Back to Journals » Journal of Experimental Pharmacology » Volume 16
Soyghurt Potentially Controls the Level of sFlt1 and PLGF in Preeclampsia Maternal Serum-Induced Placental Trophoblast Cell in vitro
Authors Khairani AF , Lantika UA, Ramadhanti J , Bashari MH , Shalannandia WA , Wikayani TP, Achadiyani A, Ritonga MA
Received 9 November 2023
Accepted for publication 2 March 2024
Published 13 March 2024 Volume 2024:16 Pages 111—122
DOI https://doi.org/10.2147/JEP.S446961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Astrid Feinisa Khairani,1 Uci Ary Lantika,2 Julia Ramadhanti,1 Muhammad Hasan Bashari,1,3 Widad Aghnia Shalannandia,1 Tenny Putri Wikayani,4 Achadiyani Achadiyani,1 Mulyanusa Amarullah Ritonga5
1Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia; 2Department of Biomedical Sciences, Faculty of Medicine, Universitas Islam Bandung, Bandung, West Java, Indonesia; 3Research Centre of Oncology and Stem Cell, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 4Culture and Cytogenetic Laboratory, Universitas Padjadjaran, Bandung, West Java, Indonesia; 5Department of Obstetrics and Gynecology, Hasan Sadikin General Hospital, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
Correspondence: Astrid Feinisa Khairani, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jalan Raya Bandung – Sumedang Km 21, Jatinangor, West Java, 45363, Indonesia, Tel +62-22-779559445363, Email [email protected]
Purpose: To observe the effect of soya yoghurt (Soyghurt), which is high in flavonoid substance, on the expression of preeclampsia biomarkers (sFLT-1 and PLGF) on preeclampsia serum-induced trophoblast primary cell culture isolated from placental tissue.
Methods: The trophoblast primary culture was induced by preeclampsia serum (10%). The Soyghurt treatment was performed with 2.5%, 5%, and 7.5% Soyghurt supernatant concentrations in culture media. The expression of preeclampsia markers, sFLT-1 and PLGF, were evaluated using ELISA.
Results: Expression of sFLT-1 on preeclampsia-induced cell culture treated with Soyghurt was significantly lowered compared to the untreated group (p< 0.01). However, no significant difference was observed in the PLGF levels of all groups induced by preeclampsia serum (p> 0.05).
Conclusion: This study demonstrates the potential effect of Soyghurt’s in balancing preeclampsia marker expression by inhibiting the expression of sFLT-1 in preeclampsia serum -induced trophoblast cells.
Keywords: preeclampsia, PLGF, sFLT-1, soya yoghurt, trophoblast
Introduction
Soybeans are unique legumes with high protein and dietary fiber levels. It is also a potent antioxidant source due to a concentrated amount of phenolic compounds, such as flavonoids and isoflavones, in them. Apart from its role as an antioxidant, flavonoids, and other phenolic compounds also regulate blood pressure and anti-inflammation.1,2 Isoflavone substances were also reported to reduce the risk of developing degenerative diseases such as osteoporosis and cancer.3
Due to its nutritional content, many functional foods were developed using soybeans as their raw material, such as soymilk and soya-based yoghurt (soyghurt), since it is convenient to consume. Soyghurt is a symbiotic drink that consists of probiotics as the fermentation agent, which could increase the beneficial effect of soybeans. The probiotics used in this process are commonly lactic acid bacteria (LAB), which produce lactic acid bacteria during their fermentation process. LAB also synthesized various beneficial nutritional compounds during the yoghurt fermentation process, such as vitamins, several types of protein, and antioxidants.4,5
Preeclampsia is one of the contributors to mortality in pregnant women and their children worldwide. The etiology of this medical condition currently is still unknown. One of the theories mentions that one of the underlying causes of preeclampsia development is the failure of trophoblast invasion. This condition can happen because of several factors, including genetic factors, immunological factors, and oxidative stress. Those three factors inhibit adequate trophoblast invasion into the mother’s endometrium, leading to endothelial dysfunction. The endothelium dysfunction will disrupt the uteroplacental flow, resulting in ischemic conditions.6,7 This condition will induce the trophoblast cells to secrete inflammatory mediators and generate chronic oxidative stress. Escalation of inflammation, oxidative stress, and endothelial dysfunction are the aspects that promote an imbalance condition of angiogenic substance (sFLT-1) and antiangiogenic (PLGF) in preeclampsia.6–9
An excessive sFLT-1 level can be found in pregnant women with preeclampsia compared to those with standard conditions. sFLT-1 is an antiangiogenic substance that operates by inhibiting PLGF and VEGF activity. sFLT-1 acts as an antagonist of VEGFR by binding with VEGF-A, VEGF-B, and PLGF, hence interfering with the activation of angiogenesis through the VEGF pathway.10 The increase of sFLT-1 serum level can be detected approximately 4–6 weeks before the appearance of the symptoms of preeclampsia.6–9 Besides the level of sFLT-1, the PLGF level has also been used as a diagnostic marker for preeclampsia. PLGF is an angiogenic agent that stimulates vascular proliferation by elevating the synthesis of NO. Pregnant women with preeclampsia tend to have lower PLGF serum levels than women with normal pregnancies.11–13
Unfortunately, the most common treatment used to overcome preeclampsia is by terminating the pregnancy and removing the fetus and placenta. However, the possibility of poor prognosis due to complications from preeclampsia before childbirth can also happen.14 Therefore, a primary preventive measure in managing preeclampsia is required, and all the efforts to find an effective prevention method are needed. One of the prospective ways to prevent preeclampsia is by utilizing functional food such as soyghurt.
In vitro study in drug discovery study is essential. Previous studies on preeclampsia used trophoblast cells isolated from placental tissue.15 One of the studies reported decreased preeclampsia markers (TNF-alfa and s-Eng) in trophoblast cell culture induced by preeclampsia serum.16 Therefore, this study aims to evaluate the effect of soyghurt supernatant on other preeclampsia markers (sFLT-1 and PLGF) levels in the primary placenta culture, which had been induced by preeclampsia serum.
Materials and Methods
Soya Yoghurt Production
The production of soyghurt utilized Lactobacillus bulgaricus ATCC 11842 bacterial culture. The purified bacteria culture was inoculated into 5 mL lactose broth and incubated for 24 h at 37°C. Bacteria were subcultured on MRS (de Mann, Rogosa, and Sharpe) agar (OXOID, CM0361), then incubated for 24 h at 37°C.
The raw material used in the production process was soybeans collected from a Tasikmalaya, West Java, Indonesia farm. Initially, cleaned soybeans were soaked in 5 L water with 0.25–0.5% NaHCO3 for 12–24 h. Afterward, the beans were rinsed and drained well before peeling the skin. Then, the peeled soybeans were crushed using a blender and mixed with 2.5 L hot water (80°C-100°C) for 7 min until it reached porridge texture. Subsequently, the soybean porridge was filtered using a cloth filter to collect the raw soymilk. After that, the filtered soymilk was mixed with 125 g granulated sugar and then sterilized using a vertical autoclave at 121°C with high-pressure steam at 1 atm (15lbs) for 10 min.
After the soymilk was ready, the L. bulgaricus ATCC 11842 bacteria culture was inoculated into 100 mL soymilk to make the starter soyghurt. The culture was incubated in a 5% CO2 incubator at 37°C for 48 h. The soyghurt was then produced by adding 10% (v/v) of starter soyghurt into soymilk and incubating for 48 h. The total bacteria colony and the product’s pH level had met the standard production of fermented drinks in Indonesia (SNI).
Preparation of Soyghurt Supernatant
The supernatant of the soyghurt was used to reach optimal intervention on the primary cell. The supernatant was harvested by centrifugation of soyghurt product with the speed of 4500 r/min at 4°C for 10 min. The collected supernatant was then filtered using a 0.2 µm filter membrane to eliminate the remaining debris and bacteria. The filtration was done twice.17
Phytochemical Screening Evaluation
The phytochemical screening on soyghurt supernatant was done based on the standard procedure from the previous study.18
Flavonoid Test
Soyghurt supernatant (1 mL) was added to 100 mL of hot water. Later on, the mixture was brought to a boil for 5 min before it was filtered. Then, magnesium powder and hydroxide acid were added to the filtrate. Following that, amyl alcohol was added and mixed vigorously. Let the mixture sit and separate naturally. The formation of reddish-orange or reddish-purple indicates the presence of flavonoids.
Saponin Test
Soyghurt supernatant (1 mL) was added to 100 mL of hot water. Later on, the mixture was brought to a boil for 5 min before it was filtered. The filtrate inside the reaction tube was vertically shaken for about 10s and then let sit for 10 min. The foam formation for around 1–10 cm suggested that the test was positive. A further test was done by adding several drops of HCl 2N into the foam. If the foam disappeared, it means that the test turned out negative. On the other hand, if the foam remains, it means the test is positive.
Alkaloid Test
In the water bath, 3 mL of soyghurt supernatant was mixed with 3 mL HCl 1%. Afterward, 1 mL of the mixture was placed into two tubes. In the first tube, several drops of Dragendroff’s reagent were added into the mix, then the change was observed. The formation of orange-red precipitation indicates a positive result. As for the second tube, Mayer’s reagent was added to the mix. A formation of buff-colored precipitation is an indication of the presence of alkaloids.
Sesquiterpene/Monoterpene Test
The soyghurt supernatant was added to the mixture of 10% vanillin and concentrated HCl. The appearance of colors indicated the presence of monoterpenoid and sesquiterpene.
Triterpenoid Test
Lieberman-Burchard’s reagent was added to 100 mL of soyghurt supernatant. A purple color appearance demonstrated the triterpenoid positive. Meanwhile, the presence of steroids was indicated by a greenish-blue color appearance.
Quinone Test
Soyghurt supernatant (100 mL) was added to 5% potassium hydroxide. Yellow to red color appearance indicated the positive result of the quinone test.
Tanin Test
Around 100 mL of soyghurt supernatant was transferred into the reaction tube, and water was added to the supernatant. After that, the mixture was heated up before it was filtered. Gelatin solution (1%) was added to the filtrate. The positive result was indicated by white precipitation in the mixture.
Isolation of Trophoblast Cells from Placental Tissue
Trophoblast primary culture cells were isolated from the placental tissue of a mother with normal pregnancy who had accepted the informed consent, which complied with the Declaration of Helsinki and was reviewed and approved by the ethical committee of the Faculty of Medicine, Universitas Padjadjaran (No.858/UN6.C.10/PN/2017). The placental tissue was harvested during caesarian section childbirth with the size of 2×3 x 1 cm on the maternal side, which then was cleaned using 0.9% NaCl solution. Subsequently, the tissue was chopped finely and added to 0.25% trypsin-EDTA (Gibco 25200056) before being incubated for 10 min at 37°C. The undigested placental tissue was re-incubated with 0.25% trypsin-EDTA for another 15–20 min. Then, trypsin-EDTA was inactivated using complete media (Amniomax C-100, basal media (Gibco, 171001074) + Amniomax C-100 supplement (Gibco 12556023)). Later, the digested tissue was filtered by a 100 µm strainer.
The supernatant was taken and centrifuged with 1500 r/min for 10 min. Next, the pellet was resuspended into complete media. The Ficoll gradient as put slowly into the cell solution and then centrifuged with 1500 r/min for 30 min. The cells in the buffy coat were then transferred into PBS solution and centrifuged for 5 min. The pellet was collected and transferred into a cell culture flask with Amniomax C-100 basal media supplemented by Amniomax C-100 supplement, antibiotic, and antimycotic. Cytokeratin-7 staining was performed to confirm the presence of trophoblast (Figure 1).
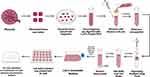 |
Figure 1 The procedure of primary trophoblast cells isolation. |
Serum Preparation
The serum was obtained from women’s blood with normal pregnancy and women with preeclampsia. The pregnancy was considered normal when there were no medical and obstetric complications during the pregnancy. Meanwhile, pregnancy with preeclampsia was determined when the mother’s systolic pressure was ≥ 160 mmHg, diastolic pressure ≥ 80 mmHg, and positive proteinuria. All the criteria followed the standard operational and procedure in Hasan Sadikin Central General Hospital, Bandung; which had been reviewed and approved by the ethical committee of the Faculty of Medicine, Universitas Padjadjaran (No.858/UN6.C.10/PN/2017).
The serum was separated from the whole blood by using the centrifugation method. Initially, the whole blood was allowed to clot by leaving it at room temperature. Afterward, the clotted blood was centrifuged with 3000 r/min for 15 min before the serum was harvested.
Induction of Preeclampsia Serum on the Trophoblast Cells
Primary trophoblast cells (5x104) were cultured in a 24-well plate until confluent. The cells were induced by 10% of preeclampsia serum and incubated for 24 h at 37°C. Afterward, the viability test was conducted before the confirmation test on the preeclampsia marker was performed by evaluating the sFLT-1 and PLGF levels using ELISA.
Dosage Determination of Soyghurt Supernatant on Trophoblast Cells Induced by Preeclampsia Serum
Primary trophoblast cells (1x104) were seeded into the 96-well plate and incubated for 24 hours in a 5% CO2 incubator at 37°C temperature. Following that, the cells were intervened by various concentrations of Soyghurt supernatant (1–20%) and incubated for another 24 hours. The MTT assay was conducted the following day to evaluate the cytotoxicity of the Soyghurt supernatant. The used media in the wells were discarded and replaced with 100µL of fresh medium with 5mg/mL MTT reagent. The cells were then incubated for 2–4 hours at 37°C in a CO2 incubator until the crystal formazan was formed. After that, 100µL of SDS in HCl was added into each well to dilute the crystal formazan. The absorbances were evaluated using a microplate reader using 450–550 nM wavelength and finally, the viability cells were determined using statistical analysis.
Soyghurt Supernatant Intervention
After 24 h of incubation, the cells were divided into five groups:
ELISA sFLT-1 Level
The measurement of the sFLT-1 level was conducted after incubation with preeclampsia serum for 24 h and following the protocol of Elisa sFLT-1 kit (Thermofisher, no. cat. BNS268-3). Each well was filled with 100 µL of supernatant. The biotin solution in conjugated diluent (1:100) was added and incubated at 18–25°C on a microplate shaker with 400 r/min speed for 2 h. After that, the well was washed, and then each was added to the streptavidin solution (1:300). Subsequently, the sample was incubated on a microplate shaker for one hour at 18–25°C. After the incubation and washing, the TMB substrate solution was put into the wells and incubated for 30 min on a microplate shaker with the same temperature. In the last step, the stop solution was added to the samples before reading the result using a spectrophotometer in 450 nm wavelength.
ELISA PLGF Level
PLGF level was measured after induction and incubation of cells with preeclampsia serum for 24 h. The procedure used followed the ELISA PLGF kit protocol (Thermofisher, no. cat. EHPGF). The standard and sample (100 µL) were put into 96-well plates, followed by incubation for 24 h before washing the wells. Afterward, the biotinylated antibody was diluted in assay diluent with a ratio of 1:80. Subsequently, 100 µL of the solution was put inside the well and incubated for 1 h on a microplate shaker. Later, the cells were washed, and 100 µL TMB substrate was added before the plate was put on a microplate shaker and incubated in the darkroom for 30 min. Stop-solution was then added after the incubation before reading the result using a spectrophotometer in 450 nm wavelength.
Statistical Analysis
The presented data was the average ± SEM (Standard Error Mean). The evaluation results were analyzed using one-way ANOVA and a post hoc Tukey multiple comparison tests. The result data with p<0.05 indicated a significant outcome, while p<0.01 indicated a very significant outcome. Statistical analysis was conducted using GraphPad Prism 10.
Results
Soyghurt and Supernatant Soyghurt Characteristic
This study used lactic acid bacteria, Lactobacillus bulgaricus, to ferment soya milk into soyghurt. The experiment showed that L. bulgaricus could grow in soya milk with the bacteria characteristic of a bacillus gram-negative bacterium with a negative result on the catalase test. The product contained 2×108 CFU/mL of bacteria with a pH level of 5.39. The bacteria amount and pH level of soyghurt complied with the national standard of Indonesia (SNI).
The soyghurt supernatant was used to intervene in the trophoblast primary cell culture. The phytochemical screening on the soyghurt supernatant demonstrated the presence of saponin, sesquiterpene/monoterpene, and quinone (Table 1).
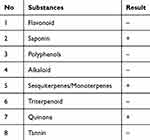 |
Table 1 Phytochemistry Screening of Soyghurt |
Induction of Preeclampsia Serum to Trophoblast Primary Cell Culture Increases sFLT-1 Expression While Decreasing PLGF Expression
The cells isolated from the placenta showed the morphological characteristic of trophoblast cells (Figure 2). The brownish color in the cytoplasm indicated the expression of CK7, which is a biomarker for trophoblast.
Trophoblast primary cell culture was cultured into three groups, and two of them were induced by preeclampsia serum (PS) and normal serum (NS) as control, respectively. This study chose 10% as the dosage for preeclampsia serum induction based on a previous study.19 After induction, the preeclampsia environment of the cell culture was verified by evaluating the sFLT-1 and PLGF levels using ELISA. The group induced by preeclampsia serum showed a higher level of sFLT-1 (33.15±0.453) than normal serum (29.793±0.768), which indicated that the induction of preeclampsia serum to trophoblast cell culture elevated the sFLT-1 level (Figure 3A). On the other hand, the PLGF level of the PS group (4.83±0.514) was significantly lower (p<0.01) than the NS group (31.21±0.751) and the control group (Figure 3B). The result validated that a preeclampsia-like condition could be demonstrated by induction of preeclampsia serum into trophoblast cell culture.
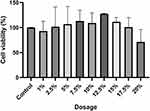
The cytotoxicity of the Soyghurt supernatant was tested using the MTT assay to determine the dosages that were used in this study. The result indicated decreasing cell viability in a dose-dependent manner, particularly in the group with a dosage over 12.5%. Furthermore, the group’s viability induced by 20% of the Soyghurt supernatant showed around 75% viability. Nevertheless, the administration of Soyghurt supernatant within the 1–7.5% concentration range was considered safe (Figure 4). Hence, this study chose 2.5%, 5%, and 7.5% as the dosage of Soyghurt supernatant intervention.
The Decrease of sFLT-1 Level in Preeclampsia Serum Induced-Trophoblast Cell Culture Upon Intervention by Supernatant Soyghurt
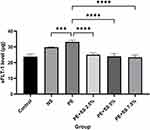
The group intervened by supernatant soyghurt was divided into three subgroups with soyghurt dosage: 2.5%, 5% and 7.5%. The sFLT-1 levels of all the intervention groups were lower than those of other groups (p<0.01), which were induced preeclampsia serum or normal serum. The data also exhibited the dosage of supernatant yoghurt affecting the decrease of the sFLT-1 level. The sFLT-1 level was lower when the dosage of supernatant yoghurt was higher (Figure 5).
The Decreased of PLGF Level in Preeclampsia Serum Induced-Trophoblast Cell Culture Upon Intervention by Supernatant Soyghurt
The result showed that the PLGF level of the PE group (4.83±0.514) was lower than the NS group (31.21±0.751). Further, the PLGF level of all groups intervened by supernatant Soyghurt was significantly lower than the control group (PE and NS), with a p-value < 0.01. The lowering of PLGF level showed a dose-dependent manner: 2.5% (2.34±0.466, 5% (2.06±0.589), dan 7.5% (1.7±0.531). This finding demonstrated that the intervention by supernatant Soyghurt on preeclampsia serum-induced trophoblast cells lowered the PLGF level (Figure 6).
Discussion
This study used soya milk as an animal milk replacement to make a fermented product. The use of soya milk in fermentation-based milk products did not affect the number of lactic acid bacteria (LAB) growing in the product (Shori 2013). The number of LAB colonies in Soyghurt was within the normal range based on the national standard in Indonesia (SNI). The fermentation process of soya milk produces lactic acid, which decreases the pH level of the fermented product. Thus, it further resulted in the coagulation of the milk, making them more solid and stable consistency.20 The pH level of the Soyghurt product used in this study was 5.39, within the normal range of acidic levels for fermented soya milk.20
Soybean, as the raw material of soya milk, contains flavonoid, which has antioxidant properties.21 Further, the fermentation process of raw materials such as soybeans could enhance the product’s nutrition content.22 The phytochemical screening of the Soyghurt showed the presence of saponin, monoterpene, and quinone in the product. Those phytochemical substances also hold antioxidant properties, making this product a potential candidate for functional food that could prevent preeclampsia development.
Preeclampsia is a pathological condition of pregnancy developed through various pathways, which can be identified by the increase of sFLT-1 level and decrease of PLGF.13,23 This study aimed to evaluate the potential of Soyghurt to prevent preeclampsia through in vitro experiments. The cells used were primary trophoblast cells cultured from the human placenta. The isolated trophoblast cells were obtained from the placental tissue of a mother with normal pregnancy and were confirmed as trophoblast cells by CK 7 expression in the cells.24 The expression of CK 7 was marked by the brownish color of the cytoplasm (Figure 2).
A previous study reported that the induction of preeclampsia serum in the pregnant mouse model could increase sFLT serum and s-Eng as well as cause hypoxia at the maternal-fetal interface.19 Preeclampsia serum induction also increased TNF-alpha and endothelin-1 levels while decreasing NO levels.25,26 This study induced the preeclampsia serum into trophoblast primary cell culture, which showed the increase of sFLT-1 level and decreased PLGF level (Figure 3). This condition was aligned with the result obtained from the ELISA evaluation of serum from a mother with preeclampsia.27 Therefore, it indicated that the administration of preeclampsia serum into trophoblast primary cell culture demonstrated preeclampsia-like condition.
The Soyghurt supernatant was administered to the trophoblast cell culture 24 hours after the induction of preeclampsia serum into the culture. The result demonstrated the decrease in sFLT-1 level in the group that intervened with Soyghurt (Figure 5). The decrease of the sFLT-1 level was expected to decrease its activity as an anti-VEGF receptor. Thus, it could increase the angiogenic factor. However, the result showed no elevation in the PLGF level.
The phytochemical substances such as isoflavones, flavonoids, saponins, and phenolic compounds are reported to influence the balance in angiogenic factors.4,28 Evidence demonstrates the suppressive effect of luteolin, a flavonoid, on sFlt-1 secretion through the HIF-1α/sFLT-1 pathway.29 The phytochemistry screening of Soyghurt showed the positive presence of saponin, quinone, and monoterpene (Table 1). These bioactive compounds, especially saponin, were suspected to be the substances that inhibit the production of sFLT-1. Saponin is a steroid glycosidic compound that is abundantly present in the plant. The saponin compound inside soybean has been reported to have antioxidant activity.30 Previous study on Chinese medicine Astragalus, which is rich in Saponins, flavonoids, and polysaccharides, displayed favorable improvement of preeclampsia condition in an in vivo rat model. The study reported the anti-inflammatory potential effect of the bioactive compound by suppressing the expression of inflammatory cytokines, including IL-6 and IL-1β via the TLR-4/NF-κB signaling pathway.31 Another bioactive compound found in soygurt is quinone. Quinone or pyrroloquinoline quinone (PQQ) is a phytochemical compound with an antioxidant effect that defense against oxidative stress of the mitochondria. Previous preeclampsia in vivo studies demonstrate the antioxidative effect of PQQ by elevation of NRF2 expression in NF- κB/Nrf2 pathway.32,33 Besides the active compounds, Soyghurt also contains probiotic components. Previous studies stated that probiotics have a role as an immunomodulatory by modulating the immune system through a “cross-talk” mechanism.34,35 Besides, probiotics could also inhibit the production of pro-inflammatory cytokines. Yeganegi et al reported that supplementation of L. rhamnosus GR-1 could inhibit IL-10 production in lipopolysaccharides-induced trophoblast cells.17 In addition, a clinical study in Norway disclosed that the administration of probiotics lowered the risk of developing preeclampsia.36
In our study, the intervention groups showed no elevation in their PLGF level. This condition occurred due to the inability of the active compound or probiotic in the product to modulate the cascade mechanism to increase the production of PLGF. This case might also happen because the preeclampsia serum was more effective in suppressing the PLGF production.
Nevertheless, many missing links exist between how the Soyghurt mechanism and probiotics affect the markers in the cascade of preeclampsia development. Hence, further study is needed to explore more of those mechanisms related to the active compounds in Soyghurt. Thus, more study at the genomic level is also suggested to get a more extensive and comprehensive analysis of the effect of Soyghurt products on preeclampsia development.
Conclusion
The administration of preeclampsia serum could induce imbalance expression of angiogenic markers (sFLT-1 and PLGF), while did not affect the cell viability. The administration of Soyghurt as a functional food could assist in attenuating the preeclampsia marker, specifically by decreasing the sFLT-1 level.
Acknowledgments
This study was conducted at the Cell Culture and Cytogenetic Laboratory, Faculty of Medicine, Universitas Padjadjaran, Indonesia. We would like to appreciate the laboratory technicians for their technical assistance during the experimental research.
Author Contributions
All the authors made substantial and proportional contributions to the design, conducting research, acquisition of data, and data analysis. All authors took part in drafting the article or critically revising for important intellectual content. All authors agreed to submit to this journal, given final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding
This work was supported by Internal Grant, Universitas Padjadjaran, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peiretti P, Karamać M, Janiak M, et al. Phenolic composition and antioxidant activities of Soybean (Glycine max (L.) Merr.) plant during growth cycle. Agronomy. 2019;9(3):153. doi:10.3390/agronomy9030153
2. Šibul F, Orčić D, Vasić M, et al. Phenolic profile, antioxidant and anti-inflammatory potential of herb and root extracts of seven selected legumes. Ind Crops Prod. 2016;83:641–653. doi:10.1016/j.indcrop.2015.12.057
3. Fauziah PN, Nurhajati J. Daya Antibakteri Filtrat Asam Laktat dan Bakteriosin Lactobacillus bulgaricus KS1 dalam Menghambat Pertumbuhan Klebsiella pneumoniae Strain ATCC 700603, CT1538, dan S941 [Antibacterial Effect of Lactic Acid Filtrate and Bacteriocins of Lactobacillus bulgaricus KS1 on Inhibiting the Growth of Klebsiella pneumoniae ATCC 700603, CT1538, and S941 Strains]. Majalah Kedokteran Bandung. 2015;47(1):35–41. doi:10.15395/mkb.v47n1.395
4. Fakri E, Lim S, Musa N, Hazizul Hasan M, Adam A, Ramasamy K. Lactobacillus fermentum LAB 9-fermented soymilk with enriched isoflavones and antioxidants improved memory in vivo. Sains Malays. 2016;45(9):1289–1297.
5. Song HH, Ryu HW, Lee KJ, Jeong IY, Kim DS, Oh SR. Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics. 2014;10(5):833–841. doi:10.1007/s11306-014-0640-3
6. James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 2010;221(4):363–378. doi:10.1002/path.2719
7. Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–1113. doi:10.2215/CJN.12081115
8. Burke SD, Zsengellér ZK, Khankin EV, et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J Clin Investig. 2016;126(7):2561–2574. doi:10.1172/JCI83918
9. Cheng M, He P, Fu J. The relationship between circulating tissue transglutaminase, soluble fms-like tyrosine kinase-1, soluble endoglin and vascular endothelial growth factor in pre-eclampsia. J Hum Hypertens. 2016;30(12):788–793. doi:10.1038/jhh.2016.32
10. Ngene NC, Moodley J. Role of angiogenic factors in the pathogenesis and management of pre-eclampsia. Int J Gynecol Obstet. 2018;141(1):5–13. doi:10.1002/ijgo.12424
11. Fan X, Rai A, Kambham N, et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Investig. 2014;124(11):4941–4952. doi:10.1172/JCI76864
12. Grill S, Rusterholz C, Zanetti-Dällenbach R, et al. Potential markers of preeclampsia – a review. Reprod Biol Endocrinol. 2009;7(1):70. doi:10.1186/1477-7827-7-70
13. Tang P, Xu J, Xie B-J, et al. Use of serum and urinary soluble sFlt-1 and PLGF in the diagnosis of preeclampsia. Hypertens Pregnancy. 2017;36(1):48–52. doi:10.1080/10641955.2016.1237642
14. Song J, Li Y, An R. Vitamin D restores angiogenic balance and decreases tumor necrosis factor-α in a rat model of pre-eclampsia. J Obstetrics Gynaecol Res. 2017;43(1):42–49. doi:10.1111/jog.13186
15. Li L, Schust DJ. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod Biol Endocrinol. 2015;13(1):71. doi:10.1186/s12958-015-0070-8
16. Vioretti R, Khairani AF, Fauziah PN, Hilmanto D. An evaluation of soyghurt potential on tumor necrosis factor-α and soluble endoglin levels in preclampsia maternal serum-induced placental trophoblast cell in vitro. Int Food Res J. 2018;25(4):13971402.
17. Yeganegi M, Watson CS, Martins A, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200(5):532.e1–532.e8. doi:10.1016/j.ajog.2008.12.032
18. Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1966;55:225–276. doi:10.1126/science.151.3712.874
19. Kalkunte S, Boij R, Norris W, et al. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177(5):2387–2398. doi:10.2353/ajpath.2010.100475
20. Sato K, Idogawa S, Fujii T. Influence of acidity regulators on the stability of a soymilk colloidal dispersion system. Jpn J Food En. 2017;18(4):177–184. doi:10.11301/jsfe.17491
21. Josipovic A, Sudar R, Sudaric A, Jurkovic V, Matosa Kocar M, Markulj Kulundzic A. Total phenolic and total flavonoid content variability of soybean genotypes in eastern Croatia. Croat J Food SciTechnol. 2016;8(2):60–65. doi:10.17508/CJFST.2016.8.2.04
22. Mukherjee R, Chakraborty R, Dutta A. Role of fermentation in improving nutritional quality of soybean meal — a review. Asian-Australas J Anim Sci. 2015;29(11):1523–1529. doi:10.5713/ajas.15.0627
23. Lecarpentier E, Tsatsaris V. Angiogenic balance (sFlt-1/PlGF) and preeclampsia. Ann Endocrinol (Paris). 2016;77(2):97–100. doi:10.1016/j.ando.2016.04.007
24. Kaitu’u-Lino TJ, Tong S, Beard S, et al. Characterization of protocols for primary trophoblast purification, optimized for functional investigation of sFlt-1 and soluble endoglin. Pregnancy Hypertens. 2014;4(4):287–295. doi:10.1016/j.preghy.2014.09.003
25. Barokah L, Baktiyani SCW, Kalsum U. Protective effect of Theobroma cacao on nitric oxide and endothelin-1 level in endothelial cells induced by plasma from preeclamptic patients: in silico and in vitro studies. Eur J Integr Med. 2016;8(1):73–78. doi:10.1016/j.eujim.2015.11.023
26. Pramatirta AY, Laksono B, Fauziah PN, et al. Effects of low dose aspirin on caspase 3, TNF-α and apoptotic index levels in preclampsia maternal serum-induced placental trophoblast cell line in vitro. Int J Pharmtech Res. 2016;9:47–53.
27. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi:10.1056/NEJMoa055352
28. Mazurakova A, Koklesova L, Samec M, et al. Flavonoids exert potential in the management of hypertensive disorders in pregnancy. Pregnancy Hypertens. 2022;29:72–85. doi:10.1016/j.preghy.2022.06.007
29. Eddy AC, Chiang CY, Rajakumar A, et al. Bioflavonoid luteolin prevents sFlt-1 release via HIF-1α inhibition in cultured human placenta. FASEB J. 2023;37(8). doi:10.1096/fj.202300611R
30. Lee JH, Jeon JK, Kim SG, Kim SH, Chun T, Imm JY. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int J Food Sci Technol. 2011;46(12):2513–2519. doi:10.1111/j.1365-2621.2011.02775.x
31. Tuerxun D, Aierken R, Zhang Y, et al. Astragaloside IV alleviates lipopolysaccharide-induced preeclampsia-like phenotypes via suppressing the inflammatory responses. Kaohsiung J Med Sci. 2021;37(3):236–244. doi:10.1002/kjm2.12313
32. Wang H, Li M, Chen P, Shi X. Anti-inflammatory and antioxidant effects of pyrroloquinoline quinone in L-NAME-induced preeclampsia-like rat model. Reprod Sci. 2022;29(2):578–585. doi:10.1007/s43032-021-00743-8
33. Tossetta G, Fantone S, Piani F, et al. Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells. 2023;12(11):1545. doi:10.3390/cells12111545
34. Kemgang TS, Kapila S, Shanmugam VP, Kapila R. Cross-talk between probiotic lactobacilli and host immune system. J Appl Microbiol. 2014;117(2):303–319. doi:10.1111/jam.12521
35. Purchiaroni F, Tortora A, Gabrielli M, et al. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17(3):323–333.
36. Brantsaeter AL, Myhre R, Haugen M, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2011;174(7):807–815. doi:10.1093/aje/kwr168
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.