Back to Journals » Drug Design, Development and Therapy » Volume 9
Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: a randomized clinical trial in the People’s Republic of China
Received 10 November 2014
Accepted for publication 2 December 2014
Published 30 January 2015 Volume 2015:9 Pages 687—694
DOI https://doi.org/10.2147/DDDT.S77270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Tong Lin, Lan Gong
Department of Ophthalmology, Eye and ENT Hospital of Fudan University, Shanghai, People’s Republic of China
Purpose: To evaluate the effectiveness and safety of 0.3% sodium hyaluronate (HA) compared to recombinant bovine basic fibroblast growth factor (rb-bFGF) for the treatment of corneal epithelial abrasion caused by mechanical damage in Chinese patients.
Methods: Thirty patients were randomly assigned to the HA or rb-bFGF treatment group. The HA group was treated with 0.3% HA and 0.5% levofloxacin, and the rb-bFGF group was treated with topical rb-bFGF and 0.5% levofloxacin. The primary endpoint was the clinical effectiveness rates at day 3. Secondary endpoints were the dimensions of the wound area and the percentage of wound closure.
Results: After 3 days of treatment, the clinical effectiveness rates of the HA group and the rb-bFGF group were 86.67% (13/15) and 93.33% (14/15), respectively. The dimensions of the wound area were reduced from 9.83±8.50 to 0.02±0.06 mm2 for the HA group at day 7, and from 10.58±9.94 to 0.02±0.07 mm2 for the rb-bFGF group. At day 3, the wound closure was almost complete in both groups; 94.73% in the HA group compared to 95.77% in the rb-bFGF group (P>0.05).
Conclusion: Topical 0.3% HA provided a promising treatment for superficial corneal abrasion caused by mechanical damage in a manner similar to rb-bFGF.
Keywords: sodium hyaluronate, superficial corneal abrasion, bovine basic fibroblast growth factor
Introduction
Sodium hyaluronate (HA) is a high molecular weight and linear polysaccharide composed of b-1,3-N-acetyl glucosamine and b-1,4-glucuronic acid repeating disaccharide units, which is found in the vitreous body and is the primary glycosaminoglycan in the interfibrillar space of cross-linked collagen matrix in the cornea.1,2 HA has been shown to increase tear film stability, reduce the tear evaporation rate, and relieve dry eye symptoms such as ocular irritation and burning.3,4 Therefore, HA is now widely used as tear substitutes for dry eyes. Unlike other tear substitutes, HA was found to accelerate ocular surface wound healing, in addition to its water retention properties.5,6 The wound healing properties of HA are not mediated only by its mechanical protective role on epithelial cells because of its viscoelasticity, but also by its positive biological functions on corneal epithelial cells.7,8 The effect of HA on intracellular signaling and cell behavior is managed by binding to specific cell-surface receptors, including CD44 and the receptor for hyaluronan-mediated motility, and the activation of these receptors modulate cell proliferation and migration.7 Indeed, these receptors have been found in the human cornea and may be involved in the wound healing property of HA.9,10 Based on this evidence, HA tends to be used for promoting corneal reparation in different experimental and clinical situations.11,12
Corneal wounds caused by trauma, surgery, or disease are very common. Inadequate healing of epithelial injuries can lead to corneal haze, ulcers, perforations, persistent epithelial defect or even blindness.13 Therefore, the need for a quick and complete wound healing led to the application of growth factors such as basic fibroblast growth factor (bFGF). It is well-known that bFGF can stimulate the proliferation of corneal epithelial cells, stromal fibroblasts, and endothelial cells, and enhance corneal epithelial wound healing in vitro and in vivo.14–19 Topical recombinant bovine basic fibroblast growth factor (rb-bFGF) is extensively applied for the treatment of corneal wounds caused by trauma, surgery, or other corneal diseases in the People’s Republic of China.
To the authors’ knowledge, few clinical studies have been designed to investigate the effect of HA eye drops on corneal epithelial abrasion caused by mechanical damage. In our current study, we performed a randomized, open, parallel-group analysis of topical applications of 0.3% HA or rb-bFGF both combined with 0.5% levofloxacin for the treatment of corneal epithelial abrasion caused by mechanical damage in Chinese patients.
Material and methods
Study population
Participants aged ≥18 years, <70 years of age, with corneal epithelial abrasion caused by mechanical damage were selected. The corneal epithelial abrasions were mainly caused by common injuries including nails, branches, contact lenses, etc, which may lead to symptoms (stabbing ophthalmalgia, photesthesia, lacrimation, foreign body sensation) and signs (conjunctival congestion and positive corneal fluorescein staining). Of these, patients with overall score of symptoms and signs ≥5 points were enrolled. The patients who had the injuries involved more than half of the cornea or deep into the corneal stroma, or had concomitant infection of the injured cornea, or had severe blepharitis, corneal endothelial decompensation and hypophasis, etc were excluded. The study was conducted in the Eye, Ear, Nose, and Throat (EENT) Hospital of Fudan University, from May 2013 to August 2014.
Study design
In a randomized parallel-group interventional clinical study conducted in the People’s Republic of China, the efficacy of 0.3% HA (Santen Pharmaceutical Co., Ltd, Ishikawa, Japan) was compared to topical rb-bFGF (Zhuhai Yisheng Biological Pharmaceutical, Zhuhai, People’s Republic of China), both combined with 0.5% levofloxacin (Santen Pharmaceutical). This study was conducted in compliance with the Declaration of Helsinki, and approved by the Ethics Committee of the EENT Hospital of Fudan University. All of the participants provided written informed consent before participation in our study.
In the beginning of the treatment period, 30 subjects were randomized corresponding to allocation codes generated for the treatment group and control group using the permuted block method, by 1:1 ratio. The study period was 7 days. During treatment period, either 0.3% HA or rb-bFGF were given as study drugs to the treatment group and control group. Dosing frequency was four times daily for both drugs. Both groups were also treated with an ophthalmic solution containing 0.5% levofloxacin three times per day in the “study eye”. During the clinical study, concomitant use of any treatment which may affect efficacy assessment was prohibited, including other ophthalmic drugs, topical corticosteroids, or remedial contact lenses.
Follow-up examinations were performed at 1, 3, and 7 days after the first treatment. All of the patients underwent subjective symptom evaluation (stabbing ophthalmalgia, photesthesia, lacrimation, foreign body sensation); slit lamp microscopy examinations including fluorescein staining of the cornea, conjunctival congestion evaluation, and digital image. Visual acuity (VA) was evaluated with logarithm of the minimum angle of resolution (logMAR) VA chart at each follow-up examination. In addition, safety was assessed and compared.
Efficacy and safety evaluation
The primary endpoint was the clinical effectiveness rates (CERs) at day 3. The effectiveness of both groups was evaluated according to the changes of the total scores of clinical indicators (TSI) and therapeutic index. The criteria are shown in Table 1.
The CER was calculated according to the following equation:
CER (%) = (number of patients indicating significant effectiveness+ numbers of patients indicating effectiveness)/total number of patients. | (1) |
The clinical indicators consisted of the symptoms (stabbing ophthalmalgia, photesthesia, lacrimation, foreign body sensation) and signs (conjunctival congestion and corneal fluorescein staining). The subjective symptoms were scored on a 4-point scale, with scores of 0 to 3 (with increasing severity). Conjunctival congestion was graded on a 5-point scale, with scores of 0 to 4 (with increasing severity). Fluorescein staining scores were measured on a 0 to 6 point scale: 0 (no staining), 2 (some staining), 4 (staining in more than half of the area), 6 (staining in the whole area).
Secondary endpoints were the dimensions of the wound area and the percentage of wound closure. Epithelial wound healing images were taken during the slit lamp examination with an integrated digital camera system (SL-D4 and DC-3; Topcon Medical Systems, Tokyo, Japan), and the dimensions of the wound area were measured using image analysis software (Image J; Scion Corp, Frederick, MD, USA). The percentage of wound closure was calculated using the following formula:
A0−Ax/A0 * 100 (%), | (2) |
where A0 is the dimension of the epithelial abrasion area at the baseline, and Ax is the dimension of the wound area on day 1, 3 and 7.
Safety parameters were incidences of adverse events, and other ophthalmological examination (slit lamp microscopy, VA).
Statistics
All data have been analyzed with the SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Wilcoxon rank-sum test or the χ2 test was conducted on the demographic and clinical characteristics of the participants at the baseline. The Fisher’s exact test was utilized to compare the indicators of the effectiveness and CER for both groups. The TSI at different examination time points were compared using a Wilcoxon rank-sum test. The tolerance rates were compared with Fisher’s exact test. A P-value of <0.05 was considered significant.
Results
Patient baseline characteristics
There were no significant differences between the baselines of age, sex, TSI, the dimensions of the wound area, and VA (P>0.05) (Table 2). The average age of patients in the treatment group was 42.33±13.17 years, and that of patients in the control group was 39.33±14.18 years (P=0.3829). The TSI of the treatment and control groups before administration were 13.80±3.14 and 13.73±4.46, respectively (P=0.9832). The dimensions of the wound area before administration were 9.83±8.50 mm2 and 10.58±9.94 mm2 for the treatment group and the control group, respectively (P=0.8195). The average baseline VA of the treatment group and the control group was 0.300±0.053 and 0.298±0.055 (P=0.9734), respectively.
Effectiveness analysis
Clinical effectiveness
After 3 days of treatment, the CERs of the treatment group and the control group were 86.67% (13/15) and 93.33% (14/15), respectively (P=0.500) (Table 3). All subjects were completely cured at day 7, therefore both groups shared the same CER of 100% at this visit time point.
  | Table 3 Clinical effectiveness of treatment group and control group at day 3 |
Change of TSI
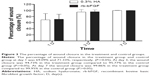
The TSI at baseline were 13.80±3.14 and 13.73±4.46 for the treatment group and control group, respectively. The corresponding scores at visit time points of 1, 3, and 7 days decreased to 8.53, 3.06, and 0.62 for the treatment group, and 7.93, 2.13, and 0.50 for the control group, respectively (Figure 1). However, there were no significant differences between the two groups at all visit time points (P>0.05).
Corneal epithelial wound healing
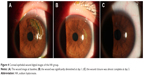
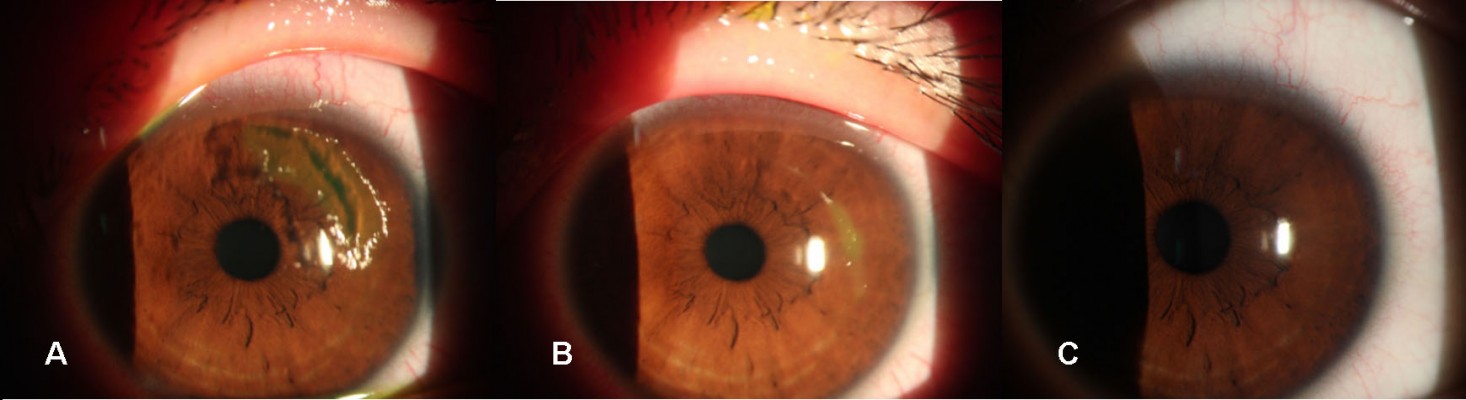
The dimensions of the wound were reduced significantly after administration of 0.3% HA or rb-bFGF in the treatment group and the control group. The damaged areas were 9.83±8.50, 3.25±4.12, 0.54±0.87, and 0.02±0.06 mm2 for the treatment group at baseline and each visit time point of 1, 3, and 7 days, and 10.58±9.94, 3.24±3.52, 0.73±1.85, and 0.02±0.07 mm2 for the control group, respectively (Figure 2). However, there were no significant differences between the two groups at all visit time points (P>0.05). Quantification of the digital images from the slit lamp camera indicated that wound closure at day 1 in the treatment group was 69.09%, and wound closure in the control group was 71.33% (P>0.05). At day 3, the wound closure was almost complete in both groups; 94.73% in the treatment group compared to 95.77% in the control group (P>0.05) (Figure 3, Figure 4).
Safety profile
No serious adverse events were observed in the treatment and control groups. In addition to assessing symptom responses to the therapies, participants were asked about discomfort sensations related to the study drop instillations. At each visit time point of 1, 3, and 7 days, the percentage of patients complaining about transient eye burning was 33.33% (five of 15 subjects), 20.00% (three of 15 subjects) and 0.00% (zero of 13 subjects) in the treatment group, and 40.00 % (six of 15 subjects), 13.33% (three of 15 subjects) and 0.00% (zero of 12 subjects) in the control group (P>0.05) (Table 4).
  | Table 4 Rates of patients reporting eye irritation |
Discussion
HA serves not only as an essential extracellular matrix component, but also as an important extracellular signal to the cells in many biological phenomena, such as wound healing.20 This function seems to be dependent on the concentration of HA.21–23 It was found that the addition of HA to the culture medium increased the length of the path of the corneal epithelial layer in a dose-dependent fashion in in vitro experiment.22 Faster wound reparation was found when HA was administered as eye drops after corneal epithelial wound in a rabbit.11,21 Results from the study by Camillieri et al23 showed HA enhanced corneal epithelial healing in certain concentrations in a dose-dependent manner: the two low concentrations, 0.015% and 0.1%, were less effective than the high concentrations, 0.2% and 0.4%. The maximum effect was observed with the 0.4% HA concentration, although the two high concentrations, 0.2% and 0.4%, were not significantly different in the amount of time needed to complete corneal wound healing. We suspect that the higher concentration of HA had no effect because the concentration of 0.4% provides sufficient HA to induce maximal effect of accelerating wound healing. Thus, these data suggest that the clinical application of HA in corneal epithelial alterations should take into account the concentration of this substance in eye drop solutions. In the current study, HA (0.3%) was used to treat superficial corneal abrasion caused by mechanical damage in Chinese patients.
After 1 day of treatment, the reduction percentage of corneal wound closure already reached 69.09% and 71.33% for the treatment group and the control group, respectively. However, Yang et al24 assessed the effect of cross-linked derivative of hyaluronan (CMHA-SX) on the rabbit corneal epithelial wound and found that the wound closure in CMHA-SX treated eyes was 82.8% complete at day 1. There are two potential reasons for this difference. Firstly the hydrogel-typed CMHA-SX was formulated to remain in place for a longer time which showed better adhesiveness than HA eye drops.25 Secondly species difference is also one of the reasons for the different responses to the same ingredient of HA.
The corneal wound closure percentages were 94.73% and 95.77% for the treatment group and the control group at day 3, which suggested that the time course of corneal epithelium wound closure is approximately 3 days for 0.3% HA or rb-bFGF treatment. These results could potentially be explained by evidence from previous studies, both in vitro and in vivo. When topically instilled into the eye, HA has been shown to promote physiological wound healing by stimulating corneal epithelial migration and proliferation of keratocytes and to reduce the healing time of corneal epithelium,22,26 which is similar to the action of bFGF. Another important feature of HA, with high molecular weight and anionic biopolymer, is its mucoadhesivity, which provides effective coating and long-lasting protection of the cornea as well as extended residence times on the ocular surface.27–29 However, the study from Schulze et al found that the mean corneal wound closure time was 7.1 days in the HA treatment group,30 which was much longer than our finding. The differences can be explained by several potential reasons. As well known, corneal wound healing can be delayed in diabetic patients,31 which could be the main reason for the longer wound closure time in Schulze et al’s study. In the current study, all the subjects did not have a history of diabetic disease. Secondly, the different concentrations of HA (0.18% in Schulze et al’s study; 0.3% in our study) may also cause the different effect on corneal wound healing, because the wound healing property of HA has been shown to be dependent on the concentration.21–23
The corneal epithelium, several layers thick, is a barrier composed of a tightly linked network of cells attached by hemidesmosomes and gap junctions, serving as the eye’s first line of immunological defense.32 Following corneal abrasion, the eye typically epithelializes and resurfaces the wound uneventfully. However, under certain clinical conditions, such as chemical injury, healing of the corneal epithelium is delayed, leaving the underlying stroma vulnerable to infection and ulceration.33– In fact the therapeutic use of growth factors in corneal disease needs to be defined not only to accelerate and modulate cell proliferation in the corneal districts, but also to provide a preferable micro-environment for corneal re-epithelialization without complications such as infection, keratitis, or corneal haze. HA and rb-bFGF as topical eye drops for faster wound reparation are beneficial to prevent corneal haze, corneal infection, and persistent epithelial defect in relation to inadequate healing of corneal injuries. However, Yan et al found that rb-bFGF had a promotive effect on corneal neovascularization except for its wound healing property.34 Corneal neovascularization may not only reduce VA but also worsen the prognosis of subsequent penetrating keratoplasty.35–37 Therefore, HA could be used as an alternative drug to promote corneal wound healing without the limitation of rb-bFGF.
No serious adverse events were found in this study. In terms of the transient eye burning related to the study drop instillations, it can be explained by the fact that superficial corneal abrasion exposes more corneal nerves under the epithelium which could make the injured cornea more sensitive to the eye drops.38
A limitation of our study was the relatively small number of participants, who have been evaluated in only one center, and the efficacy of 0.3% HA for superficial corneal abrasion requires further verification in a larger study population.
In conclusion, the present preliminary study shows promising results for the use of topically applied HA (0.3%) for the treatment of superficial corneal abrasion caused by mechanical damage in Chinese patients.
Acknowledgment
The authors gratefully acknowledge Naiqing Zhao of the Department of Biostatistics, School of Public Health, Fudan University, for help with the statistical analyses.
Disclosure
The authors report no conflicts of interest in this work. The authors had no financial interests in this study.
References
Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6(7):2397–2404. | ||
Balazs EA, Armand G. Glycosaminoglycans and proteoglycans of ocular tissues. In: Varma RS, Varma R, editors. Glycosaminoglycans and Proteoglycans in Physiological and Pathological Processes of Body Systems. Basel: Karger; 1982:480–499. | ||
Aragona P, Di Stefano G, Ferreri F, Spinella R, Stilo A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br J Ophthalmol. 2002;86(8):879–884. | ||
Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):109–112. | ||
Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86(2):181–184. | ||
Yu F, Liu X, Zhong Y, et al. Sodium hyaluronate decreases ocular surface toxicity induced by benzalkonium chloride-preserved latanoprost: an in vivo study. Invest Ophthalmol Vis Sci. 2013;54(5):3385–3393. | ||
Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. | ||
Underhill CB. The interaction of hyaluronate with the cell surface: The hyaluronate receptor and the core protein. Ciba Found Symp. 1989;143:87–99. | ||
Zhu S, Nolle B, Dunker G. Expression of the adhesion molecule CD44 on human corneas. Br J Ophthalmol. 1997;81(1):80–84. | ||
Goa KL, Benfield P. Hyaluronic acid: A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47(3):536–566. | ||
Sugiyama T, Miyauchi S, Machida A, et al. The effect of sodium hyaluronate on the migration of rabbit corneal epithelium. II. The effect of topical administration. J Ocul Pharmacol. 1991 Spring;7(1):53–64. | ||
Inoue M, Katakami C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1993;34(7):2313–2315. | ||
Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: a review. Acta Ophthalmol Scand. 2002;80(3):238–247. | ||
Hoppenreijs VP, Pels E, Vrensen GF, et al. Basic fibroblast growth factor stimulates corneal endothelial cell growth and endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994;35(3):931–944. | ||
Hecquet C, Morisset S, Lorans G, Plouet J, Adolphe M. Effects of acidic and basic fibroblast growth factors on the proliferation of rabbit corneal cells. Curr Eye Res. 1990;9(5):429–433. | ||
Scalinci SZ, Scorolli L, Meduri A, et al. Effect of basic fibroblast growth factor and cytochrome c peroxidase combination in transgenic mice corneal epithelial healing process after excimer laser photoablation. Clin Ophthalmol. 2011;5:215–221. | ||
Hu C, Ding Y, Chen J, et al. Basic fibroblast growth factor stimulates epithelial cell growth and epithelial wound healing in canine corneas. Vet Ophthalmol. 2009;12(3):170–175. | ||
Rieck P, Assouline M, Savoldelli M, et al. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: corneal wound healing effect and pharmacology. Exp Eye Res. 1992;54(6):987–998. | ||
Fredj-Reygrobellet D, Plouet J, Delayre T, et al. Effects of aFGF and bFGF on wound healing in rabbit corneas. Curr Eye Res. 1987;6(10):1205–1209. | ||
Turley EA. Hyaluronan and cell locomotion. Cancer Metastasis Rev. 1992;11(1):21–30. | ||
Nakamura M, Hikida M, Nakano T. Concentration and molecular weight dependency of rabbit corneal epithelial wound healing of hyaluronan. Curr Eye Res. 1992;11(10):981–986. | ||
Nishida T, Nakamura M, Mishima H, et al. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991;53(6):753–758. | ||
Camillieri G, Bucolo C, Rossi S, Drago F. Hyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effect. J Ocul Pharmacol Ther. 2004;20(6):548–553. | ||
Yang G, Espandar L, Mamalis N, Prestwich GD. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol. 2010;13(3):144–150. | ||
Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25(7–8):1339–1348. | ||
Tani E, Katakami C, Negi A. Effects of various eye drops on corneal wound healing after superficial keratectomy in rabbits. Jpn J Ophthalmol. 2002;46(5):488–495. | ||
Saettone MF, Monti D, Torracca MT, et al. Mucoadhesive ophthalmic vehicles: evaluation of polymeric low-viscosity formulations. J Ocul Pharmacol. 1994 Spring;10(1):83–92. | ||
Greaves JL, Wilson CG. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv Drug Delivery Rev. 1993;11:349–383. | ||
Saettone MF, Chetoni P, Torraca MT, et al. Evaluation of muco-adhesive properties and in vivo activity of ophthalmic vehicles based on hyaluronic acid. Int J Pharm. 1989;51:203–212. | ||
Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142(2):207–211. | ||
Chen WL, Lin CT, Ko PS, et al. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology. 2009;116(6):1038–1047. | ||
Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit affects epithelial properties. Exp Neurol. 1980;69(1):196–201. | ||
Ambrosone L, Guerra G, Cinelli M, et al. Corneal epithelial wound healing promoted by verbascoside-based liposomal eyedrops. Biomed Res Int. 2014;2014:471642. | ||
Yan L, Wu W, Wang Z, et al. Comparative study of the effects of recombinant human epidermal growth factor and basic fibroblast growth factor on corneal epithelial wound healing and neovascularization in vivo and in vitro. Ophthalmic Res. 2013;49(3):150–160. | ||
Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12(4):242–249. | ||
Cursiefen C, Kuchle M, Naumann GO. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea. 1998;17(6):611–613. | ||
Dana MR, Schaumberg DA, Kowal VO, et al. Corneal neovascularization after penetrating keratoplasty. Cornea. 1995;14(6):604–609. | ||
Menghini M, Knecht PB, Kaufmann C, et al. Treatment of traumatic corneal abrasions: a three-arm, prospective, randomized study. Ophthalmic Res. 2013;50(1):13–18. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.