Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Spectrum of and Factors Associated with Eye Disorders among Rheumatoid Arthritis Patients Attending Tertiary Hospital in Uganda
Authors Headcraph E , Atukunda I, Kaddumukasa M , Nakiyingi L, Lusobya RC, Ampaire-Musika A, Otike C, Nagawa E , Juma P, Msonge F, Otiti-Sengeri J
Received 22 March 2023
Accepted for publication 6 July 2023
Published 13 July 2023 Volume 2023:15 Pages 103—111
DOI https://doi.org/10.2147/OARRR.S413697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Eunice Headcraph,1 Immaculate Atukunda,1 Mark Kaddumukasa,2 Lydia Nakiyingi,2 Rebecca Claire Lusobya,1 Anne Ampaire-Musika,1 Caroline Otike,3 Elizabeth Nagawa,1 Paul Juma,1 Fransisco Msonge,1 Juliet Otiti-Sengeri1
1Department of Ophthalmology, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; 2Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; 3Clinical Epidemiology Unit, School of Public Health, College of Health Sciences, Makerere University, Kampala, Uganda
Correspondence: Eunice Headcraph, Department of Ophthalmology, College of Health Sciences, Makerere University, P.O. Box 7072, Kampala, Uganda, Tel +256783378619, Email [email protected]
Background: Ocular morbidities associated with rheumatoid arthritis (RA) have not received much attention in Africa, particularly in sub-Saharan Africa. They are among the commonest (40%) extra-articular organ involvement in RA. If undiagnosed, there is a potential risk of them causing visual impairment or blindness. There is no documented study in Uganda on the magnitude of eye disorders among RA patients.
Aim: To determine the spectrum of eye disorders and associated factors among patients with RA attending Mulago National Referral Hospital.
Methods: A hospital based cross-sectional study was conducted among adults with RA attending the rheumatology clinic between July 2021 and September 2021. Clinical and sociodemographic data were collected, and ophthalmologic examinations were performed on all consenting participants. Modified Poisson regression with robust standard error was used to determine factors associated with eye disorders.
Results: Overall, 105 patients with RA were enrolled, of which, 53 (50.5%) had eye disorders. The commonest disorder (54.7%, n=29) was dry-eye syndrome. Factors that were significantly associated with eye disorders were age 36– 55 years (aPR 1.56, p=0.015), duration of RA > 5 years (aPR 1.81, p=0.001), use of hydroxychloroquine > 5 years (aPR 1.77, p=0.041), dose of oral steroids > 10 mg/day (aPR 1.49, p=0.034), and history of both diabetes and hypertension (aPR 1.87, p=0.014).
Conclusion: The prevalence of eye disorders among patients with RA was high, with the commonest being dry-eye syndrome. We recommend that ocular examinations be performed on every patient at the time of RA diagnosis for early detection of eye disorders.
Keywords: rheumatoid arthritis, eye disorders, sub-Saharan Africa
Background
Globally, the prevalence of rheumatoid arthritis (RA) is estimated to be 1%–2% of the adult population, affecting more women than men. In Africa, 0.13%–0.9% of the general population is estimated to be affected,1–3 and there has been an increase in RA reports; however, there are no published data on the prevalence of RA in Uganda.4,5
Ocular involvement is one of the commonest extra-articular manifestations in RA patients.6,7 This results from either the disease process or drugs used to treat the disease.8 Most studies report the commonest ophthalmic manifestation in RA is dry-eye syndrome; however, other manifestations, such as episcleritis, scleritis, peripheral ulcerative keratitis, uveitis, raised intraocular pressure (IOP), cataract, retinal toxicity, and retinal vasculitis, have been reported.9 If untreated, these manifestations can lead to visual impairment and blindness, which negatively impacts the daily activities of patients and leads to loss of productivity.10,11
Globally, the prevalence of these ocular manifestations varies from 25% to 46% in different parts of the world; however there is limited information on sub-Saharan Africa.6,12,13 Furthermore, these eye disorders put an additional economic burden not only on patients but also health-care system and society as a result of direct medical costs and costs owing to loss of workdays. Therefore, early detection of these conditions allows for appropriate treatment that will prevent potentially sight-threatening complications and improve the quality of life of these patients and and benefit society.
Currently, there are limited data on eye disorders in RA in Uganda. This will contribute to the World Health Organization’s key objective of Vision 2020, which focuses on controlling diseases that affect eye health.14 We thus evaluated the prevalence, patterns, and factors associated with eye disorders among patients with RA attending the Mulago National Referral Hospital (MNRH) medical rheumatology clinic.
Methods
Study Design and Setting
In a cross-sectional study conducted between July 2021 and September 2021, 105 study participants with confirmed RA from the MNRH rheumatology medical clinic were enrolled. MNRH, which serves as a teaching hospital for Makerere University College of Health Sciences, is located approximately 5 km northeast of Kampala. The rheumatology clinic, run by a rheumatologist and three nurses, is open once a week. Currently, no ophthalmologist is attached to the unit and ocular examination is not part of routine management in RA. About 30–60 patients with different rheumatic conditions are seen in each clinic day and RA is the commonest (30%–40%) condition.
Study Population
We enrolled patients who provided written informed consent and fulfilled the study inclusion criteria: aged >18 years, a confirmed diagnosis of RA, and attending the rheumatology clinic regularly. We excluded patients who were too ill to withstand ocular assessment and those who had previously participated in the study. A consecutive sampling technique was used. Patients who met the inclusion criteria were enrolled consecutively until the desired sample size was achieved.
Study Procedure
Using a semistructured pretested interviewer-administered questionnaire, we collected sociodemographic characteristics, disease duration, and details of medications patients were on from patients’ hospital medical records. Patients had a complete ophthalmic examination in the following sequence: visual acuity testing, refraction, testing for dry eye using tear-breakup time and Schirmer’s test, anterior-segment examination, applanation tonometry, and dilated fundal examination with a +90 D lens.
Visual acuity was assessed in all participants using a Snellen chart placed 6 m from the participant or an E chart for illiterate participants. All patients with visual acuity <6/6 were retested using pinhole. Anterior-segment examination was done using torchlight and a portable slit-lamp biomicroscope. IOP was measured using a handheld iCare tonometer. For this study, normal IOP was taken as 10–21 mmHg and raised IOP >21 mmHg. Visual field was tested by the confrontational method.
Before the Schirmer test, the procedure was explained to the patient. A drop of topical anesthetic agent was instilled into each eye. Excess tears were wiped off using cotton wool. A Schirmer strip (folded at the 5 mm point at the upper end) was gently placed at the junction between the inner two-thirds and outer third of the lower eyelid. The patient was instructed to gently close their eyes. The strip was removed after 5 min and the amount of wetting read off the strip. Wetting of <6 mm was taken as abnormal.15 Ocular surface involvement was assessed by fluorescein staining. Pupil dilatation was achieved using tropicamide 1% eyedrops, fundus examination was done using a +90 D lens indirect slit-lamp biomicroscope to assess any posterior segment changes, and the results were documented.
For the purposes of this study, the following operational definitions were used for eye disorders.
Ocular manifestations: eye conditions including keratoconjunctivitis sicca or dry-eye syndrome, episcleritis, scleritis, peripheral ulcerative keratitis, cataract, retinal toxicity, and retinal vasculitis that had directly or indirectly resulted from a disease process from another part of the body or were drug-induced.
Visual impairment: distance-vision impairment is classified as mild, moderate, severe, or blindness. In this study, visual impairment was defined as presenting visual acuity <6/18 in the better eye.16
Normal or mild visual impairmen: presenting visual acuity equal to or better than 6/18 in the better eye.
Moderate visual impairment: presenting visual acuity worse than 6/18 to equal or better than 6/60 in the better eye.
Severe visual impairment: presenting visual acuity worse than 6/60 to equal or better than 3/60 in the better eye.
Blindness: presenting visual acuity worse than 3/60 in the better eye.
Dry eye: itching, photophobia, gritty feeling, frequent blinking, redness and dryness, tear-film breakup time <10 seconds, and/or Schirmer’s test result ≤10 mm/5 minutes.
Episcleritis: mild eye pain, redness, photophobia, and/redness that resolves after installation of topical phenylephrine.
Scleritis: redness, eye pain, photophobia and/or signs of scleral edema, and dilatation of both superficial and deep episcleral blood vessels.
Peripheral ulcerative keratitis: combination of peripheral crescentic ulceration, superimposed epithelial defect, loss of stroma, and infiltrates at the limbus with an overhanging edge.
Cataract: presence of lens opacity.
Data Management and Analysis
For descriptive statistics, frequencies and percentages were used for qualitative variables, whereas means ± SD were used for quantitative variables. Modified Poisson regression with robust standard error was used to determine factors associated with eye disorders. Factors that have been shown by literatures as known risk factors for eye disorders in RA were considered for multivariate analysis, and those with p<0.05 were considered statistically significant. Stata 15.0 (StataCorp, College Station, TX, USA) was used for statistical analysis.
Ethical Considerations
This study was conducted under the ethical principles stated in the Declaration of Helsinki (1996), the National Guidelines of Research Involving Humans and Research Participants, and the applicable guidelines on Good Clinical Practice. The School of Medicine Research and Ethics Committee (SOMREC) approved the study (reference Mak-SOMREC-2021-119). Administrative clearance was acquired from MNRH. The study objectives were explained to the study participants, who were assured of confidentiality through anonymous data storage and reporting.
Results
Population Characteristics
A total of 105 study participants attending the rheumatology medical outpatient clinic at MNRH were sampled. Mean age was 45.5±15.8 years with a range of 18–82 years. A majority (97 of 105) were women. Overall, 63% (n=66) reported having been diagnosed with RA <5 years prior, 60% were married, and 55.2%) were unemployed (Table 1). A majority were receiving oral methotrexate (77.1%), 23.8% oral hydroxychloroquine, 11.4% were taking oral NSAIDs, and 48.6% taking oral steroids for their attendant issues. A majority had normal vision (89.5%) with visual acuity of 6/6–6/18. Eleven participants (10.5%) had visual impairment with visual acuity of 6/24–6/60, and none had severe visual impairment or blindness.
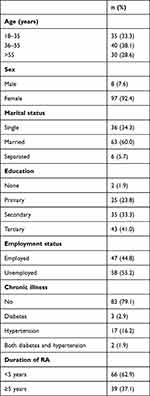 |
Table 1 Sociodemographic characteristics of study participants (n=105) |
Prevalence of Eye Disorders
Of the 105 patients screened, 53 (50.5%) had eye disorders, and thus the prevalence of eye disorders among RA patients attending the rheumatology clinic was 50.5%. A total of 42 had typical features associated with RA, while 11 had nonocular manifestations associated with RA. Almost half (47.2%, n=25) were aged 36–55 years, and 52.8% (n=28) had had RA >5 years (Table 2). None of the study participants was diagnosed with uveitis, peripheral ulcerative keratitis, retinal toxicity, or retinal retinitis.
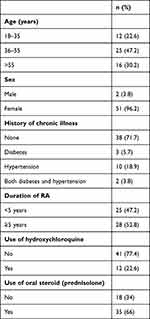 |
Table 2 Characteristics of patients with eye disorders (n=53) |
Types of Eye Disorders
The commonest ocular manifestation was dry-eye syndrome (54.7%, n=29), followed by cataract (13.2%, n=7), and the least common was scleritis (1.9%, n=1). Among the seven study participants diagnosed with cataracts, 57.1% (n=4) had non–steroid-induced cataracts (cortical and mature), while 42.9% (n=3) had posterior subcapsular cataracts. Among the patients who were receiving steroids, two had raised IOP. Five study participants (9.4%) had allergic conjunctivitis (Table 3).
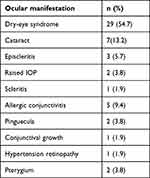 |
Table 3 Patterns of eye disorders (n=53) |
Factors Associated with Eye Disorders
Age 36–55 years (aPR 1.56, p=0.015), with a history of hypertension and diabetes comorbidity (aPR 1.87, p=0.014), having been diagnosed with RA ≥5 years prior (aPR 1.81, p=0.001), use of hydroxychloroquine >5 years (aPR 1.77, p=0.041), and receiving >10 mg/day of oral steroids (aPR 1.49, p=0.034) were significantly associated with eye disorders among the patients (Table 4).
 |
Table 4 Factors associated with ocular manifestations |
Discussion
This study set out to determine the spectrum of eye disorders and associated factors among patients with RA patients attending the MNRH medical rheumatology clinic. Our study reveals 50.5% of our study participants had eye disorders, especially those aged 36–55 years with a history of RA and hydroxychloroquine use >5 years, hypertension and diabetes comorbidity, and those receiving >10 mg/day of oral steroids. This is the first study in Uganda to explore ocular problems among patients with RA.
A majority of the study participants in this study were female, as observed in other studies.17,18 This is in keeping with the described incidence of autoimmune diseases. Women have been reported to be affected more due to female reproductive hormones, genetic factors, and environmental exposure coupled with better health-seeking behavior compared to male patients.17,19 The prevalence of RA also predominantly affects the female sex.18
Our study included adults aged 18–82 years with mean age of 45.5±15.8, years which is similar to an earlier study by Akintayo et al.6 Older age and disease duration >5 years were associated with eye disorders. In our study, the prevalence of eye disorders in RA patients was 50.5%, which is slightly higher than earlier studies from Nigeria, which reported 41.2% and 46% among study participants with RA.6,20 This might be attributed to the fact that this was a hospital-based study, and as it is a referral hospital, a majority of the patients attending the rheumatology clinic tend to have severe forms of RA that might be associated with eye disorders.
Generally, dry-eye syndrome was the commonest ocular manifestation (54.7%). This finding matches a study done in Egypt, which also found a prevalence of 54.7%, but differed from other studies, which reported a prevalence of 10%–39%.9,21 This could be due to differences in study populations. Earlier studies finding lower prevalence excluded patients aged >60 years, those with a history of ocular surgery, and those who were on hydroxychloroquine, unlike our study, which included everyone.
The second-commonest eye disorder in our study population was cataracts, with a prevalence of 13.2%. This is lower than earlier studies, which reported 18.9% and 26%.6,20 This may be due to having fewer elderly RA patients in our study than their studies. However, our finding is much higher than that of a study reported from India, with prevalence of 3.5%.22 This may be attributed to diabetes mellitus comorbidity (2.9%) and steroid use (48.6%) in our RA patients, which have been reported as risk factors for cataract development.23,24 In this study, a daily dose >10 mg oral prednisolone was more associated with eye disorders compared to those not taking steroids. This is similar to a study that was done in Egypt. The risk of ocular manifestations in RA patients on steroid treatment increased with dose of prednisolone ≥7.5 mg/day for >1 year.25 In our study, the mean steroid dose was 15.1±3.6 mg/day, and those who had been on steroids >2 years had 1.03 times the prevalence of ocular manifestations than those who were not; however, this was not statistically significant.
In this study, patients with visual impairment constituted 10.5% of the population, although these may be incidental findings, as they are not typically associated with RA. The prevalence of episcleritis was 5.7%, raised IOP 3.8%, and scleritis 1.9%, which is similar to most other studies.9,26–28 Patients aged 36–55 years had a higher prevalence of eye disorders. In agreement with our findings, Akintayo et al also found that age >45 years was significantly associated with ocular manifestations in RA.6 However, Markovitz et al did not find any significant association between age and prevalence of ocular manifestations in RA.29 According to the literature, increased age is associated with a defective adaptive immune system in RA patients contributing to the breakdown of immunological tolerance and hence increased disease severity.30 Furthermore, disease severity increases the risk of ocular manifestations.31 Therefore, these factors may possibly make RA patients in these age-groups more prone to ocular manifestations.
The current study found that having had RA >5 years was significantly associated with eye disorders compared <5 years. This is consistent with the studies done in Egypt and India. This may be due to the fact that patients who have had the disease for longer are more likely to have been on treatment. It is also known that some disease-modifying antirheumatic drugs increase the risk of ocular manifestations.32 There was a statistically significant association between eye disorders and use of hydroxychloroquine >5 years in RA patients, which differed from the study done in Nigeria, in which they did not find an association of ocular manifestations with hydroxychloroquine use in RA.6 Most studies did not report any association of ocular manifestations and use of hydroxychloroquine because they explored only ocular manifestations related to RA itself, but not those that may have been drug-induced.9,33
Limitations
This study had several limitations. First, due to it being a hospital-based study, we might have missed a substantial number of patients with eye disorders, as only those with severe forms of RA might have been referred due to survivorship bias. Also, these findings may not be generalizable to the general population. Consecutive sampling methods have a selection bias in which a variable that is associated with the outcome under investigation may occur more frequently or less in those sampled in this period compared to the general population. We also note that as this was a cross-sectional study, it was not possible to establish causal relationships between RA and ocular manifestations. A well-designed study with a large sample might be needed to explore this.
Conclusion
The prevalence of ocular manifestations among RA patients was high, 50.5% with dry-eye syndrome being the commonest. A focused ophthalmologic examination should be performed among RA patients aged 36–55 years with a disease duration or receiving hydroxychloroquine ≥5 years and on a daily dose >10 mg prednisolone.
Abbreviations
IOP, intraocular pressure; MNRH, Mulago National Referral Hospital; RA, rheumatoid arthritis.
Data Sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Permission to conduct this study was sought from the head of the rheumatology unit, Department of Ophthalmology at MNRH, and approval was obtained from the School of Medicine Research and Ethics Committee (SOMREC) of Makerere University (reference Mak-SOMREC-2021-119). Voluntary written informed consent was sought from the participants before they joined the study.
Acknowledgment
The authors would like to thank the clinical team at the rheumatology unit in the medical department and ophthalmology department of Mulago National Referral Hospital for their support during the study. The authors express their gratitude towards the participants that took part in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
No funding sources.
Disclosure
The authors declare no competing interests.
References
1. Dowman B, Campbell RM, Zgaga L, Adeloye D, Chan KY. Estimating the burden of rheumatoid arthritis in Africa: a systematic analysis. J Glob Health. 2012;2(2):020406. doi:10.7189/jogh.02.020406
2. Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(2 Suppl 1):1–12. doi:10.2165/00019053-200422001-00002
3. Oton T, Carmona L. The epidemiology of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(5):101477. doi:10.1016/j.berh.2019.101477
4. Mody GM. Rheumatology in Africa-challenges and opportunities. Arthritis Res Ther. 2017;19(1):49. doi:10.1186/s13075-017-1259-3
5. Gouda HN, Charlson F, Sorsdahl K, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–e87. doi:10.1016/S2214-109X(19)30374-2
6. Akintayo RO, Adelowo OO, Egajifo O, et al. The impact of ocular manifestations of rheumatoid arthritis on the health-related quality of life and the functional ability of black Africans. Int Ophthalmol. 2019;39(5):1003–1012. doi:10.1007/s10792-018-0902-6
7. Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2):102735. doi:10.1016/j.autrev.2020.102735
8. Ausayakhon S, Louthrenoo W, Aupapong S. Ocular diseases in patients with rheumatic diseases. Med Assoc Thai. 2002;85:855–862.
9. Eldaly ZH, Saad SA, Hammam N. Ocular surface involvement in patients with rheumatoid arthritis: relation with disease activity and duration. Egypt Rheumatol. 2020;42(1):5–9. doi:10.1016/j.ejr.2019.05.004
10. Sims J. Scleritis: presentations, disease associations and management. Postgrad Med J. 2012;88(1046):713–718. doi:10.1136/postgradmedj-2011-130282
11. Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a US normative sample. Invest Ophthalmol Vis Sci. 2005;46(1):46–50. doi:10.1167/iovs.03-0915
12. Gaubitz M. Epidemiology of connective tissue disorders. Rheumatology. 2006;45(Suppl 3):iii3–4. doi:10.1093/rheumatology/kel282
13. Domngang Noche C, Kagmeni G, Dohvoma V, Bella AL, Ebana Mvogo C, Singwe-Ngandeu M. Ophthalmic manifestations in chronic inflammatory rheumatic diseases at a referral Hospital of Yaounde, Cameroon. Ocul Immunol Inflamm. 2018;26(2):259–264. doi:10.1080/09273948.2016.1212078
14. Holland P, Resnikoff S. Beyond VISION 2020: universal eye health coverage and the elimination of trachoma. Community Eye Health. 2019;32(107):60.
15. Subcommittee of the International Dry Eye Workshop. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5:108–152.
16. Buckholtz R. Looking at the new ICD-10-CM codes for blindness. 2017.
17. Sen HN, Davis J, Ucar D, Fox A, Chan CC, Goldstein DA. Gender disparities in ocular inflammatory disorders. Curr Eye Res. 2015;40(2):146–161. doi:10.3109/02713683.2014.932388
18. Choudhary MM, Hajj-Ali RA, Lowder CY. Gender and ocular manifestations of connective tissue diseases and systemic vasculitides. J Ophthalmol. 2014;2014:403042. doi:10.1155/2014/403042
19. Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17:38. doi:10.1186/s12875-016-0440-0
20. Abdullahi MH, Pam V, Oladigbolu KK, Umar AA, Muhammad RC. Prevalence and spectrum of eye disorders among patients with rheumatoid arthritis and systemic lupus erythematosus in a tertiary hospital in Northern Nigeria. J West Afr Coll Surg. 2022;12(1):48–54. doi:10.4103/jwas.jwas_59_22
21. Almaliotis D, Zakalka M, Gerofotis A, et al. Ocular manifestations in rheumatoid arthritis. Open J Ophthalmol. 2016;06(03):170–175. doi:10.4236/ojoph.2016.63024
22. Kaur P, Singh Bal B, Singh B, Duggal A, Kaur I. To study the prevalence of ocular manifestations in rheumatoid arthritis and their correlation with anti cyclic citrullinated peptide antibodies and rheumatoid factor. J Dent Med Sci. 2016;15(09):58–63. doi:10.9790/0853-1509105863
23. Black RJ, Hill CL, Lester S, Dixon WG. The association between systemic glucocorticoid use and the risk of cataract and glaucoma in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166468. doi:10.1371/journal.pone.0166468
24. Taseer Z, Khan MA, Afzal S, Gillani SA, Sarwar S. Cataract; diabetes and smoking as a major risk factor for cataract in the community population of residents of Lahore Cantt. Prof Med J. 2019;26(2):54.
25. Da Silva JA, Jacobs JW, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65(3):285–293. doi:10.1136/ard.2005.038638
26. Promelle V, Goeb V, Gueudry J. Rheumatoid arthritis associated episcleritis and scleritis: an update on treatment perspectives. J Clin Med. 2021;10(10):2118. doi:10.3390/jcm10102118
27. Halder P, Dutta U, Ghosh B, Maiti P. A study of ophthalmological manifestations of rheumatoid arthritis in Eastern India. Int J Sci Study. 2019;7(5):8–11.
28. Al-Bedri K, Al-Quriashi NK, Gorial FI, Younis HA. Ocular manifestations in rheumatoid arthritis: a descriptive cross-sectional study from Iraq. J Nat Sci Res. 2016;6(8):57.
29. Markovitz E, Perry ZH, Tsumi E, Abu-Shakra M. Ocular involvement and its’ manifestations in rheumatoid arthritis patients. Harefuah. 2011;150(9):713–8, 51.
30. Serhal L, Lwin MN, Holroyd C, Edwards CJ. Rheumatoid arthritis in the elderly: characteristics and treatment considerations. Autoimmun Rev. 2020;19(6):102528. doi:10.1016/j.autrev.2020.102528
31. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica. 2010;5(4):286.
32. Polanska V, Hlinomazova Z, Fojtik Z, Nemec P. Dry eye syndrome in rheumatoid arthritis patients. Ceska a slovenska oftalmologie. 2007;63(6):422–430.
33. Vignesh APP, Srinivasan R. Ocular manifestations of rheumatoid arthritis and their correlation with anti-cyclic citrullinated peptide antibodies. Clin Ophthalmol. 2015;9:393–397. doi:10.2147/OPTH.S77210
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
