Back to Journals » International Journal of Women's Health » Volume 16
Superb Micro-Vascular Imaging in Prenatal Ultrasound Diagnosis of Placental Infarction: A Case Report
Received 6 October 2023
Accepted for publication 25 February 2024
Published 1 March 2024 Volume 2024:16 Pages 325—330
DOI https://doi.org/10.2147/IJWH.S440522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Supplementary video of “Micro-Vascular Imaging in Placental Infarction” [440522].
Views: 59
Yun-Zhu Wu,1,2 Qing-Yun Song1,2
1Department of Diagnostic Ultrasound, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Key Laboratory of Obstetric & Gynecologic and Pediatric Diseases and Birth Defects of Ministry of Education, Chengdu, People’s Republic of China
Correspondence: Qing-Yun Song, Department of Diagnostic Ultrasound, West China Second University Hospital, Sichuan University, Chengdu, Sichuan Province, 610041, People’s Republic of China, Email [email protected]
Background: Placental infarction refers to a localized area of ischemic villous necrosis resulting from the interruption of maternal blood flow to the intervillous space, which can be attributed to spasm, stenosis, or occlusion of the decidual spiral artery caused by systemic or localized maternal vascular disease. The presence of large placental infarcts may pose significant risks to fetal well-being, including intrauterine growth retardation, fetal distress, and even fetal demise. Although placental infarction is commonly identified during postnatal pathological examinations, its prenatal diagnosis through ultrasound remains challenging and has been rarely reported.
Case Presentation: This report presents a case of acute placental infarction diagnosed by prenatal ultrasound using Superb Micro-vascular Imaging (SMI) technology. At 23 weeks’ gestation, the ultrasound revealed that the placenta was attached to the left lateral and posterior walls of the uterus, showing localized thickening. Within this area of thickening, there were observed inhomogeneous hypoechoic regions. Superb Micro-vascular Imaging (SMI) revealed an abnormal echogenic region within the thickened placental tissue that lacked microvascular blood flow signals, but showed surrounding vascularity. Visually, this elliptical-shaped echogenic region enveloped by microvascular blood flow. From the 29th weeks of gestation onward, ultrasound suggested that the fetus was small for gestational age. A live baby weighing 2360g was delivered by cesarean section at 37 weeks’ gestation. The placenta was approximately 20× 18 × 3 cm with large grayish-yellow infarcts.
Conclusion: SMI allows rapid screening of large placental infarcts and easy detection of regions without normal vessel trees, thereby reducing missed diagnoses. Infarct area is easily measured by measuring the area surrounded by small blood vessels, especially in acute placental infarction, which is very helpful in accurately determining infarct size.
Keywords: SMI, placental infarction, prenatal ultrasound diagnosis
Background
Placental infarction is common in pregnant women with gestational hypertension, chronic nephritis, primary hypertension, and diabetes. It refers to a localized area of ischemic villous necrosis due to the interruption of the maternal blood flow into the intervillous space due to spasm, stenosis, or occlusion of the decidual spiral artery caused by the systemic or localized maternal vascular disease. Placental infarction can be divided into acute (red) and chronic (white) infarction. Large placental infarcts may pose a threat to the fetus and result in intrauterine growth retardation, fetal distress, fetal death, etc. Placental infarction is often found in pathological placental examinations after birth, without a high diagnostic rate in prenatal ultrasound. Prenatal diagnosis of large placental infarcts has been rarely reported.
Case Presentation
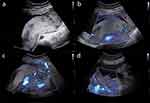
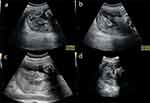
A 28-year-old female patient, gravida 1 para 0, received a 5-day-old blastocyst in our hospital for “bilateral fallopian tube obstruction” and visited our hospital regularly for antenatal care after the blastocyst survived. Her blood pressure, oral glucose tolerance, thyroid function, liver function, and renal function tests were within the normal range. Ultrasound at 23 weeks’ gestation showed no abnormalities in fetal size and structure. The placenta was found to be attached to the left lateral and posterior wall of the uterus, exhibiting localized thickening (Figure 1a). Within this area of thickening, there were observed inhomogeneous hypoechoic regions (Video S1). Superb Micro-vascular Imaging (SMI) revealed an abnormal echogenic region within the thickened placental tissue that exhibited a lack of microvascular blood flow signals, but demonstrated surrounding vascularity. Visually, this elliptical-shaped echogenic region was observed to be enveloped by microvascular blood flow (Figure 1b, Video S2). The maximum longitudinal and anterior-posterior measurements in the sagittal plane, along with the maximum transverse measurements in the transverse plane, were determined for the elliptical region encompassed by microvascular blood flow, measuring approximately 8.3×3.7×8.2 cm. Branching micro-vascular blood flow was observed in the normal placental tissue adjacent to the lesion (Figure 1c and d, Video S3). The lesion was diagnosed as an acute placental infarction. The patient was informed that if the lesion remained stable in size, close monitoring could be conducted; however, there might be potential risks of fetal growth restriction and other related complications in the future. Subsequent ultrasound images revealed a gradual transformation of the lesion into a localized hypoechoic area within the placenta (Figure 2a), accompanied by focal heterogeneous strong echoes (Figure 2b), which gradually diminished in size. The dimensions were approximately 7.0×3.4×5.0 cm at 29 weeks (Figure 2c) and approximately 3.9×3.9×4.2 cm at 36+6 weeks (Figure 2d). From the 29th week of gestation, the ultrasound suggested that the fetus was small for gestational age according to NICHD Asian standards (Figure 3). The estimated fetal weight (EFW) at 29 weeks of gestation was 1021g (0.9%). The systolic/diastolic (S/D) ratio of the fetal umbilical artery flow ranged from 3.97 to 5.82 (95.1–99.8%), and the umbilical artery pulsatility index (UAPI) ranged from 1.35 to 1.61 (97.4–99.9%). At 36+6 weeks of gestation, the EFW was 2334g (6.1%), the S/D ratio of the fetal umbilical artery flow ranged from 2.84 to 3.55(85.3–98.5%), and the UAPI ranged from 1.05 to 1.25(89.2–99.3%). The patient underwent amniocentesis, which revealed no evidence of chromosomal abnormalities. A cesarean section was performed at 37 weeks’ gestation and a live baby was delivered with a length of 46 cm, a weight of 2360 g, an Apgar score of 10-10-10, and a clear amniotic fluid of approximately 700 mL. The placenta measured approximately 20×18 cm and weighed 450 g, with large grayish-yellow infarct (Figure 4).
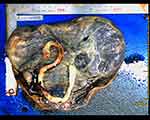 |
Figure 4 Image of the placenta. The placenta was approximately 20×18 cm in size and 450 g in weight, with large grayish-yellow infarcts. |
Discussion and Conclusions
Placental infarction is a condition where a specific area of the placenta experiences ischemic villous necrosis. This occurs due to the interruption of the maternal blood flow into the intervillous space which can be caused by spasm, stenosis, or occlusion of the decidual spiral artery. The underlying cause of this condition is often systemic or localized vascular disease in the pregnant woman. In the early stages, placental infarction appears granular and deep red, also known as red infarction. Under a microscope, the villi are tightly squeezed, causing the intervillous space to almost entirely disappear and the villus capillaries to dilate and congest. Old infarcts are yellowish-white, also known as white infarcts. Under a microscope, a large number of squeezed villi are visible, and the syncytiotrophoblast cells of the villi degenerate to varying degrees, while the capillary occlusion disappears. Naeye’s study of 39,215 placentas found that placental infarction had an incidence of approximately 1/200 and was common in pregnant women with pregnancy complications and comorbidities.1 The incidence of placental infarction, particularly acute red infarction, was high in patients with gestational hypertension and was also common in pregnant women with chronic nephritis, autoimmune disease, and thrombophilia.2–4 Research has shown that placental infarction on the maternal side is highly associated with recurrent miscarriage from an epidemiological perspective. Ischemia or infection-induced and damage to the decidua basalis may trigger infarction. Naeye’s study found that 50% of pregnant women with placental infarction on the maternal side had previously experienced miscarriage or stillbirth.1
Placental infarction typically occurs at the placental edge, often appearing as a thick yellow-white fibrinoid ring that is multifocal.5 While large of complete placental infarctions are rare, those exceeding 10% pose a threat to the fetus. Infarctions of less than 5% do not affect placental function. Placental infarction is associated with fetal growth restriction, increased fetal perinatal morbidity, and a higher risk of fetal death.6 Research has shown that placental infarction is associated with fetal death and stillbirth in up to 17% of patients.7
SMI is a novel technique for imaging blood flow that visualizes low-velocity flow in small vessels. It uses a unique algorithm to minimize motion artifacts. SMI can significantly reduce motion artifacts and display low-velocity blood flow in small vessels compared to traditional blood flow imaging methods such as color Doppler. Research has indicated that the SMI of healthy placentas clearly displays the cotyledon structure, including the branched vessels and surrounding villous tree. In contrast, the villous tree is absent in the SMI of placentas with infarction.8 Although SMI may not differentiate small sinusoids from placental infarcts, this is not a primary concern since small placental infarcts do not impact placental functions. Thus, it was found that SMI can quickly identify large placental infarcts, particularly in cases of acute placental infarction. During acute placental infarction, the infarcted area is not easily distinguishable from the normal placental parenchyma the two-dimensional images and color Doppler ultrasound images, as the infarcted area has similar echoes to the normal placental parenchyma. Additionally, the placenta often attaches to the lateral wall and locally thicken, making infarction difficult to detect during examinations. If the entire placenta is scanned directly using SMI, it will be easier to identify the area without a normal vascular tree, thereby reducing the likelihood of missed diagnoses. The area affected by infarction experiences high tension due to hemorrhage. As a result, small blood vessels surrounding the infarcted area become stretched and form a ring-shaped image. This characteristic can be used to distinguish infarcts from large sinusoids. The villi around the sinusoids maintain a tree structure, instead of surrounding the sinusoids in a ring. The size of the infarcted area can be accurately determined by measuring the area surrounded by blood vessels which is very helpful.
In this case, fetal growth was normal when abnormal placenta was discovered. However, as gestational age increased, the fetus developed abnormal umbilical blood flow and restricted intrauterine growth. The patient initially expressed her concerns about the fetal conditions due to conceiving through IVF. However, after receiving a detailed explanation from the doctor, her anxiety was relieved, and she fully cooperated with the follow-up examinations, including amniocentesis. Finally, a healthy baby was born. Early and timely detection of large placental infarctions can not only help clinicians promptly determine the relevant causes but also provide timely psychological counseling to patients and improve their emotional state.
Abbreviations
SMI, Superb Micro-vascular Imaging; IVF, in vitro fertilization; S/D, systolic/diastolic; EFW, estimated fatal weight; UAPI, umbilical artery pulsatility index; NICHD, National Institute of Child Health and Human Development.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
The need for approval was waived and written consent was obtained.
Consent for Publication
Written consent for publication was obtained from the patient. There are no identifying images or other personal or clinical details of the patient that compromise anonymity in this manuscript.
Acknowledgments
We acknowledge our colleagues from Department of Diagnostic Ultrasound, West China Second University Hospital, Sichuan University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Naeye RL. Maternal floor infarction. Hum Pathol. 1985;16(8):823–828. doi:10.1016/S0046-8177(85)80254-9
2. Bendon RW, Hommel AB. Maternal floor infarction in autoimmune disease: two cases. Pediatr Pathol Lab Med. 1996;16(2):293–297. doi:10.1080/15513819609169291
3. Vinnars MT, Nasiell J, Ghazi S, Westgren M, Papadogiannakis N. The severity of clinical manifestations in preeclampsia correlates with the amount of placental infarction. Acta Obstet Gynecol Scand. 2011;90(1):19–25. doi:10.1111/j.1600-0412.2010.01012.x
4. Taweevisit M, Thorner PS. Maternal floor infarction associated with oligohydramnios and cystic renal dysplasia: report of 2 cases. Pediatr Dev Pathol. 2010;13(2):116–120. doi:10.2350/09-06-0669-CR.1
5. Kondo T. Placental infarction probably associated with late term premature delivery. J Surg Case Rep. 2014;2014(1):rjt125–rjt125. doi:10.1093/jscr/rjt125
6. Blair E, de Groot J, Nelson KB. Placental infarction identified by macroscopic examination and risk of cerebral palsy in infants at 35 weeks of gestational age and over. Am J Obstet Gynecol. 2011;205(2):124 e121–127. doi:10.1016/j.ajog.2011.05.022
7. Vernof KK, Benirschke K, Kephart GM, Wasmoen TL, Gleich GJ. Maternal floor infarction: relationship to X cells, major basic protein, and adverse perinatal outcome. Am J Obstet Gynecol. 1992;167(5):1355–1363. doi:10.1016/S0002-9378(11)91716-5
8. Hasegawa J, Suzuki N. SMI for imaging of placental infarction. Placenta. 2016;47:96–98. doi:10.1016/j.placenta.2016.08.092
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.