Back to Journals » OncoTargets and Therapy » Volume 17
Taking the Next Step in Double Refractory Disease: Current and Future Treatment Strategies for Chronic Lymphocytic Leukemia
Received 1 November 2023
Accepted for publication 28 February 2024
Published 8 March 2024 Volume 2024:17 Pages 181—198
DOI https://doi.org/10.2147/OTT.S443924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Manabu Hayama, John C Riches
Centre for Haemato-Oncology, Barts Cancer Institute, Queen Mary University of London, London, EC1M 6BQ, UK
Correspondence: John C Riches, Centre for Haemato-Oncology, Barts Cancer Institute, Queen Mary University of London, Charterhouse Square, London, EC1M 6BQ, United Kingdom, Tel +44 20 7882 3825, Email [email protected]
Abstract: Chronic lymphocytic leukemia (CLL) is a monoclonal B-cell lymphoproliferative disease with a high annual incidence in Western countries. As B-cell receptor (BCR) signaling and intrinsic apoptotic resistance play critical roles in the development and survival of CLL cells, therapeutic approaches targeting these pathways have been extensively investigated to tackle this incurable disease. Over the last decade, several Phase 3 trials have confirmed the superior efficacy of covalent Bruton tyrosine kinase inhibitors (cBTKis) and venetoclax, a selective B-cell lymphoma 2 (BCL2) inhibitor, over chemoimmunotherapy. This has been demonstrated in both the treatment-naïve and relapsed/refractory (RR) settings and includes patients with high-risk molecular features. However, these drugs are not curative, with patients continuing to relapse after treatment with both cBTKis and BCL2is, and the optimal treatment strategy for these patients has not been defined. Several novel agents with distinct mechanisms have recently been developed for CLL which have demonstrated efficacy in patients who have previously received cBTKis and BCL2i. In particular, novel BCR-signaling targeting agents have shown promising efficacy in early-phase clinical trials for RR-CLL. Furthermore, cancer immunotherapies such as bispecific antibodies and chimeric antigen receptor T-cells have also shown anti-tumor activity in patients with heavily pretreated RR-CLL. Personalised approaches with these novel agents and combination strategies based on the understanding of resistance mechanisms have the potential to overcome the clinical challenge of what to do next for a patient who has already had a cBTKi and venetoclax.
Keywords: CLL, BTK inhibitor, BCL2 inhibitor, bispecific antibody, CAR-T cell therapy
Introduction
Chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) is an indolent hematological malignancy, characterized by the clonal proliferation and accumulation of functionally incompetent B cells in the blood, bone marrow, lymph nodes and spleen.1 CLL and SLL are considered to be the same disease with different manifestations. The clinical presentation of CLL is primarily that of a peripheral lymphocytosis, whereas patients with SLL only manifest lymphadenopathy and organomegaly.2 CLL is the most common adult leukemia in Western countries.3 Traditional chemotherapeutic agents such as bendamustine, chlorambucil, fludarabine and cyclophosphamide used in combination with anti-CD20 monoclonal antibodies (mAbs) could induce long-term remissions, with these chemoimmunotherapy (CIT) regimens representing the standard treatment options for CLL until the advent of targeted therapies.4–6
As B-cell receptor (BCR) signaling mediates the development and differentiation of normal B cells and plays a crucial role in tumorigenesis of B-cell malignancies including CLL, therapeutic approaches targeting key molecules in the BCR-signaling pathways such as Bruton tyrosine kinase (BTK) and phosphatidylinositol-3-kinase (PI3K) were investigated.7 In 2014, ibrutinib, the first-in-class covalent BTK inhibitor (cBTKi) was approved by the US Food and Drug Administration (FDA) for the treatment of relapsed/refractory (RR) CLL based on its remarkable clinical efficacy.8 Following this, more selective next-generation cBTKis such as acalabrutinib and zanubrutinib were developed to improve the efficacy and safety of ibrutinib.9 Several phase 3 trials confirmed the superior efficacy of these cBTKis as monotherapy or in combination with an anti-CD20 mAb to CITs in the frontline and subsequent treatment settings.10–13 Another important treatment target is an anti-apoptotic protein B-cell lymphoma 2 (BCL2), which is overexpressed in CLL cells.14 Venetoclax, an oral selective BCL2 inhibitor (BCL2i), in combination with an anti-CD20 mAb also showed significant improvements in clinical outcomes compared to CITs.15,16
These targeted therapies have dramatically transformed the treatment landscape of CLL and long-term survival can be achieved for many patients even those with high-risk features such as TP53 dysruption and complex karyotypes. However, CLL remains an incurable disease and patients experience disease progression after clinical responses to cBTKis and venetoclax. In this situation, they have limited treatment options with poor clinical outcomes, which highlights a significant unmet medical need.17 Based on the molecular understanding of clinical resistance to cBTKi and venetoclax, several novel agents that could overcome the resistance mechanisms have entered the clinical investigation. The objectives of this article are to review the biological backgrounds and clinical applications of targeted therapies for CLL, to discuss current clinical challenges in the treatment of CLL, and to explore potential treatment strategies for patients who progressed after both cBTKi- and venetoclax-based therapies.
The Biological Backgrounds of Targeted Therapies for CLL
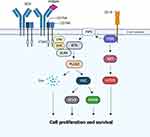
The BCR complex with CD79A and CD79B regulates the development and differentiation of B cells by tonic or antigen-dependent BCR-signaling. Tonic BCR-signaling is considered independent of antigen binding and mediated by PI3K/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathways.7 When BCR is engaged by antigen, spleen tyrosine kinase (SYK) and LYN recruit other signaling partner proteins such as BTK, B-cell linker (BLNK), and phospholipase C gamma 2 (PLCG2).18 BTK and SYK phosphorylate PLCG2, inducing calcium flux and the activation of the protein kinase C beta (PKCβ), mitogen-activated protein kinase (MAPK) and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) pathways. Furthermore, the BCR co-receptor CD19 is phosphorylated by LYN to enable the recruitment and activation of PI3K, promoting downstream activation of AKT and mTOR. PI3K generates a second messenger, phosphatidylinositol-3,4,5-triphosphate (PIP3), which activates BTK. The combination of these BTK- and PI3K-dependent signaling pathways regulates the proliferation and survival of B cells (Figure 1).19
In CLL, BCR-signaling via antigen binding and homotypic interaction plays a crucial role in the development of malignant B cells, consistent with the clinico-pathological features of CLL. Patients with unmutated immunoglobulin heavy chain variable region (IGHV) genes have polyreactive low-affinity BCRs that can recognise various foreign antigens and autoantigens, leading to the proliferation and survival of CLL cells. In contrast, CLL cells with mutated IGHV genes have high-affinity BCRs with restricted reactivity: therefore, those patients generally show stable disease courses.20 Furthermore, the fact that one-third of CLL patients have identical or near-identical “stereotyped” BCRs provides evidence for selection pressure by specific antigens.21
Apart from the activation of BCR-signaling, dysregulation of intrinsic apoptosis plays another important role in the development and survival of CLL cells.14 Intrinsic mitochondrial apoptosis is regulated by the balance and interactions between BCL2 family member proteins, including pro-survival proteins such as BCL2, myeloid cell leukemia-1 (MCL1) and B-cell lymphoma-extra large (BCL-XL), pro-apoptotic BCL2 homology domain 3 (BH3)-only proteins such as BCL2 interacting mediator of cell death (BIM) and BCL2 associated agonist of cell death (BAD), and apoptosis effector proteins: BCL2 associated X-protein (BAX) and BCL2 antagonist/killer (BAK).22 In certain types of tumors, particular pro-survival proteins such as BCL2 in CLL are overexpressed and the balance of these interactions tips towards cell survival. Therefore, small molecules that mimic the action of the BH3-only proteins have been developed, leading to the clinical application of venetoclax, a selective BCL2i, for the treatment of CLL.23
Initial Treatment Options for CLL
Ibrutinib
The molecular understanding of BCR-signaling pathways and intrinsic apoptosis regulation in B-cell malignancies promoted the development of multiple small molecule drugs that inhibit these pathways. Over the last 10–15 years, significant advances have been made in BTK- and BCL2-targeted therapies, which revolutionized treatment strategies for CLL.24,25 Ibrutinib is the first-in-class, orally bioavailable, highly potent BTK inhibitor, which covalently binds to a cysteine residue (C481) at the ATP binding site of BTK, to inhibit the phosphorylation of BTK and downstream signaling proteins including PLCG2, extracellular signal-regulated kinase (ERK), and NF-κB without affecting T-cell survival.26,27 Furthermore, ibrutinib inhibits integrin and chemokine-mediated cell adhesion and migration, which is reflected the rapid reduction of lymphadenopathy and transient lymphocytosis in ibrutinib-treated patients.28,29 In the Phase 1b/2 trial, ibrutinib monotherapy demonstrated full occupancy of BTK and an overall response rate (ORR) of 71% for heavily pretreated RR-CLL patients.30 In February 2014, ibrutinib was granted accelerated approval from the FDA for the treatment of RR-CLL.8 The phase 3 RESONATE trial directly compared ibrutinib and ofatumumab, an anti-CD20 mAb, in patients with RR-CLL at risk for a poor outcome. Ibrutinib significantly improved ORR (42.6% vs 4.1%, p < 0.001) and median progression-free survival (mPFS) (not reached [NR] vs 8.1 months; hazard ratio [HR] 0.22, 95% confidence interval [CI] 0.15–0.32) compared to ofatumumab. The benefit of ibrutinib was also observed in a subgroup of patients with del(17p).31
In the frontline setting, several phase 3 trials have reported remarkable clinical outcomes with ibrutinib as monotherapy and in combination with an anti-CD20 mAb (rituximab or obinutuzumab). In the phase 3 RESONATE-2 trial, 269 treatment-naïve (TN) CLL/SLL patients aged ≥65 years without del(17p) were randomly assigned to receive ibrutinib or chlorambucil. Ibrutinib showed significantly higher ORR (86% vs 35%, p < 0.001), longer PFS (mPFS NR vs 18.9 months; HR 0.16, p < 0.001), and longer overall survival (OS) (2-year-OS 98% vs 85%; HR 0.16, p = 0.001).32 Subsequent phase 3 trials showed the superiority of ibrutinib with or without an anti-CD20 mAb to CIT regimens.33–36 For example, in the phase 3 ECOG-ACRIN E1912 trial, 529 TN-CLL patients aged ≤70 years without del(17p) were randomly assigned to receive ibrutinib + rituximab (IbR) or fludarabine + cyclophosphamide + rituximab (FCR). IbR prolonged PFS (HR 0.35, 95% CI 0.22–0.56) and OS (HR 0.17, 95% CI 0.05–0.54) compared to FCR.33 The long-term follow-up results confirmed the PFS improvement in patients with both mutated (HR 0.27, p < 0.001) and unmutated IGHV (HR 0.27, p < 0.001).37 In the phase 3 iLLUMINATE trial with CLL/SLL patients aged ≥65 years or <65 years with co-existing comorbidities, ibrutinib + obinutuzumab (IbObi) showed significantly longer PFS (mPFS NR vs 22 months; HR 0.25, 95% CI 0.16–0.39) and higher undetectable minimal residual disease (uMRD) rate (38% vs 25%) compared to chlorambucil + obinutuzumab (ChlObi). The PFS benefit was also observed in patients with high-risk features of del(17p), TP53 mutation, del(11q), and unmutated IGHV.34 In the phase 3 Alliance A041202 trial with TN-CLL patients aged ≥65 years, both ibrutinib and IbR significantly improved PFS compared to bendamustine + rituximab (BR) (HR for ibrutinib vs BR 0.39, 95% CI 0.26–0.58; HR for IbR vs BR 0.38, 95% CI 0.25–0.59). IbR did not improve PFS compared to ibrutinib and there was no significant difference in OS between the three groups.35
Acalabrutinib
Despite the remarkable clinical efficacy, patients may discontinue ibrutinib due to its characteristic adverse events (AEs) such as atrial fibrillation and bleeding, which can be explained by the off-target inhibition of other kinases such as C-terminal Src kinase and Tec kinase.38,39 Therefore, more selective cBTKis were developed to overcome these challenges. Acalabrutinib is a potent and selective irreversible BTK inhibitor.40 As the bioavailability of the acalabrutinib capsule is impaired by acid-suppressing therapies, a new tablet form of acalabrutinib, which can be coadministered with proton pump inhibitors, has also been developed.41 The clinical efficacy of acalabrutinb for CLL has been shown in phase 3 trials. In the phase 3 ASCEND trial for RR-CLL, acalabrutinib significantly prolonged PFS compared to the investigator’s choice of IdR (idelalisib + rituximab) or BR (mPFS NR vs 16.8 months; 42-month-PFS 62% vs 19%; HR 0.28, 95% CI 0.20–0.38). Acalabrutinib also showed a favourable trend of OS advantage (median overall survival [mOS] NR vs NR; 42-month-OS 78% vs 65%; HR 0.69, 95% CI 0.46–1.04).10 In the phase 3 ELEVATE-RR trial, 533 RR-CLL patients with del(17p) or del(11q) were randomised to receive acalabrutinib or ibrutinib. Acalabrutinib was determined to be non-inferior to ibrutinib (mPFS 38.4 months in both arms; HR 1.00, 95% CI 0.79–1.27). Total cardiac events (24.1% vs 30.0%), hypertension (9.4% vs 23.2%), and atrial fibrillation/flutter (9.4% vs 16.0%) were less frequent with acalabrutinib than ibrutinib. Treatment discontinuations due to AEs were less frequent with acalabrutinib (14.7%) than ibrutinib (21.3%).42
In the frontline setting, the phase 3 ELEVATE-TN trial evaluated acalabrutinib + obinutuzumab (AcalaObi), acalabrutinib alone, or ChlObi. PFS was significantly prolonged with both AcalaObi (mPFS NR vs 22.6 months; HR 0.1, 95% CI 0.06–0.17) and acalabrutinib (mPFS NR vs 22.6 months; HR 0.20, 95% CI 0.13–0.3) compared to ChlObi. Cytopenia, infusion-related reactions, and grade 3 (G3) or higher infections were observed more frequently with AcalaObi than with acalabrutinib.11 In the long-term follow-up analysis, AcalaObi prolonged PFS compared to acalabrutinib (HR 0.58, 95% CI 0.39–0.86).43 Although there has not been direct comparison data of acalabrutinib and other cBTKis in the frontline setting, indirect comparisons have been conducted. A network meta-analysis of the iLLUMINATE, ELEVATE-TN, and CLL14 trials with a total of 1191 patients evaluated the efficacy and safety of frontline IbObi, venetoclax + obinutuzumab (VenObi), AcalaObi and acalabrutinib. No significant differences in PFS were observed between IbObi, VenObi, and acalabrutinib. In contrast, AcalaObi showed significantly improved PFS compared to IbObi (relative risk [RR] 0.43, 95% CI 0.22–0.87) and VenObi (RR 0.29, 95% CI 0.15–0.56). There were no significant differences in the frequency of AEs between these 4 groups.44 Davids et al conducted a matching-adjusted indirect comparison (MAIC) of acalabrutinib with targeted comparators (ibrutinib, IbObi, and VenObi) using data from the ELEVATE-TN, RESONATE-2, iLLUMINATE and CLL14 trials. After matching baseline characteristics, neither acalabrutinib or AcalaObi significantly improved PFS compared to any other comparators, although both acalabrutinib and AcalaObi showed improved safety outcomes.45
Zanubrutinib
Zanubrutinib is another potent and highly selective cBTKi. In the phase 1 study, BTK occupancy in peripheral blood mononuclear cells (PBMCs) was >95% at 4-hour post doses in almost all patients at all doses.46 In the phase 3 ALPINE trial for RR-CLL, zanubrutinib showed significantly longer PFS (2-year-PFS 78.4% vs 65.9%; HR 0.65, 95% CI 0.49–0.86), and higher ORR (83.5% vs 74.2%), lower incidence of cardiac disorders (21.3% vs 29.6%) than ibrutinib. Zanubrutinib also showed the PFS benefit in the subgroup of patients with del(17p) or TP53 mutation.12 The long-term follow-up analysis confirmed the sustained PFS benefits of zanubrutinib over ibrutinib (3-year-PFS 65.8% and 54.3%; HR 0.67, 95% CI 0.52–0.86).47 In the frontline setting, the phase 3 SEQUOIA trial evaluated 590 older or frail patients with TN-CLL/SLL. Among them, 479 patients without del(17p) were randomised to receive zanubrutinib or BR, and 111 patients with del(17p) were assigned to receive zanubrutinib. Compared to BR, zanubrutinib significantly prolonged PFS (2-year-PFS 86% vs 70%; HR 0.42, 95% CI 0.28–0.63). The PFS benefit of zanubrutinib was not observed in the subgroup of patients with mutated IGHV. In those with del(17p), zanubrutinib showed a 2-year-PFS rate of 89%.13 The phase 3 clinical trials of cBTKis are summarised in Table 1.
 |
Table 1 A Summary of the Phase 3 Clinical Trials of Covalent BTK Inhibitors |
Venetoclax-Based Therapies
Small molecules that mimic the action of the BH3-only proteins have been developed to target the intrinsic apoptosis dysregulation in B-cell malignancies. Venetoclax, an oral selective BCL2i, as monotherapy showed high response rates for heavily pretreated CLL patients including those with del(17p).48,49 As it was found that an anti-CD20 mAb might be able to overcome resistance to venetoclax, the combination of these two agents has been investigated. The phase 3 MURANO trial compared venetoclax + rituximab (VenR) and BR in patients with RR-CLL. The 2-year-PFS rate was significantly higher with VenR than with BR (84.9% vs 36.3%; HR 0.17, p < 0.001). The ORRs were 92.3% with VenR and 72.3% with BR. The rate of MRD clearance at the 9-month point in peripheral blood was higher with VenR than with BR (62.4% vs 13.3%). G3 or 4 tumor lysis syndrome was observed in 3.1% of patients with VenR.50 Long-term follow-up results confirmed the PFS (4-year-PFS 57.3% vs 4.6%; HR 0.19, 95% CI 0.14–0.25) and OS benefits (4-year-OS 85.3% vs 66.8%; HR 0.41, 95% CI 0.26–0.65) with VenR compared to BR.15
In the frontline setting, the phase 3 CLL14 trial compared VenObi and ChlObi in 432 TN-CLL patients with coexisting conditions. VenObi significantly prolonged PFS (2-year-PFS 88.2% vs 64.1%; HR 0.35, 95% CI 0.23–0.53) compared to ChlObi. The PFS benefit was also observed in those with TP53 deletion/mutation and in those with unmutated IGHV. The rates of uMRD in peripheral blood and bone marrow were higher with VenObi than with ChlObi (75.5% vs 35.2%, and 56.9% vs 17.1%, respectively).16 The long-term follow-up analysis reported that the 6-year PFS rates were estimated as 53.1% and 21.7%, respectively.51 In the phase 3 GAIA-CLL13 trial, VenR and VenObi were evaluated in TN-CLL patients without del(17p) or TP53 mutation. VenObi significantly improved PFS (3-year-PFS 87.7% vs 75.5%; HR 0.42, p < 0.001) and uMRD (86.5% vs 52.0%, p < 0.001) compared with FCR/BR, whereas VenR did not.52
Treatment Choices and Sequences of cBTKis and Venetoclax
As several phase 3 trials confirmed the superior efficacy of cBTKi- and venetoclax-based therapies to CITs as described above, these two treatments should be used as a first- or second-line treatment for most patients. The patient’s symptoms and comorbidities as well as the genetic risk of the disease should be considered for the choice of these agents.25,53 Previous small studies reported treatment sequences of cBTKi and venetoclax in the first- and second-line setting. In a small Phase 2 trial, venetoclax treatment showed an ORR of 65% and a mPFS of 23.5 months in RR-CLL patients who had previously received ibrutinib.54 In a small subset of patients who received subsequent ibrutinib therapy after VenR in the MURANO trial, the response rate was 87.5%.55 Furthermore, a small retrospective study reported that BTKis (ibrutinib or zanubrutinib) showed durable remissions after progression on venetoclax with mPFS of 34 months and mOS of 42 months.56 Venetoclax retreatment can be considered after a certain period of durable remission by a prior venetoclax-based therapy. An international retrospective study reported the efficacy and safety data of 46 CLL patients treated with venetoclax retreatment. Eighteen patients had received a cBTKi prior to the initial venetoclax therapy. The median duration between the completion of the initial and second venetoclax therapies was 16.1 months. The ORR and mPFS of the second venetoclax therapy were 79.5% and 25 months. In a subgroup of patients with a prior cBTKi, the ORR and mPFS were 56.3% and 15 months.57
Based on the results from indirect and direct comparisons between ibrutinib and newer cBTKis, acalabrutinib or zanubrutinib are preferred as the first choice cBTKi for the treatment of TN- and RR-CLL.12,44,45 Regarding the choice between acalabrutinib and zanubrutinib, there has been no direct comparison. Based on individual patient data of acalabrutinib from the phase 3 ASCEND trial and published aggregated data of zanubrutinib, an unanchored MAIC was performed to compare acalabrutinib and zanubrutinib in the frontline setting. Although the PFS data were similar between acalabrutinib and zanubrutinib (HR 0.90, 95% CI 0.60–1.36), acalabrutinib was found to be associated with lower risks of serious AEs and dose reductions.58
Clinical Challenges in the Era of Targeted Therapies
Double Refractory Disease
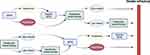
Even though the treatment sequence with cBTKi and venetoclax-based therapies including retreatment approaches are effective, many patients eventually progress after treatment with both classes of targeted therapy (Figure 2).57,59 Prospective clinical data on this “double refractory disease”, are very limited.60 Most of the clinical evidence derives from retrospective studies and the definitions of clinical resistance to targeted therapies are not consistent, particularly for fixed-duration venetoclax-based therapies. Aronson et al defined “double refractory” disease as a clinical situation where the patient (i) was treated with both a BTKi and venetoclax-based therapy, (ii) experienced disease progression while on a BTKi, and (iii) experienced disease progression while on venetoclax or within 24 months after venetoclax discontinuation, or became resistant to venetoclax retreatment.61 The prognosis of patients with double refractory disease is poor due to the lack of effective treatments. A retrospective study with 17 double refractory CLL patients reported the mOS was 3.6 months, and the majority of them died from disease progression including 8 patients with Richter’s syndrome (RS).62 Another retrospective study with 125 patients who had received both a cBTKi and venetoclax (double-exposed) reported the efficacy of subsequent treatments. The ORRs and mPFS were 40.9% and 5 months with PI3K inhibitors, and 31.8% and 3 months with CITs, respectively.63
Resistance Mechanisms
Even though cBTKis can achieve long-term remissions, many patients develop secondary resistance. The mechanisms of secondary resistance have extensively been studied in patients progressing on cBTKis, particularly ibrutinib. Most of those who progressed on ibrutinib develop mutations affecting the C481 residue in the kinase domain of BTK, which hinders the covalent binding of ibrutinib to BTK.64 Although mutations from cysteine to serine (C481S) are the most common, other mutations such as C481Y/R/F (tyrosine/arginine/phenylalanine) are also observed.65 The next most common resistance mechanism is gain-of-function mutations of PLCG2.66 Recently, secondary resistance mechanisms to newer cBTKis have been reported. As with the cases of ibrutinib, BTK C481 mutations are the most commonly observed in patients who progressed on acalabrutinib.67 However, BTK L528W mutations (from leucine to tryptophan) have been reported as the most common resistance mechanisms to zanubrutinib.68,69 Recent data with a small number of patients suggest that L528W mutations also show cross-resistance with pirtobrutinib, a novel non-covalent BTK inhibitor.69,70
Acquired mutations affecting the BH3-binding domain of BCL2 have also been reported as the main resistance mechanisms to venetoclax. Blombery et al reported that BCL2 G101V mutations were identified by next-generation sequencing (NGS) in 7 out of 15 patients who had progressed on continuous venetoclax therapy. The binding of venetoclax to BCL2 was markedly reduced by this mutation.71 In addition to G101V mutations, other BCL2 mutations have been reported (eg D103Y), which, along with overexpression of BCL-XL and MCL1, have been associated with clinical resistance to venetoclax.72,73 A recent study with 24 patients who had progressed on BTKi and received continuous venetoclax monotherapy reported that BCL2 G101V mutations were rare and BTK and PLCG2 mutations persisted or increased during venetoclax treatment.74 However, as these data largely derive from continuous venetoclax monotherapy, the clinical implications are not clear for the majority of patients receiving fixed-duration venetoclax-based regimens.
cBTKi and BCL2i Combinations
The fact that cBTKi and BCL2i have distinct and complementary mechanisms of action, combined with preclinical observations showing that BTK inhibition increases functional dependence on BCL2, paved the way for synergistic combinations of these classes.75,76 The increase of BCR-pathway mutations during venetoclax therapy after the discontinuation of cBTKi also provides a rationale to delay the emergence of resistance mutations by combination strategies.74 Furthermore, the cBTKi and venetoclax combination can achieve deep and durable responses with a fixed duration of treatment. As the clearance of MRD is correlated with longer PFS and OS, the uMRD status can predict long-term remissions, highlighting the importance of MRD as a clinical trial endpoint and a biomarker which can guide treatment approaches.77,78 The phase 2 CAPTIVATE trial investigated different treatment regimens based on the uMRD status after 3 cycles of ibrutinib lead-in followed by 12 cycles of ibrutinib + venetoclax (IbVen) in TN-CLL. In the MRD cohort of this trial, patients who achieved uMRD after the fixed-duration IbVen combination were randomly assigned to ibrutinib or placebo. The 1-year disease-free survival rates were not significantly different between them (100% vs 95%, respectively) (p = 0.15).77 The updated results of this trial showed that no BTK mutations were found at disease progression after the fixed-duration IbVen and the ORR of ibrutinib retreatment was 86%.78 Another single-arm phase 2 trial investigated the combination treatment with zanubrutinib, obinutuzumab and venetoclax (BOVen), which was discontinued after 8–24 cycles when uMRD was met. Thirty-three patients (89%) reached the pre-specified uMRD status with a median of 10 cycles.79 Currently, fixed-duration and MRD-guided cBTKi and BCL2i combination with or without an anti-CD20 mAb are being investigated in phase 3 trials (Table 2). In the phase 3 GLOW trial, 211 patients with TN-CLL/SLL were randomly assigned to receive fixed-duration IbVen (3 cycles of ibrutinib lead-in, then 12 cycles of IbVen) or ChlObi (6 cycles). IbVen significantly prolonged PFS compared to ChlObi (HR 0.22, 95% CI 0.13–0.36). The rate of sustained uMRD in the peripheral blood for 3 to 12 months after the end of treatment was 84.5% for IbVen and 29.3% for ChlObi. AEs G3 or higher occurred in 75.5% of those who received IbVen and 69.5% of those who received ChlObi.80 The 54-month PFS and OS rates were reported as 65.8% and 84.5% for IbVen and 19.1% and 63.1% for ChlObi, respectively.81 IbVen was approved as the frontline treatment for TN-CLL by the European Commission in August 2022.82 In the phase 3 FLAIR trial, IbVen (with MRD-guided duration) and ibrutinib alone arms were added in 2017. The recent analysis showed that the 4-year-PFS rates were 93.5% for IbVen and 64.8% for FCR (HR 0.13, 95% CI 0.07–0.24) and the 4-year-OS rates were 94.9% for IbVen and 87.3% for FCR (HR 0.31, 95% CI 0.15–0.67).83 The phase 3 GAIA-CLL13 trial compared VenR, VenObi, ibrutinib + obinutuzumab + venetoclax (IbObiVen) (ibrutinib was discontinued or extended based on the uMRD status), or CIT (FCR or BR) in 926 fit TN-CLL patients without del(17p) or TP53 mutation. IbObiVen improved 3-year-PFS (90.5% vs 75.5%, HR 0.32, p < 0.001) and uMRD at 15 months (92.2% vs 52.0%, p < 0.001) compared to CIT. However, the addition of ibrutinib to VenObi did lead to an increase in G3 or 4 infections (21.2% vs 13.2%).52
 |
Table 2 Phase 3 Clinical Trials for BTKi and BCL2i Combinations |
Novel Targeted Therapies for Double Refractory Disease
Non-Covalent BTK Inhibitors
As described above, the most common secondary resistance mechanism to cBTKi are BTK C481 mutations. Therefore, novel agents that non-covalently bind to BTK at non-C481 sites have been developed with promising clinical efficacy. Pirtobrutinib (LOXO-305) is a novel oral non-covalent BTK inhibitor (ncBTKi), with activity against both wild-type and C481-mutated BTK.84 Currently, the phase 1/2 BRUIN trial is investigating the safety and efficacy of pirtobrutinib for B-cell malignancies including CLL/SLL. A recent analysis of the ongoing BRUIN trial included 282 CLL/SLL patients who had received a previous BTKi with a median number of prior therapies of 4 (range 1–11). The ORR, ORR including partial response with lymphocytosis (PR-L) and mPFS were 72%, 82% and 19.4 months. In the subgroup of those who had received prior BTKi and BCL2i, the ORR including PR-L and mPFS were 79.7% and 15.9 months.85 In December 2023, pirtobrutinib achieved accelerated approval from the FDA for the third or later-line treatment of CLL/SLL.86
Nemtabrutinib (MK-1026, formerly ARQ-531) is another ncBTKi with high potency against both wild-type and C481S-mutated BTK. In a recent analysis of the phase 1/2 BELLWAVE-001 trial, 57 patients with CLL/SLL were treated at the recommended phase 2 dose of 65 mg. Among them, 54 patients (95%) had a prior BTKi, 24 patients (42%) had both prior BTKi and venetoclax, and 36 patients (63%) had BTK C481S mutation. With the median follow-up of 8.1 months, ORR, median duration of response (mDoR) and mPFS were 56%, 24.4 months and 26.3 months, respectively. In those with prior BTKi and venetoclax, ORR, mDoR and mPFS were 58%, 8.5 months and 10.1 months, respectively.87
BTK Degraders
Another BTK-targeted approach to overcome the resistance to cBTKis and ncBTKis is the degradation of the BTK protein itself rather than the inhibition of the BTK function. BTK degraders induce catalytic ubiquitination of BTK via recruitment of the cereblon E3 ubiquitin ligase complex, leading to BTK degradation by the proteasome.88 Recent studies reported acquired BTK mutations including T474I gatekeeper mutations on the treatment with ncBTKis, and BTK degraders can bind to BTK proteins with T474 mutations.89 NX-2127 is a novel oral BTK degrader that also degrades IKAROS family zinc finger 1 (IKZF1) and IKZF3, inducing immunomodulatory activity.90 Recently, preliminary data of the phase 1 NX-2127-001 trial have been reported. Among 17 RR-CLL patients, all had received a prior BTKi and 13 patients (76.5%) had also received venetoclax. In 12 response-evaluable CLL patients, although the best ORR was 33%, ORR increased with longer follow-up (16.7% at 2 months, 42.9% at 4 months, and 50% at 6 months). Responses were also observed in double-refractory patients and those who progressed on a ncBTKi.91
NX-5948 is an oral small molecule that selectively degrades BTK. In a preclinical study, NX-5948 catalysed the rapid degradation of BTK and potently inhibited B-cell activation in human PBMCs. Oral administration of NX-5948 demonstrated the degradation of BTK in mice and cynomolgus monkeys.92 Preliminary data of the ongoing phase 1 NX-5948-301 trial showed rapid, robust and sustained BTK degradation with NX-5948 at 50 mg and 100 mg dose levels in patients with RR B-cell malignancies.88 In addition to NX-2127 and NX-5948, other BTK degraders: BGB-16673 (NCT05006716 and NCT05294731), ABBV-101 (NCT05753501), and AC676 (NCT05780034) are also currently under clinical investigation for B-cell malignancies including CLL.
Novel BCL2 Targeted Therapies
Novel BCL2is are currently under development and there are some preliminary data for RR-CLL, although the clinical data among patients who progressed on venetoclax are very limited. Lisaftoclax is a highly selective and potent BCL2i. Preliminary data with 141 RR-CLL/SLL patients in the phase 2 trial have recently been reported. Seventeen patients (12%) had progressed on a BTKi (n = 15) and/or venetoclax (n = 3). The ORRs with lisaftoclax monotherapy, lisaftoclax + acalabrutinib, and lisaftoclax + rituximab were 65%, 98%, and 87%, respectively.93 Sonrotoclax (BGB-11417) is a novel highly selective and potent BCL2i with a favourable pharmacokinetics profile and a broad therapeutic index.94 According to the preliminary data for CLL/SLL in the phase 1 BGB-11417-101 trial, the ORRs with sonrotoclax monotherapy and in combination with zanubrutinib were 67% (6/8) and 95% (19/25), respectively.95 LOXO-338 is a novel BCL2i developed to achieve selectivity over BCL-XL.96 The phase 1 LOXO-BCL-20001 trial is currently evaluating LOXO-338 monotherapy and in combination with pirtobrutinib in RR B-cell malignancies including CLL/SLL (NCT05024045).
Immunotherapeutic Approaches
Recent advancements in cancer immunotherapy have dramatically changed the treatment landscape in hematological malignancies as well as solid tumors.97 In CLL, observations regarding the immunological effect of allogenic hematopoietic stem cell transplantation (graft-versus-leukemia effect) provide a rationale to develop immunotherapeutic approaches to boost anti-tumor immune responses. Currently, several novel antibody-based and adoptive immunotherapies have shown promising efficacy as a monotherapy or in combination with targeted therapies in heavily pre-treated RR-CLL.98
Monoclonal Antibodies (mAbs)
CD20 has been considered a safe and effective target for B-cell malignancies including CLL.99 Rituximab and obinutuzumab have been widely used for the treatment of CLL in combination with chemotherapies and targeted therapies.17 Ofatumumab is a fully humanised anti-CD20 mAb, which has shown efficacy in combination with chemotherapies and PI3K inhibitors.100–102 Although these anti-CD20 mAbs can be used as monotherapy in later lines, the clinical efficacy is very limited for patients who progressed on venetoclax treatment, which is usually combined with an anti-CD20 mAb.59 Another commonly targeted antigen is CD19, which is continuously expressed on the surface of all stages of B cells.103 Tafasitamab, a novel anti-CD19 mAb with enhanced CD16 affinity, has shown promising efficacy in RR-CLL. In a cohort of the phase 2 COSMOS trial, 11 patients who had received a prior BTKi were treated with tafasitamab in combination with idelalisib. One patient also had received previous treatment with venetoclax. The best ORR was 90.9% and uMRD was achieved in 2 of 8 patients.104 Currently, tafasitamab in combination with zanubrutinib for TN-CLL (NCT05718869), in combination with parsaclisib, a next-generation PI3Kδ inhibitor, for RR-NHL or CLL (NCT04809467), and in combination with acalabrutinib and obinutuzumab for TN-CLL (NCT05943496) are under clinical investigation.
Immune checkpoint inhibitors (ICIs) block inhibitory receptors on immune cells or their ligands on tumor cells such as programmed cell death protein 1 (PD1) and programmed death-ligand 1 (PD-L1) to overcome tumor immune escape mechanisms, leading to remarkable long-term tumor responses in many types of solid cancers.98 Anti-PD1/PD-L1 antibodies showed significant efficacy results in multiple hematological malignancies such as Hodgikin’s lymphomas and diffuse large B-cell lymphoma (DLBCL).105,106 However, the clinical efficacy of ICI monotherapy for CLL is disappointing which is likely to reflect the pseudo-exhausted state of T-cells in CLL.107 In a small phase 2 trial with pembrolizumab, an anti-PD1 antibody, the ORRs in 16 RR-CLL and 9 RS patients were 0% and 44%, respectively.108 For the treatment of RS, combination strategies with targeted therapies have shown promising results. Nivolumab, an anti-PD1 antibody, in combination with ibrutinib reported an ORR of 46%.109 Furthermore, atezolizumab, an anti-PD-L1 antibody, in combination with venetoclax and obinutuzumab showed a 100% response rate for previously untreated RS.110
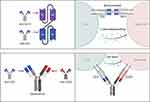
Bispecific Antibodies
A novel promising immunotherapeutic approach has been the development of bispecific antibodies (bsAbs), which redirect T cells to tumor cells, leading to potent tumor cell lysis (Figure 3). Currently, many bsAbs based on a variety of platforms, are under clinical development in hematological malignancies as well as solid cancers.111 Blinatumomab, a CD3/CD19 bispecific T cell engager (BiTE), was the first bsAb evaluated for the treatment of CLL. In a small phase 2 trial with 9 heavily pre-treated patients with RS, blinatumomab treatment was well tolerated with no G3 or higher cytokine release syndrome (CRS) and 1 case of G3 neurotoxicity. However, the ORR and mPFS were only 22% and 1.9 months.112 Currently, phase 1 studies are evaluating blinatumomab and lenalidomide combination in non-Hodgkin lymphoma (NHL) including SLL (NCT02568553) and blinatumomab-expanded T cells in indolent NHL and CLL (NCT03823365).
CD3/CD20-targeting bsAbs have also shown promising efficacy for RS. Epcoritamab is a subcutaneously administered CD3/CD20 bsAb, redirecting and activating T cells to kill CD20-expressing tumor cells. The phase 1b/2 EPCORE CLL-1 trial is investigating epcoritamab monotherapy and in combination with venetoclax for RR-CLL and epcoritamab monotherapy and in combination with lenalidomide or R-CHOP for RS.113 Initial data included 10 patients with RS who were treated with epcoritamab monotherapy. No cases of G3 or more CRS and no case of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. The ORR was 60% with a complete response rate of 50%.114 Epcoritamab was granted accelerated approval from the FDA for the treatment of adult RR-DLBCL including DLBCL arising from indolent lymphoma in May 2023.115 Glofitamab, a CD3/CD20 bsAb with two anti-CD20 binding domains, also showed promising efficacy for RS. In the phase 1/2 trial with 11 patients with RS, the ORR and complete response rate were 63.6% and 45.5%, respectively. Glofitamab showed a manageable safety profile with 2 cases of G3 or 4 CRS and 1 case of G3 ICANS.116 Glofitamab was also granted accelerated approval from the FDA for the treatment of adult RR-DLBCL in June 2023.117
Chimeric Antigen Receptor T Cell Therapies
In addition to antibody-based therapies, chimeric antigen receptor (CAR)-T cell therapies have shown remarkable results with long-term complete remissions in hematological malignancies such as DLBCL and B-cell acute lymphoblastic leukemia (ALL).118–120 In autologous CAR-T cell therapies, T cells derived from the patient are genetically engineered to express CARs on the cell surface. A CAR is composed of a single chain variable fragment (scFV) that recognises a specific tumor antigen and intracellular T-cell activation domains. The CAR-T cells are infused back into the patient and recognise the target antigens on the tumor cells, inducing downstream signals to kill the tumor cells. Although the first experimental use of CAR-T cells was reported as the treatment of CLL in 2011,121 further development was outpaced by other hematological malignancies and CAR-T therapies are still in the investigational stage for CLL.122
The phase 1/2 TRANSCEND CLL004 trial has recently reported the preliminary data on lisocabtagene maraleucel (liso-cel), an autologous CD19-targeting CAR-T cell product, with 118 RR-CLL/SLL patients. All patients had received a previous BTKi. In the primary efficacy analysis set, 50 patients received liso-cel at a target dose of 100 × 106 CAR T cells after lymphodepleting chemotherapy. Among them, the rate of complete response or remission (CR) and CR with incomplete marrow recovery was 20%.123 In order to improve the clinical outcomes of CAR-T cell therapies for CLL, there are some challenges to overcome. As the loss of CD-19 represents one of the main causes of relapse, other target molecules are being investigated.124 MB-106 is a third-generation CD20-targeted CAR-T cell product. In the initial report from the ongoing phase 1/2 trial for high-risk B-NHL and CLL, 16 patients including 1 CLL patient were treated with MB-106. The ORR was 94% and the CLL patient had a complete response and uMRD in peripheral blood and bone marrow.125 Other potential target antigens that are highly expressed on CLL cells include receptor tyrosine kinase-like orphan receptor 1 (ROR1) and Fc receptor for immunoglobulin M (FcμR).126,127 Furthermore, as ibrutinib has beneficial effects on the tumor microenvironment and can expand CAR-T cells, a combination strategy may overcome the resistance to CD19-targeted CAR-T therapies.128 In the phase 1/2 TRANSCEND CLL004 trial, ibrutinib and liso-cel combination showed an ORR of 95% in 19 RR-CLL patients who had previously received ibrutinib.129
Conclusion
In the last 10–15 years, small molecule agents targeting key proteins in BCR-signaling and intrinsic apoptotic pathways have significantly improved clinical outcomes of CLL. Current treatment algorithms with cBTKi- and venetoclax-based therapies based on patient characteristics and treatment responses can bring long-term survival to patients with this incurable disease. Extensive biological research has revealed the mechanisms that underlie resistance to cBTKis and venetoclax, enabling the clinical development of novel targeted agents. The promising preliminary data of ncBTKis and BTK degraders suggest that the use of these BTK-targeted agents may be the next treatment approach for double refractory disease. In addition, novel cancer immunotherapies such as bsAbs and CAR-T therapies can expand the treatment armamentarium for RR-CLL including double-refractory disease. Considering the distinct mechanisms of action, combination strategies with immunotherapies and targeted therapies are also being extensively investigated. As described in this article, there are a wide array of potential new treatment strategies for double-refractory disease (Figure 4). In order to accelerate the clinical application of these treatments, further research on reliable biomarkers for appropriate patient selection and treatment sequence is necessary. Furthermore, effective collaboration among academia, industry, regulatory agents, and patients in multiple regions is increasingly important to efficiently conduct patient-centered clinical trials of novel treatments for CLL.
Abbreviations
AcalaObi, acalabrutinib + obinutuzumab; AE, adverse event; AKT, protein kinase B; ALL, acute lymphoblastic leukemia; BAD, BCL2 associated agonist of cell death; BAK, BCL2 antagonist/killer; BAX, BCL2 associated X-protein; BCL2, B-cell lymphoma 2; BCL2i, BCL2 inhibitor; BCL-XL, B-cell lymphoma-extra large; BCR, B-cell receptor; BH3, BCL2 homology domain 3; BIM, BCL2 interacting mediator of cell death; BiTE, bispecific T cell engager; BLNK, B-cell linker; BOVen, zanubrutinib + obinutuzumab + venetoclax; BR, bendamustine + rituximab; bsAb, bispecific antibody; BTK, Bruton tyrosine kinase; cBTKi, covalent BTK inhibitor; CAR, chimeric antigen receptor; ChlObi, chlorambucil + obinutuzumab; CI, confidence interval; CIT, chemoimmunotherapy; CLL, chronic lymphocytic leukemia; CR, complete response or remission; CRS, cytokine release syndrome; ERK, extracellular signal-regulated kinase; FCR, fludarabine + cyclophosphamide + rituximab; FCμR, Fc receptor for immunoglobulin M; FDA, US Food and Drug Administration; G, grade; HR, hazard ratio; IbObi, ibrutinib + obinutuzumab; IbR, ibrutinib + rituximab; IbVen, ibrutinib + venetoclax; ICI, immune checkpoint inhibitor; ICANS, immune effector cell-associated neurotoxicity syndrome; IdR, idelalisib + rituximab; IGHV, immunoglobulin heavy chain variable region; IKZF, IKAROS family zinc finger; mAb, monoclonal antibody; MAIC, matching-adjusted indirect comparison; MAPK, mitogen-activated protein kinase; MCL1, myeloid cell leukemia-1; mDoR, median duration of response; mOS, median overall survival; mPFS, median progression free survival; mTOR, mammalian target of rapamycin; ncBTKi, non-covalent BTK inhibitor; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; NGS, next-generation sequencing; NHL, non-Hodgkin’s lymphoma; NR, not reached; ORR, overall response rate; OS, overall survival; PBMC, peripheral blood mononuclear cell; PD1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphatidylinositol-3-kinase; PIP3, phosphatidylinositol-3,4,5-triphosphate; PKCβ, protein kinase C beta; PLCG2, phospholipase C gamma 2; PR-L, partial response with lymphocytosis; ROR1, receptor tyrosine kinase-like orphan receptor 1; RR, relapsed or refractory; RR, relative risk; RS, Richter’s syndrome; scFV, single chain variable fragment; SLL, small lymphocytic lymphoma; SYK, spleen tyrosine kinase; TN, treatment naïve; uMRD, undetectable minimal residual disease; VenObi, venetoclax + obinutuzumab; VenR, venetoclax + rituximab.
Acknowledgments
The authors thank Jeff K. Davies for his comments on the manuscript. The figures were created in Biorender.
Disclosure
MH is a former employee of AstraZeneca. JCR reports speakers’ fees from Janssen and expenses from NURIX. The authors report no other conflicts of interest in this work.
References
1. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
2. Santos FP, O’Brien S. Small lymphocytic lymphoma and chronic lymphocytic leukemia: are they the same disease? Cancer J. 2012;18(5):396–403. doi:10.1097/PPO.0b013e31826cda2d
3. Yao Y, Lin X, Li F, Jin J, Wang H. The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: analysis based on the global burden of disease study 2019. Biomed Eng Online. 2022;21(1):4. doi:10.1186/s12938-021-00973-6
4. Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi:10.1182/blood-2015-06-651125
5. Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. doi:10.1016/s1470-2045(16)30051-1
6. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. doi:10.1056/NEJMoa1313984
7. Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148–167. doi:10.1038/nrc.2017.121
8. de Claro RA, McGinn KM, Verdun N, et al. FDA approval: ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2015;21(16):3586–3590. doi:10.1158/1078-0432.ccr-14-2225
9. Eyre TA, Riches JC. The evolution of therapies targeting Bruton tyrosine kinase for the treatment of chronic lymphocytic leukaemia: future perspectives. Cancers. 2023;15(9):2596. doi:10.3390/cancers15092596
10. Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801. doi:10.1097/hs9.0000000000000801
11. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/s0140-6736(20)30262-2
12. Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2023;388(4):319–332. doi:10.1056/NEJMoa2211582
13. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–1043. doi:10.1016/s1470-2045(22)00293-5
14. Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10(3):456–459.
15. Kater AP, Wu JQ, Kipps T, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO Phase III study. J Clin Oncol. 2020;38(34):4042–4054. doi:10.1200/jco.20.00948
16. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi:10.1056/NEJMoa1815281
17. Mato AR, Hess LM, Chen Y, et al. Outcomes for Patients With Chronic Lymphocytic Leukemia (CLL) previously treated with both a covalent BTK and BCL2 Inhibitor in the United States: a Real-World Database Study. Clin Lymphoma Myeloma Leuk. 2023;23(1):57–67. doi:10.1016/j.clml.2022.09.007
18. Rolli V, Gallwitz M, Wossning T, et al. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10(5):1057–1069. doi:10.1016/s1097-2765(02)00739-6
19. Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–1184. doi:10.1182/blood-2012-02-362624
20. Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592–601. doi:10.1016/j.it.2013.07.002
21. Stamatopoulos K, Agathangelidis A, Rosenquist R, Ghia P. Antigen receptor stereotypy in chronic lymphocytic leukemia. Leukemia. 2017;31(2):282–291. doi:10.1038/leu.2016.322
22. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi:10.1038/nrc883
23. Bennett R, Thompson E, Tam C; SOHO State of the Art Updates and Next Questions. Mechanisms of resistance to BCL2 inhibitor therapy in chronic lymphocytic leukemia and potential future therapeutic directions. Clin Lymphoma Myeloma Leuk. 2022;22(11):795–804. doi:10.1016/j.clml.2022.07.013
24. Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–1705. doi:10.1002/ajh.26367
25. Stephens DM. NCCN guidelines update: chronic lymphocytic leukemia/small lymphocytic lymphoma. J Natl Compr Canc Netw. 2023;21(5.5):563–566. doi:10.6004/jnccn.2023.5007
26. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075–13080. doi:10.1073/pnas.1004594107
27. Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi:10.1182/blood-2011-01-328484
28. Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi:10.1182/blood-2011-10-386417
29. de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi:10.1182/blood-2011-11-390989
30. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi:10.1056/NEJMoa1215637
31. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi:10.1056/NEJMoa1400376
32. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. doi:10.1056/NEJMoa1509388
33. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi:10.1056/NEJMoa1817073
34. Moreno C, Greil R, Demirkan F, et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: final analysis of the randomized, phase III iLLUMINATE trial. Haematologica. 2022;107(9):2108–2120. doi:10.3324/haematol.2021.279012
35. Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi:10.1056/NEJMoa1812836
36. Hillmen P, Pitchford A, Bloor A, et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(5):535–552. doi:10.1016/s1470-2045(23)00144-4
37. Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112–120. doi:10.1182/blood.2021014960
38. Xiao L, Salem J-E, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal src kinase. Circulation. 2020;142(25):2443–2455. doi:10.1161/CIRCULATIONAHA.120.049210
39. Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332. doi:10.1056/NEJMoa1509981
40. Herman SEM, Montraveta A, Niemann CU, et al. The Bruton Tyrosine Kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831–2841. doi:10.1158/1078-0432.ccr-16-0463
41. Sharma S, Pepin X, Burri H, et al. Bioequivalence and relative bioavailability studies to assess a new acalabrutinib formulation that enables coadministration with proton-pump inhibitors. Clin Pharmacol Drug Dev. 2022;11(11):1294–1307. doi:10.1002/cpdd.1153
42. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized Phase III trial. J Clin Oncol. 2021;39(31):3441–3452. doi:10.1200/jco.21.01210
43. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naive chronic lymphocytic leukemia: 6-year follow-up of elevate-TN. Blood. 2023;142(Suppl 1):636. doi:10.1182/blood-2023-174750
44. Molica S, Giannarelli D, Montserrat E. Comparison between venetoclax-based and Bruton tyrosine kinase inhibitor-based therapy as upfront treatment of Chronic Lymphocytic Leukemia (CLL): a systematic review and network meta-analysis. Clin Lymphoma Myeloma Leuk. 2021;21(4):216–223. doi:10.1016/j.clml.2020.10.012
45. Davids MS, Telford C, Abhyankar S, Waweru C, Ringshausen I. Matching-adjusted indirect comparisons of safety and efficacy of acalabrutinib versus other targeted therapies in patients with treatment-naïve chronic lymphocytic leukemia. Leuk Lymphoma. 2021;62(10):2342–2351. doi:10.1080/10428194.2021.1913144
46. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi:10.1182/blood.2019001160
47. Brown JR, Eichhorst BF, Lamanna N, et al. Extended follow-up of ALPINE randomized Phase 3 study confirms sustained superior progression-free survival of zanubrutinib versus ibrutinib for treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma (R/R CLL/SLL). Blood. 2023;142(Suppl 1):202. doi:10.1182/blood-2023-174289
48. Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778. doi:10.1016/s1470-2045(16)30019-5
49. Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2015;374(4):311–322. doi:10.1056/NEJMoa1513257
50. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi:10.1056/NEJMoa1713976
51. Al-Sawaf O, Robrecht S, Zhang C, et al. S145: venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized CLL14 Study. Hemasphere. 2023;7(Suppl):e064430a. doi:10.1097/01.HS9.0000967492.06443.0a
52. Eichhorst B, Niemann CU, Kater AP, et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388(19):1739–1754. doi:10.1056/NEJMoa2213093
53. Hallek M. First line therapy of CLL. Hematol Oncol. 2023;41(Supple 1):129–135. doi:10.1002/hon.3145
54. Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65–75. doi:10.1016/s1470-2045(17)30909-9
55. Greil R, Fraser G, Leber B, et al. Efficacy and Safety of Ibrutinib (IBR) after Venetoclax (VEN) Treatment in IBR-Naïve Patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL): follow-up of patients from the MURANO study. Blood. 2018;132(Suppl 1):5548. doi:10.1182/blood-2018-99-118148
56. Lin VS, Lew TE, Handunnetti SM, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135(25):2266–2270. doi:10.1182/blood.2020004782
57. Thompson MC, Harrup RA, Coombs CC, et al. Venetoclax retreatment of patients with chronic lymphocytic leukemia after a previous venetoclax-based regimen. Blood Adv. 2022;6(15):4553–4557. doi:10.1182/bloodadvances.2022007812
58. Skarbnik A, Miranda M, Yong AS, et al. A matching-adjusted indirect comparison (MAIC) of the efficacy and safety of acalabrutinib (acala) versus zanubrutinib (zanu) in relapsed or refractory chronic lymphocytic leukemia (RR CLL). J Clin Oncol. 2023;41(16 Suppl):7540. doi:10.1200/JCO.2023.41.16_suppl.7540
59. Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589–3596. doi:10.1158/1078-0432.ccr-19-3815
60. Mato AR, Davids MS, Sharman J, et al. Recognizing unmet need in the era of targeted therapy for CLL/SLL: “What’s Past Is Prologue” (Shakespeare). Clin Cancer Res. 2022;28(4):603–608. doi:10.1158/1078-0432.ccr-21-1237
61. Aronson JH, Skånland SS, Roeker LE, Thompson MC, Mato AR. Approach to a patient with “double refractory” chronic lymphocytic leukemia: “Double, double toil and trouble” (Shakespeare). Am J Hematol. 2022;97(Suppl 2):S19–S25. doi:10.1002/ajh.26682
62. Lew TE, Lin VS, Cliff ER, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021;5(20):4054–4058. doi:10.1182/bloodadvances.2021005083
63. Thompson MC, Roeker LE, Coombs CC, et al. Addressing a new challenge in chronic lymphocytic leukemia: outcomes of therapies after exposure to both a covalent Bruton’s tyrosine kinase inhibitor and venetoclax. Blood. 2021;138(Suppl 1):2628. doi:10.1182/blood-2021-150751
64. Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370(24):2352–2354. doi:10.1056/NEJMc1402716
65. Nakhoda S, Vistarop A, Wang YL. Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br J Haematol. 2023;200(2):137–149. doi:10.1111/bjh.18418
66. Quinquenel A, Fornecker L-M, Letestu R, et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: a FILO group study. Blood. 2019;134(7):641–644. doi:10.1182/blood.2019000854
67. Woyach J, Huang Y, Rogers K, et al. Resistance to acalabrutinib in CLL is mediated primarily By BTK mutations. Blood. 2019;134(Suppl 1):504. doi:10.1182/blood-2019-127674
68. Handunnetti SM, Tang CPS, Nguyen T, et al. BTK Leu528Trp - a potential secondary resistance mechanism specific for patients with chronic lymphocytic leukemia treated with the next generation BTK inhibitor zanubrutinib. Blood. 2019;134(Suppl 1):170. doi:10.1182/blood-2019-125488
69. Blombery P, Thompson ER, Lew TE, et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: potential for pirtobrutinib cross-resistance. Blood Adv. 2022;6(20):5589–5592. doi:10.1182/bloodadvances.2022008325
70. Wang E, Mi X, Thompson MC, et al. Mechanisms of resistance to noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735–743. doi:10.1056/NEJMoa2114110
71. Blombery P, Anderson MA, Gong J-N, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342–353. doi:10.1158/2159-8290.cd-18-1119
72. Tausch E, Close W, Dolnik A, et al. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica. 2019;104(9):e434–e437. doi:10.3324/haematol.2019.222588
73. Blombery P, Thompson ER, Nguyen T, et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135(10):773–777.
74. Lucas F, Larkin K, Gregory CT, et al. Novel BCL2 mutations in venetoclax-resistant, ibrutinib-resistant CLL patients with BTK/PLCG2 mutations. Blood. 2020;135(24):2192–2195.
75. Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia. 2017;31(10):2075–2084. doi:10.1038/leu.2017.32
76. Kater AP, Slinger E, Cretenet G, et al. Combined ibrutinib and venetoclax treatment vs single agents in the TCL1 mouse model of chronic lymphocytic leukemia. Blood Adv. 2021;5(23):5410–5414. doi:10.1182/bloodadvances.2021004861
77. Wierda WG, Allan JN, Siddiqi T, et al. Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized Phase II CAPTIVATE study. J Clin Oncol. 2021;39(34):3853–3865. doi:10.1200/JCO.21.00807
78. Ghia P, Wierda WG, Barr PM, et al. Relapse after first-line fixed duration ibrutinib + venetoclax: high response rates to ibrutinib retreatment and absence of BTK mutations in patients with Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) with up to 5 years of follow-up in the Phase 2 Captivate Study. Blood. 2023;142(Suppl 1):633. doi:10.1182/blood-2023-187128
79. Soumerai JD, Mato AR, Dogan A, et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021;8(12):e879–e890. doi:10.1016/S2352-3026(21)00307-0
80. Kater AP, Owen C, Moreno C, et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. 2022;1(7):EVIDoa2200006. doi:10.1056/EVIDoa2200006
81. Moreno C, Munir T, Owen C, et al. First-line fixed-duration Ibrutinib Plus Venetoclax (Ibr+Ven) versus Chlorambucil Plus Obinutuzumab (Clb+O): 55-month follow-up from the Glow Study. Blood. 2023;142(Suppl 1):634. doi:10.1182/blood-2023-177713
82. Janssen. European Commission Approves IMBRUVICA® (ibrutinib) in a fixed-duration combination regimen for adult patients with previously untreated Chronic Lymphocytic Leukaemia (CLL) [press release]; 2022 [August 4]. Available from: https://www.jnj.com/european-commission-approves-imbruvica-ibrutinib-in-a-fixed-duration-combination-regimen-for-adult-patients-with-previously-untreated-chronic-lymphocytic-leukaemia-cll.
83. Hillmen P, Cairns DA, Bloor A, et al. Ibrutinib Plus Venetoclax with MRD-directed duration of treatment is superior to FCR and is a new standard of care for previously untreated CLL: report of the Phase III UK NCRI FLAIR Study. Blood. 2023;142(Suppl 1):631. doi:10.1182/blood-2023-178298
84. Aslan B, Kismali G, Iles LR, et al. Pirtobrutinib inhibits wild-type and mutant Bruton’s tyrosine kinase-mediated signaling in chronic lymphocytic leukemia. Blood Cancer J. 2022;12(5):80. doi:10.1038/s41408-022-00675-9
85. Woyach JA, Brown JR, Ghia P, et al. Pirtobrutinib in Post-cBTKi CLL/SLL: ~30 months follow-up and subgroup analysis with/without prior BCL2i from the Phase 1/2 BRUIN Study. Blood. 2023;142(Suppl 1):325. doi:10.1182/blood-2023-185852
86. Eli Lilly and Company. Jaypirca® (pirtobrutinib) Now Approved by U.S. FDA for the treatment of adult patients with chronic lymphocytic leukemia or small lymphocytic lymphoma who have received at least two lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor [press release]. 2023 [December 1]. Available from: https://investor.lilly.com/news-releases/news-release-details/jaypircar-pirtobrutinib-now-approved-us-fda-treatment-adult.
87. Woyach JA, Flinn IW, Awan FT, et al. Efficacy and safety of nemtabrutinib, a wild-type and C481S-mutated Bruton tyrosine kinase inhibitor for B-cell malignancies: updated analysis of the open-label Phase 1/2 Dose-Expansion Bellwave-001 Study. Blood. 2022;140(Suppl 1):7004–7006. doi:10.1182/blood-2022-163596
88. Linton K, Forconi F, Lewis D, et al. Robust Bruton’s tyrosine kinase (BTK) degradation with NX-5948, an oral BTK degrader, in a first-in-human phase 1a trial in relapsed/refractory B cell malignancies. Hematol Oncol. 2023;41(Suppl 2):573–574. doi:10.1002/hon.3164_428
89. Portelinha A, Wendel HG. The cat-and-mouse game of BTK inhibition. Blood. 2023;141(13):1502–1503. doi:10.1182/blood.2022018936
90. Mihalic JT, Brathaban N, Bravo B, et al. Abstract 3423: NX-2127: a first-in-class clinical stage degrader of BTK and IKZF1/3 for the treatment of patients with B cell malignancies. Cancer Res. 2023;83(7 Suppl):3423. doi:10.1158/1538-7445.am2023-3423
91. Mato AR, Wierda WG, Ai WZ, et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-Targeted Protein Degrader, in patients with relapsed or refractory chronic lymphocytic leukemia and B-cell malignancies. Blood. 2022;140(Suppl 1):2329–2332. doi:10.1182/blood-2022-164772
92. Robbins D, Noviski M, Tan M, et al. POS0006 NX-5948, a selective degrader of btk, significantly reduces inflammation in a model of autoimmune disease. Ann Rheum Dis. 2021;80(Suppl 1):204. doi:10.1136/annrheumdis-2021-eular.1675
93. Davids MS, Chanan-Khan A, Mudenda B, et al. Lisaftoclax (APG-2575) safety and activity as monotherapy or combined with acalabrutinib or rituximab in patients (pts) with treatment-naïve, relapsed or Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (R/R CLL/SLL): initial data from a Phase 2 Global Study. Blood. 2022;140(Suppl 1):2326–2328. doi:10.1182/blood-2022-160386
94. Soumerai JD, Lasica M, Opat S, et al. A Phase 1 Study with the Novel B-Cell Lymphoma 2 (Bcl-2) Inhibitor Bgb-11417 As Monotherapy or in Combination with Zanubrutinib (ZANU) in Patients (Pts) with Non-Hodgkin Lymphoma (NHL) or Waldenström Macroglobulinemia (WM): preliminary data. Blood. 2022;140(Suppl 1):9325–9327. doi:10.1182/blood-2022-169664
95. Cheah CY, Tam CS, Lasica M, et al. A Phase 1 Study with the Novel B-Cell Lymphoma 2 (Bcl-2) Inhibitor Bgb-11417 as monotherapy or in Combination with Zanubrutinib (ZANU) in Patients (Pts) with CLL/SLL: preliminary data. Blood. 2022;140(Suppl 1):2321–2323. doi:10.1182/blood-2022-169662
96. Brandhuber B, Ku K, Lallena MJ, et al. Abstract 1258: preclinical characterization of LOXO-338, a novel, oral and selective BCL2 inhibitor. Cancer Res. 2021;81(13 Suppl):1258. doi:10.1158/1538-7445.am2021-1258
97. Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: current progress and prospects. Front Immunol. 2022;13:961805. doi:10.3389/fimmu.2022.961805
98. Perutelli F, Jones R, Griggio V, Vitale C, Coscia M. Immunotherapeutic strategies in chronic lymphocytic leukemia: advances and challenges. Front Oncol. 2022;12:837531. doi:10.3389/fonc.2022.837531
99. Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin Hematol. 2010;47(2):107–114. doi:10.1053/j.seminhematol.2010.01.001
100. Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385(9980):1873–1883. doi:10.1016/S0140-6736(15)60027-7
101. Cortelezzi A, Sciumè M, Liberati AM, et al. Bendamustine in combination with Ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia. 2014;28(3):642–648. doi:10.1038/leu.2013.334
102. Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–e126. doi:10.1016/S2352-3026(17)30019-4
103. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. doi:10.1186/2162-3619-1-36
104. Staber PB, Jurczak W, Greil R, et al. Tafasitamab combined with idelalisib or venetoclax in patients with CLL previously treated with a BTK inhibitor. Leuk Lymphoma. 2021;62(14):3440–3451. doi:10.1080/10428194.2021.1964020
105. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N Engl J Med. 2014;372(4):311–319. doi:10.1056/NEJMoa1411087
106. Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. doi:10.1182/blood-2017-07-740993
107. Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–1621. doi:10.1182/blood-2012-09-457531
108. Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419–3427. doi:10.1182/blood-2017-02-765685
109. Jain N, Senapati J, Thakral B, et al. A phase 2 study of nivolumab combined with ibrutinib in patients with diffuse large B-cell Richter transformation of CLL. Blood Adv. 2023;7(10):1958–1966. doi:10.1182/bloodadvances.2022008790
110. Jain N, Ferrajoli A, Thompson PA, et al. Venetoclax, obinutuzumab and atezolizumab (PD-L1 Checkpoint Inhibitor) for treatment for patients with Richter transformation. Blood. 2021;138(Suppl 1):1550. doi:10.1182/blood-2021-154279
111. Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276. doi:10.3389/fimmu.2022.1035276
112. Thompson PA, Jiang X, Banerjee P, et al. A phase two study of high dose blinatumomab in Richter’s syndrome. Leukemia. 2022;36(9):2228–2232. doi:10.1038/s41375-022-01649-3
113. Eichhorst B, Eradat H, Niemann CU, et al. Epcoritamab monotherapy and combinations in relapsed or refractory chronic lymphocytic leukemia or Richter’s Syndrome: new escalation and expansion cohorts in epcore Cll-1. Hematol Oncol. 2023;41(Suppl 2):828–829. doi:10.1002/hon.3166_OT10
114. Kater AP, Ye JC, Sandoval-Sus J, et al. Subcutaneous epcoritamab in patients with Richter’s syndrome: early results from Phase 1b/2 Trial (EPCORE CLL-1). Blood. 2022;140(Suppl 1):850–851. doi:10.1182/blood-2022-158298
115. Abbvie. EPKINLY™ (epcoritamab-bysp) Approved by U.S. FDA as the first and only bispecific antibody to treat adult patients with relapsed or refractory Diffuse Large B-Cell Lymphoma (DLBCL) [press release]; 2023 [May 19]. Available from: https://news.abbvie.com/news/press-releases/epkinly-epcoritamab-bysp-approved-by-us-fda-as-first-and-only-bispecific-antibody-to-treat-adult-patients-with-relapsed-or-refractory-diffuse-large-b-cell-lymphoma-dlbcl.htm.
116. Carlo-Stella C, Hutchings M, Offner F, et al. Glofitamab monotherapy induces durable complete remissions and has a manageable safety profile in patients with Richter’s transformation. Hematol Oncol. 2023;41(Suppl 2):63–65. doi:10.1002/hon.3163_28
117. Genentech. FDA Approves Genentech’s Columvi, the first and only bispecific antibody with a fixed-duration treatment for people with relapsed or refractory diffuse large B-cell lymphoma [press release]; 2023 [June 15]. Available from: https://www.gene.com/media/press-releases/14994/2023-06-15/fda-approves-genentechs-columvi-The-firs.
118. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
119. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018;380(1):45–56. doi:10.1056/NEJMoa1804980
120. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi:10.1056/NEJMoa1709866
121. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi:10.1126/scitranslmed.3002842
122. Todorovic Z, Todorovic D, Markovic V, et al. CAR T cell therapy for chronic lymphocytic leukemia: successes and shortcomings. Curr Oncol. 2022;29(5):3647–3657. doi:10.3390/curroncol29050293
123. Siddiqi T, Maloney DG, Kenderian SS, et al. Lisocabtagene Maraleucel (liso-cel) in R/R CLL/SLL: 24-month median follow-up of TRANSCEND CLL 004. Blood. 2023;142(Suppl 1):330. doi:10.1182/blood-2023-179529
124. Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi:10.1016/j.csbj.2016.09.003
125. Shadman M, Yeung C, Redman M, et al. Safety and efficacy of third generation CD20 targeted CAR-T (MB-106) for treatment of relapsed/refractory B-NHL and CLL. Blood. 2021;138(Suppl 1):3872. doi:10.1182/blood-2021-149181
126. Hudecek M, Schmitt TM, Baskar S, et al. The B-cell tumor–associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–4541. doi:10.1182/blood-2010-05-283309
127. Faitschuk E, Hombach AA, Frenzel LP, Wendtner CM, Abken H. Chimeric antigen receptor T cells targeting Fc μ receptor selectively eliminate CLL cells while sparing healthy B cells. Blood. 2016;128(13):1711–1722. doi:10.1182/blood-2016-01-692046
128. Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–1127. doi:10.1182/blood-2015-11-679134
129. Wierda WG, Dorritie KA, Munoz J, et al. Transcend CLL 004: Phase 1 cohort of lisocabtagene maraleucel (liso-cel) in combination with ibrutinib for patients with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL). Blood. 2020;136:39–40. doi:10.1182/blood-2020-140622
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.