thirdly, we firstly found a partial mediating effect of pregnancy complications on the relationship between PTB and exercise during pregnancy.
We also have some limitations. There is recall bias due to the research method being a face-to-face questionnaire. In the multivariate analysis, we did not adjust the time spent on exercise during the 1st, 2nd, and 3rd trimester for each other, because there was collinearity between the three pregnancies. And there are some confounding factors we cannot control, such as dietary differences between regions.
Conclusion
Exercise during pregnancy can reduce the risk of PTB for women without pregnancy complications. 2.5 to 7 hours of exercise (like walking) per week may be appropriate in three trimesters of pregnancy, and the time can be extended in the 3rd trimester. To provide the basis for the formulation of exercise guidelines for pregnant women in China and Asia, more researches are needed to be done.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of Preventive Medicine of Shandong University (Approved number: 20170315) and c complies with the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from mothers of subjects.
Acknowledgments
Thanks to the Jinan Center for Disease Control and Prevention and the staff of the community vaccination clinic for the investigation. We also thank all mothers and infants for their cooperation.
Funding
This study was funded by the National Natural Science Foundation of China (81773386).
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
1. World Health Organization. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56(3):247–253.
2. UNICEF, World Bank Group, Division UP. Levels and trends in child mortality: report 2017; 2017.
3. Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health. 2019;7(1):E37–E46. doi:10.1016/S2214-109X(18)30451-0
4. Silverberg O, Park AL, Cohen E, Fell DB, Ray JG. Premature cardiac disease and death in women whose infant was preterm and small for gestational age a retrospective cohort study. JAMA Cardiol. 2018;3(3):247–251. doi:10.1001/jamacardio.2017.5206
5. Owen N, Healy GN, Dempsey PC, et al. Sedentary behavior and public health: integrating the evidence and identifying potential solutions. Ann Rev Public Health. 2020;41(1):265–287. doi:10.1146/annurev-publhealth-040119-094201
6. Takami M, Tsuchida A, Takamori A, et al. Effects of physical activity during pregnancy on preterm delivery and mode of delivery: the Japan Environment and Children’s Study, birth cohort study. PLoS One. 2018;13(10):e0206160. doi:10.1371/journal.pone.0206160
7. Perkins CC, Pivarnik JM, Paneth N, Stein AD. Physical activity and fetal growth during pregnancy. Obstetrics Gynecol. 2007;109(1):81–87. doi:10.1097/01.AOG.0000249605.11458.ac
8. Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis. Obstetrics Gynecol. 2000;95(4):623–635. doi:10.1016/s0029-7844(99)00598-0
9. Rêgo AS, Alves MT, Batista RF, et al. Physical activity in pregnancy and adverse birth outcomes. Cadernos de saude publica. 2016;32(11):e00086915. doi:10.1590/0102-311x00086915
10. Di Mascio D, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Exp Obstet Gynecol. 2016;215(5):561–571. doi:10.1016/j.ajog.2016.06.014
11. Perreault M, Atkinson SA, Mottola MF, et al. Structured diet and exercise guidance in pregnancy to improve health in women and their offspring: study protocol for the Be Healthy in Pregnancy (BHIP) randomized controlled trial. Trials. 2018;19(1):19. doi:10.1186/s13063-017-2412-7
12. Syed H, Slayman T, Thoma KD. ACOG Committee Opinion No. 804: physical activity and exercise during pregnancy and the postpartum period. Obstetrics Gynecol. 2021;137(2):375–376. doi:10.1097/AOG.0000000000004266
13. Activity P, Pregnancy ED. Committee Opinion No. 650: physical activity and exercise during pregnancy and the postpartum period. Obstetrics Gynecol. 2015;126(6):e135.
14. Evenson KR, Barakat R, Brown WJ, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. Am J Lifestyle Med. 2014;8(2):102–121. doi:10.1177/1559827613498204
15. Watson ED, Macaulay S, Lamont K, et al. The effect of lifestyle interventions on maternal body composition during pregnancy in developing countries: a systematic review. Cardiovasc J Afr. 2017;28(6):397–403. doi:10.5830/CVJA-2017-003
16. Du S, Bai SX, Zhao XD, et al. The effect and its critical window for ambient temperature and humidity in pregnancy on term low birth weight. Environ Sci Pollut Res. 2022;29(36):54531–54542. doi:10.1007/s11356-022-19512-4
17. Bai SX, Du S, Liu HP, et al. The causal and independent effect of ozone exposure during pregnancy on the risk of preterm birth: evidence from northern China. Environ Res. 2022;214:113879.
18. Engle WA. A recommendation for the definition of ”late preterm” (near-term) and the birth weight-gestational age classification system. Semin Perinatol. 2006;30(1):2–7. doi:10.1053/j.semperi.2006.01.007
19. Howson CP, Kinney MV, Mcdougall L, Lawn JE; Group BTSPBA. Born Too Soon: preterm birth matters. Reproductive Health. 2013;10(Suppl 1):S1. doi:10.1186/1742-4755-10-S1-S1
20. Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exercise. 2008;40(9):1581–1588. doi:10.1249/MSS.0b013e31817cab1
21. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exercise. 2000;32(9 Suppl):S498–S516. doi:10.1097/00005768-200009001-00009
22. Cai M, Zhang B, Yang R, et al. Association between maternal outdoor physical exercise and the risk of preterm birth: a case-control study in Wuhan, China. BMC Pregnancy Childbirth. 2021;21(1):206. doi:10.1186/s12884-021-03678-9
23. Raper MJ, McDonald S, Johnston C, et al. The influence of exercise during pregnancy on racial/ethnic health disparities and birth outcomes. BMC Pregnancy Childbirth. 2021;21(1):258. doi:10.1186/s12884-021-03717-5
24. Sorg AL, von Kries R, Klemme M, et al. Risk factors for perinatal arterial ischaemic stroke: a large case-control study. Dev Med Child Neurol. 2020;62(4):513–520. doi:10.1111/dmcn.14347
25. van der Tweel I, Schipper M. Sequential tests for gene-environment interactions in matched case-control studies. Stat Med. 2004;23(24):3755–3771. doi:10.1002/sim.2071
26. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. doi:10.1016/j.bpobgyn.2018.04.003
27. Gu H. Analysis on factors influencing shanghai career women’s participation in leisure physical exercise.
28. Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174(9):1062–1068. doi:10.1093/aje/kwr230
29. Juhl M, Andersen PK, Olsen J, et al. Physical exercise during pregnancy and the risk of preterm birth: a study within the Danish National Birth Cohort. Am J Epidemiol. 2008;167(7):859–866. doi:10.1093/aje/kwm364
30. Guendelman S, Pearl M, Kosa JL, Graham S, Abrams B, Kharrazi M. Association between preterm delivery and pre-pregnancy body mass (BMI), exercise and sleep during pregnancy among working women in Southern California. Matern Child Health J. 2013;17(4):723–731. doi:10.1007/s10995-012-1052-5
31. Magro-Malosso ER, Saccone G, Di Mascio D, Di Tommaso M, Berghella V. Exercise during pregnancy and risk of preterm birth in overweight and obese women: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2017;96(3):263–273. doi:10.1111/aogs.13087
32. Huang L, Fan L, Ding P, et al. Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J Matern Fetal Neonatal Med. 2019;32(1):109–116. doi:10.1080/14767058.2017.1372415
33. Vargas-Terrones M, Nagpal TS, Barakat R. Impact of exercise during pregnancy on gestational weight gain and birth weight: an overview. Braz J Physic Ther. 2019;23(2):164–169. doi:10.1016/j.bjpt.2018.11.012
34. Wagner CL, Baggerly C, McDonnell SL, et al. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. J Steroid Biochem Mol Biol. 2015;148:256–260. doi:10.1016/j.jsbmb.2014.11.013
35. Aran T, Pekgöz I, Bozkaya H, Osmanagaoglu MA. Association between preterm labour and pelvic floor muscle function. J Obstet Gynaecol. 2018;38(8):1060–1064. doi:10.1080/01443615.2018.1446922
36. Clapp JF, Rizk KH. Effect of recreational exercise on midtrimester placental growth. Am J Clin Exp Obstet Gynecol. 1992;167(6):1518–1521. doi:10.1016/0002-9378(92)91730-X
37. Clapp JF, Stepanchak W, Tomaselli J, Kortan M, Faneslow S. Portal vein blood flow-effects of pregnancy, gravity, and exercise. Am J Clin Exp Obstet Gynecol. 2000;183(1):167–172. doi:10.1067/mob.2000.105902
38. Steckle V, Shynlova O, Lye S, Bocking A. Low-intensity physical activity may protect pregnant women against spontaneous preterm labour: a prospective case-control study. Appl Physiol Nutr Metab. 2021;46(4):337–345. doi:10.1139/apnm-2019-0911
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
