Back to Journals » Journal of Asthma and Allergy » Volume 17
Smoking, Urban Housing and Work-Aggravated Asthma are Associated with Asthma Severity in a Cross-Sectional Observational Study
Authors Chevereau-Choquet M, Thoreau B, Taillé C, Marchand-Adam S, Morel H, Plantier L, Portel L
Received 6 June 2023
Accepted for publication 2 December 2023
Published 1 February 2024 Volume 2024:17 Pages 69—79
DOI https://doi.org/10.2147/JAA.S424546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Marie Chevereau-Choquet,1 Benjamin Thoreau,2,3 Camille Taillé,4,5 Sylvain Marchand-Adam,1,6 Hugues Morel,7 Laurent Plantier,1,6,* Laurent Portel8,*
1Service de Pneumologie et Explorations Fonctionnelles Respiratoires, CHRU de Tours, Tours, France; 2Service de Médecine Interne, National Referral Center for Rare Systemic Autoimmune Diseases, AP-HP, Hôpital Cochin, Paris, France; 3Inserm U1016, CNRS UMR8104, Université Paris Cité, Institut Cochin, Paris, France; 4Service de Pneumologie A, AP-HP Nord, Hôpital Bichat Claude Bernard, Paris, France; 5Inserm U1152, Université Paris Cité, Paris, France; 6CEPR, Inserm UMR1100, Université de Tours, Tours, France; 7Service de Pneumologie, CHR d’Orléans, Orléans, France; 8Service de Pneumologie, Centre Hospitalier Robert Boulin, Libourne, France
*These authors contributed equally to this work
Correspondence: Laurent Plantier, Service de Pneumologie et Explorations Fonctionnelles Respiratoires, CHRU de Tours, 2 Bd Tonnellé, Tours, 37044, France, Email [email protected]
Purpose: Severe asthma affects 5 to 10% of asthmatics and accounts for a large part of asthma-related morbidity and costs. The determinants of asthma severity are poorly understood. We tested the hypothesis that asthma severity was associated with 1) atopy and allergy and 2) markers associated with environmental exposure.
Patients and Methods: Data from the FASE-CPHG study, a cross-sectional, observational, multicenter investigation, were analyzed to identify markers associated with asthma severity. Asthma severity was gauged using the ASSESS score, encompassing symptom control, exacerbations, FEV1 and therapeutic load. Bivariate and multivariate analyses were used to identify patient characteristics associated with the ASSESS score.
Results: The analysis involved 948 patients, with 592 women, of which 447 patients (47%) had severe asthma. Among these, 491 patients (52%) had at least one positive aeroallergen skin prick test and 525 (55%) had at least one allergic disease among atopic dermatitis, chronic rhinitis and food allergy. The mean±SD ASSESS score was 11.2± 3.4. Characteristics associated with a higher ASSESS score were female sex, secondary or lower education, unemployed occupational status, smoking, work-aggravated asthma and urban housing. There was no association between the ASSESS score and allergic diseases, aeroallergen-specific skin prick tests and IgEs, or blood eosinophil counts.
Conclusion: While atopy and allergy were frequent among asthmatics, neither was associated with asthma severity. Modifiable environmental factors such as smoking, urban housing and work-aggravated asthma were independently associated with asthma severity.
Keywords: allergy, ASSESS, asthma, environment, severity
Introduction
Asthma is a common heterogeneous chronic disease which affects approximately 300 million people worldwide.1 Asthma etiology is multifactorial and involves individual susceptibility to the disease. At the molecular level, multiple gene polymorphisms drive personal susceptibility to asthma.2 At the clinical level, atopy is a key marker of an increased risk of asthma.3 Exposure to environmental factors also appears to play a key role in the onset of asthma. Environmental factors such as tobacco smoke, airborne allergens and non-specific irritants in the home or the workplace are of particular interest since they may modifiable, with the potential to alter the disease’s trajectory when corrected.4 The classification of asthma into distinct phenotypes, based on specific demographic, clinical, and pathophysiological characteristics, aids in understanding and managing the disease. The main clinical phenotypes of asthma are allergic asthma, non-allergic asthma, adult-onset asthma, asthma with persistent airflow limitation and asthma with obesity.5 The main pathophysiological feature associated with clinical characteristics and treatment response is type 2 inflammation.5
Although asthma is mild in most patients, 5 to 10% of patients are affected by difficult-to-treat or severe asthma.6 Better understanding of the mechanisms of asthma holds the key to refining its management. In particular, the factors contributing to asthma severity are not well known beyond the role of persistent airway inflammation.
A comprehensive evaluation of asthma severity encompasses symptoms, exacerbations, and the intensity of treatment necessary for symptom and exacerbation control. The multidimensional Asthma Severity Scoring System (ASSESS) was developed in the setting of the Severe Asthma Research Program.7 The ASSESS score expands on the Composite Asthma Severity Index, which was developed in children, and lacks precision in gauging severity at the higher end of the controller treatment spectrum.8 The ASSESS score globally quantifies asthma severity by integrating symptom control via the Asthma Control Test (ACT) score,9 the occurrence of exacerbations in the last 6 months, lung function evaluated by the FEV1, and therapeutic load assessed in a similar way to 2016 Global Initiative for Asthma (GINA) levels.
In the present work, we analyzed data from a large French multicenter cross-sectional study to identify factors associated with asthma severity. Specifically, we tested the hypothesis that the ASSESS score was associated 1) with atopy and allergy and 2) with markers indicative of environmental exposure.
Methods
Data Collection
The France Asthme Severe (FASE-CPHG) study was an observational, cross-sectional, multicenter, national study which aimed to describe the characteristics of asthma patients aged 18 or older under the care of secondary care hospital pulmonology departments in either outpatient or inpatient settings. The methodology of the FASE study has been described in detail previously.10 Briefly, patients were included if they had a pulmonologist’s diagnosis of asthma and asthma was either 1) uncontrolled or partially controlled despite treatment including either inhaled low-dose corticosteroid therapy (<500 µg beclometasone equivalent) combined with a long-acting bronchodilator or medium-dose (500 µg) inhaled corticosteroid therapy without a long-acting bronchodilator, or 2) controlled by treatment including either ≥500 µg/day of inhaled beclometasone equivalent combined with a long-acting bronchodilator or ≥1000 µg/day of inhaled beclometasone without a long-acting bronchodilator, or long-term oral corticosteroid therapy, or monoclonal antibodies. Patients with cancer or hematological malignancy and those unwilling to provide verbal consent were not included. The study was approved by the Advisory Committee on Information Processing in Health Research and was conducted in accordance with French law in force at the time of the study (CCTIRS, 15–864). Notably, the study was considered data-driven and written informed consent was not required.
Data from the FASE study were collected between May 2016 and June 2017, involving 1377 patients who visited a pulmonologist among 104 French hospital pulmonology departments. The data included social and demographic characteristics, triggers of asthma symptoms, medical history and comorbidities, as well as lung function testing data, blood cell counts, skin prick tests to aeroallergens, allergen-specific immunoglobulin E (IgE), treatments, and a self-administered questionnaire. The questionnaire covered the ACT score, the number of unscheduled visits and the number of asthma exacerbations requiring a burst of oral corticosteroids or hospitalization in the preceding 12 months. In the present study, we specifically used data on atopy and allergy, risk factors of environmental exposure, and asthma severity. Information on blood eosinophilia (defined as eosinophils > 300/mm3) and drug allergies was also obtained.
Atopy was defined by at least one positive skin prick test to aeroallergen extracts. The number of positive skin prick tests was used as an index of atopy severity. Allergy was defined by the declaration of one or more of the following conditions: food allergy, atopic dermatitis and chronic rhinitis.
Given the subjective nature of many patient-declared FASE data, the collection of environmental exposure information focused on objective items. Pet ownership, housing type (urban or rural), smoking status and smoking history expressed in pack-years, occupational status (active or unemployed or retired, and current occupation when available), and patient-declared work-aggravated asthma were employed as markers of environmental exposure relevant to asthma. Additionally, the educational level (secondary or lower education) was documented.
Asthma phenotypes were defined as follows. Allergic, early asthma was defined as the asthma onset under 18 years and at least one allergic disease. Non-allergic, late onset asthma was defined as asthma onset ≥18 years and no allergic disease. Asthma with persistent airflow limitation was defined as FEV1/FVC < 0.7. Asthma with obesity was defined as body mass index (BMI) >30.
Asthma severity was quantified by the ASSESS score7 which ranges from 0 to 22, where higher scores indicated greater severity. The ASSESS score integrated asthma control quantified by the ACT,9 lung function quantified by FEV1 expressed as a percentage of the value predicted by GLI2012 equations,11 therapeutic load, and the number of exacerbations requiring oral corticosteroids in the preceding 6 months. A dose of inhaled corticosteroids <500 µg beclometasone equivalent was considered low, while beclometasone equivalent >1000 µg/day was considered a high dose. Patients in whom missing data precluded calculation of the ASSESS score were excluded.
Statistical Analyses
The main objective was to determine whether allergy, atopy and indicators of environmental exposure were associated with the ASSESS score. Secondary objectives were to determine their association with components of the ASSESS score, and the association of asthma phenotypes with the ASSESS score.
Continuous variables were presented as means ± standard deviation. Discontinuous variables were described by number (n) and proportion (%). A univariate linear regression model followed by a multivariate linear regression model incorporating all variables with a p-value <0.05 in the univariate analysis was used to identify independent patient characteristics associated with the ASSESS score. Subsequently, for each component of the ASSESS score (ACT, exacerbations, number of unscheduled visits in the last 12 months, FEV1, dose of inhaled corticosteroids, type of controller treatment), associations with patient characteristics were sought by linear or logistic regression depending on the nature of the component (continuous or discontinuous). The ASSESS score was compared in patients with defined asthma phenotypes and the remaining patients using Student’s unpaired t-test. A value of p<0.05 was considered significant.
Results
Patient Characteristics
Out of the initial 1377 patients in the FASE-CPHG study, 429 patients were excluded due to missing data preventing calculation of the ASSESS score. Consequently, the analysis was performed on 948 patients.
The main patient characteristics are summarized in Table 1. The ratio of women to men was 1.7:1. Age was 54 ± 16 years and the age of asthma onset was 27±21 years. Body mass index was 28±6 kg/m2. At least one allergic disease was declared by 525 (55.4%) patients. Among these patients, the mean number of allergic diseases was 1.41±0.63. Food allergy was reported in 92 (9.7%) patients. Atopic dermatitis was reported in 137 (14.4%) patients. Chronic rhinitis, chronic rhinosinusitis and nasal polyposis were reported in 439 (46.3%), 241 (25.4%) patients and 179 (18.8%) patients, respectively.
 |
Table 1 Patient Characteristics |
Aeroallergen-specific prick tests were performed in 694 patients, of whom 491 patients had at least one positive prick test. Among the 694 patients who had prick tests, the average number of positive tests was 1.5±1.4. House dust mite extract was the most prevalent aeroallergen. Aeroallergen-specific IgE assays were performed in 352 patients, with positive results detected in 205 patients. All patients with aeroallergen-specific IgE also had positive prick tests, thus a total of 491 patients (51.8%) were considered atopic. The average blood eosinophil count was 447±459/mm3 with levels exceeding 300/mm3 in 399 patients (42.1%). The mean total IgE level was 530±1046 IU/L.
Occupational information was available in 919 patients. A current occupation was declared by 395 patients (43.0%), while 196 were unemployed (21.3%) and 328 were retired (35.7%). Within the subset of 120 patients (12.7%) who reported work-aggravated asthma, 63 patients were actively employed at the time of the study, 30 were unemployed, and 25 were retired. Educational attainment was known for 726 patients. Education was secondary or lower in 476 patients (65.5%). One hundred and four patients were active smokers (11.0%), 291 patients were former smokers (30.9%) and 553 patients were never-smokers (56.2%). Urban housing was reported by 465 patients (49.1%). The presence of at least one pet in the home was reported by 392 patients (41.3%). Computed tomography of the chest revealed airway wall thickening in 195 patients (20.6%), any degree of bronchiectasis in 94 patients (9.9%), and any degree of emphysema in 70 patients (7.4%).
Asthma Severity
Among the 948 patients, 447 were identified as having severe asthma based on the administration of at least high-dose inhaled corticosteroids combined with a long-acting bronchodilator (279 patients) and/or receiving oral corticosteroids for a minimum of 6 months in the preceding year (168 patients), in line with American Thoracic Society/European Respiratory Society guidelines.12 Of the remaining 501 patients, 412 had moderate asthma and 89 had mild asthma (treated with low-dose ICS).5
The mean ASSESS score was 11.2 ± 3.4. ASSESS score components are described in Table 2. A majority of patients (641 patients – 67.6%) were treated with a combination of inhaled steroids and long-acting bronchodilators. Long-term oral corticosteroid therapy was prescribed for 168 patients (17.7%) while 266 patients (28.1%) were treated with omalizumab at the time of inclusion. Ten (10.6%) patients were treated with mepolizumab. Over the preceding 12 months, 443 (46.7%) patients experienced at least one exacerbation requiring oral corticosteroids, and 249 (26.2%) required hospitalization at least once. The mean FEV1 was 68 ± 20% predicted. FEV1 was less than 80% predicted in 340 patients (35.8%).
 |
Table 2 ASSESS Score Components |
Variables Associated with the ASSESS Score
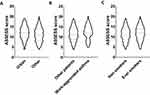
Bivariate and multivariate analysis were employed to identify variables associated with the ASSESS score (Table 3). In the bivariate analysis, significant associations were observed between the ASSESS score and female sex, increasing age, smoking, secondary or lower education, unemployed and retired occupational status, work-aggravated asthma, urban housing, and pet ownership. There was no association between the ASSESS score and the body mass index. There was also no association with personal atopy, the presence of positive aeroallergen-specific skin prick tests and their number, the presence of positive aeroallergen-specific blood IgEs and their number, and the presence of allergic diseases and their number. There was also no association with drug allergies and blood eosinophil counts. Figure 1 illustrates the bivariate associations between the ASSESS score and urban housing, work-aggravated asthma, and smoking.
 |
Table 3 Markers Associated with the ASSESS Score by Bivariate and Multivariate Analysis |
The variables that exhibited significant associations with the ASSESS score in the bivariate analysis were used in a multivariate analysis. In the multivariate analysis, the variables positively associated with the ASSESS score were female sex (β-coefficient=0.85, p=0.001), smoking history expressed in pack years (β=0.03 per unit, p=0.006), secondary or lower education (β=0.90, p=0.001), unemployed occupational status ((β=0.87, p=0.006), work-aggravated asthma (β=0.82, p=0.022), and urban housing (β=1.16, p<0.001). There was no association between the ASSESS score and either the presence of pet ownership, positive skin prick tests, the number of allergic diseases, or blood eosinophils.
Components of the ASSESS Score
Table 4 reports variables associated with the components of the ASSESS score in the multivariate analysis. Of note, both female sex and markers of environmental exposure were associated with the ACT and the frequency of exacerbations.
 |
Table 4 Variables Associated with the Components of the ASSESS Score in Multivariate Analysis |
Association of Asthma Phenotypes with the ASSESS Score
Among the 948 patients, 245 (25.8%) met the criteria for the “allergic early asthma” phenotype, 271 (28.5%) were classified as having the “non-allergic late asthma” phenotype, 469 (49.4%) exhibited persistent airflow limitation, and 289 (30.5%) were obese. Patients with persistent airflow limitation had a higher ASSESS score when compared to patients without this limitation (12.4±3.2 vs 10.5±3.3, p<0.001). The other phenotypes were not associated with higher severity of asthma.
Discussion
The main finding of this study was the strong association between asthma severity and markers of modifiable environmental exposure such as smoking, work-aggravated asthma, and urban housing. Additionally, asthma severity was associated with female sex, secondary or lower education, and unemployment. By contrast, there was no association between asthma severity and either atopy or allergy.
Several variables directly describing exposure or linked to an increased risk of exposure were independently associated with the ASSESS score. In the present study, the association of smoking with the ASSESS score was driven by its association with multiple dimensions of severity such as the ACT score, exacerbations, and lower FEV1. Smoking is well known to promote asthma exacerbations and worsen control,13 while smokers with asthma respond less to oral or inhaled corticosteroids than non-smokers.14 Smoking is a modifiable trait and is accessible to therapeutic intervention. Smoking cessation is a priority for asthmatic smokers.
Patients residing in urban areas exhibited a higher ASSESS score due to a lower ACT score, lower FEV1, and more frequent exacerbations. The association between urban housing and poor asthma control is in line with previous research.15,16 Although the mechanisms driving the association between urban living and asthma severity remain to be fully clarified, urban settings are characterized by more severe outdoor and indoor air pollution. Earlier studies show that air pollution can trigger asthma exacerbations,16 is associated with increased asthma symptoms,17 and is associated with lower lung function in population studies.18 Although modifying urban housing directly may pose challenges, mitigating its detrimental influences, especially indoor and outdoor air pollution, holds promise as a feasible intervention. The association between patient-reported work-aggravated asthma and the ASSESS score appeared driven by the link between this marker and poor asthma control. The lack of comprehensive information on occupation and occupational exposure to sensitizing or irritating agents in the FASE dataset did not allow us to explore the mechanisms of this association.
An intriguing result of this study was that, although atopy and allergy were frequent in our population, there was no association between atopy, allergy and asthma severity. Likewise, the ASSESS score of patients with the allergic, early asthma phenotype was similar to other patients and patients with the non-allergic, late onset phenotype.19 This result was consistent with literature data which suggests that atopy and allergy probably play an etiological role in the development of severe asthma but are not critical factors of poor control once the disease is established, at least in adults. Early sensitization is associated with the development of severe asthma in adulthood,20 which explains the high prevalence of positive prick tests in this category of asthmatics. However, in adult patients with late-onset severe asthma, symptoms do not seem to be aggravated by allergen exposure or associated with symptoms of allergic rhinitis symptoms, unlike in patients with mild asthma.21 Another hypothesis for the lack of an association between atopy, allergy and asthma severity may be the overall better response to inhaled corticosteroids in patients with these traits compared to patients without either atopy or allergy.22 The sensitization rate (51.8%) and the proportion of patients with late-onset asthma in our population were in alignment with other cohorts,23–25 although the mean age of patients (54±16 years) in our cohort was higher than in other cohorts such as the SARP3 cohort (44±1 years) where the ASSESS score was developed.7
Blood hypereosinophilia was a prevalent finding in this study, consistent with previous series.26 Although blood hypereosinophilia was not associated with asthma severity, this observation poses interpretative challenges. It is possible that treatments likely to reduce blood eosinophil counts were confounding factors. For instance, 168 patients (18%) received long-term oral corticosteroid therapy in the present study, which could have impacted blood eosinophil counts. It is also possible that patients with blood eosinophilia may in fact be more treatment responsive therefore reducing asthma severity, although the study took place in 2016 and 2017, when few patients were treated with monoclonal antibodies targeting interleukin-5 or its receptor and monoclonal antibodies targeting interleukins-4 and −13 or epithelial alarmins were not yet available. Interestingly, the lack of an association between age at asthma onset, allergy, blood eosinophils, and the ASSESS score suggests no direct link between major asthma phenotypes and severity.
The ASSESS score was higher in women, consistent with previous observations. This association was related to a lower ACT score and more frequent exacerbations. Women exhibit a higher prevalence of asthma, including severe asthma, compared to men,27,28 and a heightened risk of hospitalization for acute asthma.24 The mechanisms underlying the greater severity of asthma in women are poorly understood. This study strengthens the hypothesis of an independent role of female sex in asthma severity, transcending environmental factors. Among the 120 patients with work-aggravated asthma, 63 were women.
This study has limitations. Patients in the FASE study were adults, and were older than in other cohorts, thus the generalizability of our results to pediatric or young-adult populations may be limited. It is possible that some patients also had chronic obstructive pulmonary disease since 16.6% had a smoking history ≥ 20 pack years and pulmonary emphysema was reported in 7.4% patients. Although obesity is associated with poor treatment response, no association was found between BMI over 30 and the ASSESS score. This finding is difficult to interpret and may suggest the need for a more nuanced definition of obesity. Because the study was conducted before the wide availability of biologic therapies targeting the interleukin-4, −5 and −13 pathways or epithelial alarmins, and because any data on the pre-inclusion period was lacking, the issue of clinical remission29 could not be addressed. Although the association between secondary or lower education and asthma severity reproduced the previously reported poorer asthma control and higher risk of exacerbation in asthmatics with low educational attainment, the underlying mechanisms remain unclear. Although one hypothesis involves a greater likelihood of occupational exposure in patients with low education, other factors may contribute to increased asthma severity in this demographic, such as higher stress.30 The association of the persistent airflow phenotype with asthma severity is challenging to interpret, since FEV1 is a determinant of the ASSESS score. A number of items relied on patient reports, leading to missing data and declarative bias. Data on aeroallergen specific IgGs or IgEs was lacking in 63% of patients. There was no information on household income or accommodation conditions, and comprehensive comorbidity data were not available. An important limitation of a cross-sectional study is that the nature of observed associations is not explicit and does not imply causal relationship.
The main strength of this study lies in the use of the ASSESS score, which allows the compilation of several domains of asthma severity into a single metric. The finding that all components of the ASSESS score were associated with patient characteristics suggests the potential inadequacy of relying solely on control scores or the number of exacerbations to assess asthma severity. In an observational approach, such as the present study, the ASSESS score can be used to identify markers of asthma severity. The ASSESS score could also serve as an endpoint for evaluating the impact of interventions aimed at modifying asthma severity. Other strengths include the study’s large number of subjects and multicenter design.
Conclusion
In this cross-sectional study conducted in 948 adult asthmatics with a high proportion of severe asthma, we identified smoking, urban housing and patient-reported work-aggravated asthma as modifiable markers associated with asthma severity.
Abbreviation
ACT, Asthma Control Test; ASSESS, Asthma Severity Scoring System; LABA, Long-acting beta agonists; LAMA, Long-acting muscarinic antagonists; FEV1, Forced Expiratory Volume in 1 second; ICS, inhaled corticosteroids; SABA, Short-acting bronchodilators; FASE-CPHG, France Asthme Sévère-Collège des Pneumologues des Hôpitaux Généraux; GINA, Global Initiative for Asthma.
Acknowledgments
The FASE-CPHG study was supported by contributions made through the CPHG from ALK, AstraZeneca, Boehringer Ingelheim, GSK, and Le Nouveau Souffle. Work specifically related to the present manuscript was not funded. The authors thank the Collège des Pneumologues des Hôpitaux Généraux (CPHG, France) which initiated the FASE-CPHG study and Kappa Santé (Paris, France) for the management of collected data. The sponsors of the FASE-CPHG study funded data collection. They had no role in conducting data analysis related to the present study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Vos T, Lim SS, Abbafati C., et al.; GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond Engl. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
2. Holgate ST, Davies DE, Powell RM, Howarth PH, Haitchi HM, Holloway JW. Local genetic and environmental factors in asthma disease pathogenesis: chronicity and persistence mechanisms. Eur Respir J. 2007;29(4):793–803. doi:10.1183/09031936.00087506
3. Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344(5):350–362. doi:10.1056/NEJM200102013440507
4. de Groene GJ, Pal TM, Beach J, et al. Workplace interventions for treatment of occupational asthma: a Cochrane systematic review. Occup Environ Med. 2012;69(5):373–374. doi:10.1136/oemed-2011-100399
5. Global initiative for asthma main report; 2023. Available from: https://ginasthma.org/2023-gina-main-report/.
6. Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7(5):1477–1487. doi:10.1016/j.jaip.2018.12.029
7. Fitzpatrick AM, Szefler SJ, Mauger DT, et al. Development and initial validation of the Asthma Severity Scoring System (ASSESS). J Allergy Clin Immunol. 2020;145(1):127–139. doi:10.1016/j.jaci.2019.09.018
8. Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the Composite Asthma Severity Index--an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129(3):694–701. doi:10.1016/j.jaci.2011.12.962
9. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
10. Portel L, Parrat E, Nocent-Ejnaini C, et al. FASE-CPHG study: a panoramic snapshot of difficult-to-treat, severe asthma in French nonacademic hospitals. ERJ Open Res. 2019;5(4):00069–02019. doi:10.1183/23120541.00069-2019
11. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
12. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi:10.1183/13993003.00588-2019
13. Ioachimescu OC, Desai NS. Nonallergic triggers and comorbidities in asthma exacerbations and disease severity. Clin Chest Med. 2019;40(1):71–85. doi:10.1016/j.ccm.2018.10.005
14. Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy. 2017;10:47–56. doi:10.2147/JAA.S121276
15. Sheehan WJ, Phipatanakul W. Difficult-to-control asthma: epidemiology and its link with environmental factors. Curr Opin Allergy Clin Immunol. 2015;15(5):397–401. doi:10.1097/ACI.0000000000000195
16. O’Connor GT, Neas L, Vaughn B, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121(5):1133–1139.e1. doi:10.1016/j.jaci.2008.02.020
17. Carlsen HK, Haga SL, Olsson D, et al. Birch pollen, air pollution and their interactive effects on airway symptoms and peak expiratory flow in allergic asthma during pollen season - A panel study in Northern and Southern Sweden. Environ Health Glob Access Sci Source. 2022;21(1):63. doi:10.1186/s12940-022-00871-x
18. Guo C, Zhang Z, Lau AKH, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2(3):e114–e125. doi:10.1016/S2542-5196(18)30028-7
19. Hirano T, Matsunaga K. Late-onset asthma: current perspectives. J Asthma Allergy. 2018;11:19–27. doi:10.2147/JAA.S125948
20. Del Giacco SR, Bakirtas A, Bel E, et al. Allergy in severe asthma. Allergy. 2017;72(2):207–220. doi:10.1111/all.13072
21. Warm K, Hedman L, Lindberg A, Lötvall J, Lundbäck B, Rönmark E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol. 2015;136(6):1559–1565.e2. doi:10.1016/j.jaci.2015.06.015
22. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi:10.1164/rccm.200906-0896OC
23. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the national heart, lung, and blood institute’s severe asthma research program. J Allergy Clin Immunol. 2007;119(2):405–413. doi:10.1016/j.jaci.2006.11.639
24. Trawick DR, Holm C, Wirth J. Influence of gender on rates of hospitalization, hospital course, and hypercapnea in high-risk patients admitted for asthma: a 10-year retrospective study at Yale-New Haven Hospital. Chest. 2001;119(1):115–119. doi:10.1378/chest.119.1.115
25. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi:10.1164/rccm.201602-0419OC
26. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
27. Baldaçara RP, Silva I. Association between asthma and female sex hormones. Sao Paulo Med J Rev Paul Med. 2017;135(1):4–14. doi:10.1590/1516-3180.2016.011827016
28. McCallister JW, Mastronarde JG. Sex differences in asthma. J Asthma off J Assoc Care Asthma. 2008;45(10):853–861. doi:10.1080/02770900802444187
29. Hamada K, Oishi K, Murata Y, Hirano T, Matsunaga K. Feasibility of discontinuing biologics in severe asthma: an algorithmic approach. J Asthma Allergy. 2021;14:1463–1471. doi:10.2147/JAA.S340684
30. Cardet JC, Chang KL, Rooks BJ, et al. Socioeconomic status associates with worse asthma morbidity among Black and Latinx adults. J Allergy Clin Immunol. 2022;150(4):841–849.e4. doi:10.1016/j.jaci.2022.04.030
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.