Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Short-Term Impact of Cardiac Intervention on the Nutritional Status of Malnourished Children with Congenital Heart Disease – A Report from a Developing African Country, Ethiopia
Received 18 July 2023
Accepted for publication 28 November 2023
Published 7 December 2023 Volume 2023:14 Pages 465—475
DOI https://doi.org/10.2147/PHMT.S431164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Kidist Tesfaye,1 Temesgen Tsega2
1Tirunesh Beijing Hospital, Department of Pediatrics and Child Health, Addis Ababa, Ethiopia; 2Saint Paul Hospital Millennium Medical College, Department of Pediatrics and Child Health, Pediatric Cardiology Unit, Addis Ababa, Ethiopia
Correspondence: Temesgen Tsega, Email [email protected]
Background and Objective: Malnutrition is a common problem in infants and children with congenital heart defect and has an impact on the disease outcome. This study aimed to assess the short-term impact of corrective cardiac intervention on growth recovery of malnourished children with congenital heart defects.
Methods: A retrospective cohort study was conducted over a year period (April 2021 to April 2022) by retrieving data from pediatric corrective cardiac interventions performed over a period of 5 years (2017– 2021). We enrolled pediatric patients with congenital heart disease whose age is less than 18 years and have undergone corrective cardiac intervention. Those children identified to have malnutrition pre-operatively have been followed for 6 months postoperatively. Anthropometry measurements were used to measure the outcome, before intervention and every 3 months for a total of 6 months after correction.
Results: A total of 148 children from age 2 months to 18 years with a mean age of 5 years were included in the study. Most of the subjects had acyanotic CHD accounting for 93.2%. Magnitudes of underweight, wasting and stunting at pre intervention were 54%, 54.1% and 59.5% respectively, decreased to 40.7%, 39.2%, 49.2% and 29.3%, 25.9%, 34.8% at the 3rd and 6th month of the post intervention period respectively. Predictors of undernutrition at post intervention were type of CHD, age at correction, PAH, type of intervention and were summarized. Comparison of Z-scores for WFH and HFA on the 3rd and 6th month post intervention has shown significant improvement from baseline. Those subjects with PAH and older age at correction have a greater chance of being underweight and wasted.
Conclusion: Malnutrition is very common in children with CHD and is predicted by the presence of pulmonary hypertension and older age at correction. Corrective cardiac intervention significantly improved nutritional status during the follow-up over 6 months.
Keywords: congenital heart disease, underweight, wasting and stunting
Background
Congenital heart disease (CHD) is the most common major congenital malformation, having a prevalence of ≈9 per 1000 live births.1 It is a well-recognized fact that CHDs without timely intervention are being associated with several complications that have a major contribution for infant mortality, particularly in developing countries.2,3 Malnutrition is one of the conditions usually overlooked and exists in a significant portion of children with uncorrected congenital heart disease, the severity of which is variable ranging from mild undernutrition to severe failure to thrive.4
“Malnutrition” basically refers to a pathological state resulting from relative or absolute deficiency or excess of one or more essential nutrients. It is measured commonly with anthropometric parameters like underweight, wasting and stunting. The latter two reflect acute and chronic exposures for nutritional deficiency, respectively, while underweight reflects both acute and chronic exposures for nutritional deficiency. Several mechanisms are mentioned to explain how CHD can result in undernutrition and few of them are well elucidated as a direct etiology; otherwise in many circumstances it is multifactorial in its origin and may differ among individuals. These include the underlying cardiac illness, hemodynamic factors, degree and duration of cyanosis, insufficient calorie intake, unbalanced higher energy expenditure, vulnerability to infection and associated comorbidities or genetic syndromes.3–8
Prevalence of undernutrition is high in low and middle-income countries and the coexistence of CHD will escalate the burden both in its incidence and severity among these regions.9,10 According to the 2019 Ethiopian demographic health statistic report, 37% of children under five years of age are stunted (severe stunting of 12%), 7% are wasted (severe wasting of 1%) and 21% are underweight (severe underweight of 6%).5
A few domestic research studies have already revealed the incidence of malnutrition in pediatric cardiac patients to be significantly high. A cross-sectional hospital-based study in 2021 has shown the prevalence of wasting and stunting was 38.6% and 35.9%, respectively.11 In another cross-sectional study in a cardiac center of Ethiopia, in 2022, the overall prevalence of wasting, underweight and stunting was 41.3%, 49.1% and 43% respectively.4 In a retrospective cross-sectional study conducted from 2016 to 2019 in Hawassa University tertiary teaching hospital, severe acute malnutrition (SAM) using both mid-upper arm circumference (MUAC) and weight-for-height (WFH) was documented in 51.8% of the study subjects and stunting was reported in 29.8% of patients.12
Data collected from a total of 194 pediatric patients with CHD at Mulago Hospital in Uganda, 2013/14, has shown 31.5% of children aged 0–5 years were wasted; 42.5% of children aged 0–10 years were underweight, and 45.4% of children were stunted.13 In some research studies, like the one conducted in Nigeria, a case–control observational study published in 2011, the prevalence of malnutrition is found to be as high as 90.4% in children with CHD compared to 21.1% prevalence in other children with no known illness.14
Irrespective of multiple evidence in literature regarding its impact on the outcome, malnutrition in pediatric patients with unoperated CHD continues to be given less emphasis in terms of diagnosis and management. Especially in those who are hospitalized, it is of critical importance, as it may identify more fragile patients at greater risk of complications, with therapeutic challenges and requiring special attention.15
Poor nutritional status prior to and post intervention may result in increased morbidity and mortality in children with congenital cardiac defects, so it is essential to evaluate their nutritional profiles pre-operatively and give due consideration to optimize their nutrition before subjecting them for intervention.16 Together with their underlying cardiac illness, macronutrient and micronutrient scarcity will aggravate the adverse outcomes arising out of organ dysfunctions.17
In developing countries, like ours, due to resource limitations, corrective interventions for CHDs may not be done at all or performed later than the appropriate age. This leads to a vicious cycle of congestive heart failure (CHF) and respiratory infections that in turn result in a high prevalence of pre-operative malnutrition in patients with CHD, which can range from mild undernutrition to failure to thrive. As it has been observed in other studies, the co-occurrence of malnutrition with CHD significantly affects the outcome of cardiac intervention, increasing morbidity and mortality.18 The chance of developing malnutrition increases in those having CHD with cyanosis, multiple heart defects, heart failure, delayed intervention, anemia and pulmonary hypertension. The presence of malnutrition will prone them for infection and makes the prognosis poor, even after corrective intervention.4,13
The catch-up growth usually starts to be noticed as early as 3 months after the corrective cardiac intervention that continues up to the first year following the intervention. Then after the growth curve starts to slow and reach into a plateau this reflects that the impact of CHD no longer exists and other factors may contribute for persistence of malnutrition then after.19
In this study, we have assessed the pattern of growth response and determinant factors of the anticipated recovery over the six-month period after corrective intervention.
Methods
We conducted this research from April 27, 2021 to April 26, 2022 in a children's cardiac center in Ethiopia, the only one to provide corrective cardiac intervention primarily for children. It is a non-governmental institution located in Addis Ababa, Ethiopia and has waiting lists of more than 10,000 children with cardiac illness requiring intervention.
A retrospective cohort study was conducted over a year period (April 2021 to April 2022) by retrieving data from pediatric corrective cardiac interventions performed over a period of 5 years (2017–2021). We enrolled pediatric patients with congenital heart disease whose age is less than 18 years and have undergone corrective cardiac intervention. Those children identified to have malnutrition pre-operatively have been followed for 6 months postoperatively. Anthropometry measurements were used to measure the outcome, before intervention and every 3 months for a total of 6 months after correction.
We have excluded those patients with comorbidities, which could possibly affect the nutritional status of the child. These are those with prematurity history, small for gestational age and have associated genetic syndrome or another chronic medical illnesses. Patients who had palliative surgery like Fontan, Bidirectional Glen (BDG) and Blalock–Taussig (BT) shunts, pulmonary artery banding, etc. and those who required another corrective operation more than once during the six-month post intervention period were also excluded from the study. Following our exclusion criteria, the total number of subjects enrolled into our study was 148.
Retrospective review of medical records was conducted by pediatric residents; and validation and data cleaning were performed by a pediatrician, public health specialist and pediatric cardiologist. The dependent variable was nutritional status of a child prior to and after cardiac intervention that was assessed with serial anthropometric measurements. Independent variables including socio-demographic data, clinical variables like the type and number of cardiac lesions, pulmonary hypertension, cardiac intervention performed and the age at which it was performed were included in the questionnaire, which was adopted from previous similar research studies.
Anthropometry measurements (weight and height/length) before cardiac intervention and every 3 months for a total of 6 months after intervention were obtained and z scores were compared. Malnutrition was defined using weight-for-age, height-for-age, and weight/height (BMI for those above 5 years) z scores. Data were entered into SPSS version 25 checked for completeness, cleaned and analyzed. Descriptive statistics like frequency, percent, mean, table and graph were used for categorical variables. Wasting, underweight and stunting was defined as z-score ≤-2 and paired t-test was used to compare the mean z-scores pre and post intervention. Logistic regression analysis was used at the bivariate and multivariate level. All the variables with at least one factor having a p-value <0.2 at the bivariate level were considered for entry into the multivariate logistic regression analysis to control confounding factors and to assess the association between independent variables and post intervention nutritional status. A probability p-value <0.05 was considered statistically significant. A confidence interval of 95% was used to summarize the data.
Ethical Clearance
We obtained written consent from the patients’ parent/caregiver to review their child’s medical record and received ethical clearance from the institutional review board of Saint Paul Hospital Millennium Medical College. All data from the medical records were fully anonymised. This study adhered to the declaration of Helsinki.
Operational Definition
Anthropometry refers to comparative measurements of the human body.
- The primary measures used as indices of growth include stature (length or height), weight, and head circumference.
- The secondary measures used to estimate body composition include triceps skinfold thickness, subscapular skinfold thickness, and mid-upper arm circumference.
Malnutrition (undernutrition): if the child has one of wasting, stunting and underweight
- According to the World Health Organization (WHO), wasting, stunting, and underweight are defined as Z-scores less than ≤−2 standard deviations of weight for height (for <5 years), BMI (replacing weight for height for age 5–19 years), height for age, and weight for age (up to 10 years) as moderate malnutrition respectively and a z score of ≤−3 SD to define severe malnutrition. A z score of ≤−2 indicates that a child’s WAZ, WHZ, BMI or HAZ is 2 SD below the age- and gender-specific median for the normal population, and 3 SD below the median cut-off if the z score is ≤−3.18
Corrective cardiac intervention: a definitive repair of the heart defect using either surgery or cardiac catheterization.
Results
Socio-Demographic and Clinical Characteristics
A total of 148 study subjects were enrolled in our study and the majority of them were females, with 57.4%. The mean age was 5.0 years with the standard deviation of 3.9 years, ranging from 2 months to 18 years. Children aged between one and five years accounted for more than half (54.7%) of the subjects. Patients residing in the urban area of the country contribute 55.4% from the total proportion. Congenital heart defects classified into a major category as cyanotic and acyanotic, out of which those with acyanotic CHD (93.8%) significantly outnumber and pulmonary arterial hypertension was found in fewer than half (38.9%) of study subjects. From the study subjects 106 (71.6%) underwent surgery while the remaining (28.4%) were managed by catheter-based interventions. Some patients did not show up at their 3rd month (11 patients) and 6th month (35 patients) of postoperative follow-up dates (Table 1).
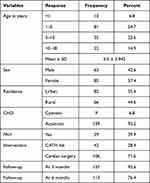 |
Table 1 Sociodemographic and Clinical Profiles of Study Participants |
The most frequent congenital heart defect category among the participants was acyanotic CHD (Table 2), of which PDA (39.8%) is the most common one, followed by VSD (19.6%) and ASD (12.2%). From the cyanotic CHD category TOF held the highest proportion, 2.7%.
 |
Table 2 Type of Cardiac Defects and Prevalence Among Study Participants |
Malnutrition in CHD Patients
Pre intervention, three-month and six-month anthropometric follow-up data of 148 study subjects were analyzed and Z score of ≤ −2 was taken to define underweight, wasting and stunting. The prevalence of underweight, wasting and stunting before intervention was 54%, 54.1% and 59.5%, respectively, which decreased to 40.7%, 39.2% and 49.2% at the 3rd month and to 29.3%, 25.9% and 34.8% at the 6th month of post intervention period, respectively (Table 3).
 |
Table 3 Magnitude of Wasting, Stunting and Underweight, Pre and Post Corrective Cardiac Intervention |
Improvement in the severity of nutritional status, across all parameters, was noticed gradually in both follow-ups (Table 4).
 |
Table 4 Severity of Under Nutrition at Pre and Post Intervention Period |
Mean values were calculated for ZWA, ZWH/BAZ and ZHA (Table 5) and paired t-test done to compare z-scores at 3 and 6 months from baseline and statistically significant improvements with p-value <0.001 were observed for ZWA at the 6th month and ZHA and ZWH/BAZ both at the 3rd and 6th months.
 |
Table 5 Comparison of the Mean ± SD of Z-Scores of ZWA, ZWH/BAZ and ZHA |
There was an improvement in the trends (Figure 1) of mean z-scores of WFA, WFH and HFA at 3 and 6-month post intervention from baseline. Z-score of WFA has shown higher improvement at 3-month post intervention but it was not statistically significant (p<0.17).
 |
Figure 1 Trends in the mean Z scores before and after corrective intervention. |
Bivariate analysis was done to check whether patient age at correction, sex, residence, PAH, type of intervention have significant association with underweight at the 6th month of post intervention period and those variables with p-value <0.2 were exported to multivariable regression analysis model and a p-value <0.05 was considered a statistically significant association. The chance of being underweight increases when the age at correction increases by one year (95% CI 0.54–0.83, p<0.001) and in addition, children with PAH were 66.2% less likely to have normal WFA or with odds of 0.338 times (95% CI 0.12–0.95) compared to children without pulmonary hypertension (Table 6).
 |
Table 6 Bivariate and Multivariate Analysis for Underweight at 6 months Post Intervention |
The candidate variables for multivariate regression analysis were patient age at correction, sex, residence, PAH and type of intervention which had significant association with wasting at the 6th month on bivariate analysis (Table 7). The delay at correction of congenital heart defects had a significant effect on wasting when the age increases (95% CI 0.69–0.90, p<0.001) and children with PAH were 71.4% less likely to have normal WFH (no wasting) compared to children without pulmonary hypertension with odds ratio of 0.286 (95% CI for AOR 0.12–0.95), at 6th month follow-up.
 |
Table 7 Bivariate and Multivariate Analysis for Wasting at 6 months Post Intervention |
No significant association was observed for bivariate and multivariable analysis of patient age at correction, sex, residence, PAH, type of intervention and type of CHD with stunting at the 6th month of post intervention (Table 8).
 |
Table 8 Bivariate and Multivariate Analysis for Stunting at 6 months Post Intervention |
Discussion
Congenital heart diseases in low and middle-income countries contribute to 90% of the disease burden across the globe and various studies have documented the high prevalence of undernutrition in children with uncorrected CHDs.3 The immediate postoperative period is characterized by a hyper-metabolic state; low total and resting energy expenditure are reported within 24 h of surgery. After 5 days, resting energy expenditure returns to pre-operative levels. Significant improvements in weight and growth occur within months after corrective surgery. Several literature studies have implicated the impact of anatomic closure on somatic growth. Significant improvements in weight and catch-up growth occurred within months, mostly in the first year after corrective surgery. This is a reflection of the correction of the hemodynamic derangements leading to lessening of the cardiac cachexia and promotion of a more anabolic state.20
Our study has specifically selected those malnourished patients with CHD and has subsequently undergone corrective cardiac intervention rather than assessing the magnitude of malnutrition in all CHD patients. We have identified the overall prevalence of undernutrition as 54% underweight, 51% wasting and 59% stunting; higher than what was observed in a study conducted in the same center (underweight, wasting, and stunting were 49.1% 41.3%, and 43% respectively) that has assessed the occurrence of undernutrition in all CHD patients prior to intervention but no further details are known whether these patients were operated on or not.4 Same wise, the prevalence in our study is higher than other similar local studies.11,12 This higher prevalence might be explained by how patients are getting the opportunity to be operated on in the center. It mainly considers their queue and gives priority for those who have been waiting for a long time, as a result of which and because of the limited capacity of the center many patients are insisted to wait for long without getting appropriate definitive intervention which makes them vulnerable to develop undernutrition. One of the major findings in our study is that the likelihood of being malnourished increases in unoperated patients when their age increases. In addition, as it is mentioned in the literature, one of the criteria to select some patients with CHD for intervention is the presence of failure to thrive and these selected patients might have been chosen for this reason.
In a retrospective study conducted in Singapore from 2012 to 2016 to assess the impact of pre-operative nutritional status on outcomes following congenital heart surgery19 they identified that the WAZ ≤ −2 (underweight) was 33.4%, BAZ ≤ −2 (wasting) of 29.1% and HAZ ≤ −2 (stunting) of 26.8%%. In this study they have restricted the age to less than ten years, which we have extended up to 18 years so that could have an impact in increasing the magnitude of malnutrition for the same reasons mentioned above.
Approximate figures in the prevalence of underweight (59%) and wasting (55.9%) except for stunting (26.3%) were observed in a prospective study conducted in a tertiary referral hospital in Southern India which included children less than 5 years of age undergoing surgical or catheter-based corrective intervention from June 2005 to June 2006.21
Significant improvement on the mean values of ZWA, ZWH/BAZ and ZHA was found in post cardiac intervention in both follow-ups (Figure 1) (p<0.001) except for the mean value for ZWA at the third month of follow-up when compared to the baseline measurement. Similarly, studies done in India and Thailand have revealed significant improvement at 3rd and 6th months of post intervention in the mean value Z-scores of WFA, WFH and HFA when it was compared with baseline.21,22 Z-score of WFA has shown higher improvement at 3-month post intervention than other parameters but it was not significant when statistically analyzed (p<0.17).
A study from Chile to assess nutritional recovery after cardiac surgery at the 3rd and 6th month after surgery in 46 pediatric patients with CHD in the year 2017 found significant improvement in all the parameters except for HAZ at the six months after intervention.23 Such a finding, no improvement in height between admission and 6th month after intervention, is also documented in other similar studies by Vaidyanathan et al from India which has enrolled 476 children with CHD18 and a study from Canada that assessed 725 patients managed at the Hospital for Sick Children, Toronto, Ontario between 1995 and 2005.24 This might be due to a longer period of recovery required to reverse the chronic malnutrition (stunting) in comparison to the acute one.
In our study more than half of study participants underwent intervention between one and five years of age. Age at correction was associated with underweight and wasting in which children with late correction have a relatively high risk of undernutrition [AOR 0.67 (95% CI 0.54–0.83, p<0.001) and AOR 0.79 (95% CI 0.69–0.90, p<0.001)] respectively. The results were consistent with the study done in India and Thailand; older age at correction was one of the predictors of malnutrition. Different literatures reveal that early surgical correction or palliation is often the most efficient way of improving the nutritional status of these infants by eliminating cardiac factors contributing to malnutrition.18,22
The other finding in this study was increased risk being of underweight and wasting in children with PAH [OR 0.338 (95% CI 0.12–0.95) and OR 0.286 (95% CI 0.12–0.95)] respectively when compared to those who do not have PAH. This finding is consistent with results obtained from the study conducted in the same setting few years back which assessed the nutritional status all children with congenital heart disease prior to intervention4 and another one from India that was mentioned earlier.18
No statistical significant correlation was observed between the type of CHD and post intervention nutritional status but it is difficult to compare the two broad categories of CHD (cyanotic vs acyanotic) as the majority of patients in our study were acyanotic ones who are relatively simpler for intervention as compared to the more complex cyanotic defects. Studies from India and Chile have also demonstrated this lack of association between the type defects and post-operative somatic growth.18,23 Comparing the mode of intervention as surgical and catheter-based with the post intervention nutritional status, no association was identified.
Conclusion
Malnutrition is very common in children with uncorrected CHD, especially in those CHDs with pulmonary hypertension and those children who have undergone corrective cardiac surgery at older age. Corrective intervention significantly improves nutritional status during the follow-up over six months.
Recommendation
As per the findings in this study we suggest that early definitive cardiac intervention is a determinant factor in reducing the development of malnutrition in patients with CHD.
A significant number of patients still remained to be malnourished, which implies the need for subsequent follow-up and study to assess the long-term impact of intervention on their nutritional status.
Routine monitoring of the nutritional status of patients with CHD is mandatory as a result of high prevalence of malnutrition and guidelines to address this problem in such a selected population need to be available.
Limitation of the Study
The fact that the study is a retrospective one, means additional contributing factors like birth weight, gestational age, parental anthropometry, detailed nutritional history, economic and parental educational status at that time, etc. were not assessed, which could be overcome by a prospective study.
The majority of patients were with acyanotic CHDs as a result of which it was difficult to compare the outcome with cyanotic ones.
Few patients did not show up at their 3rd month (11 patients) and 6th month (35 patients) follow-up dates and we excluded them in subsequent analysis.
Abbreviations
ASD, Atrial septal defect; AS, Aortic stenosis; AVSD, Atrioventricular septal defect; BAZ, Z score of Body mass index for age; CHD, Congenital heart defect; COA, Coarctation of aorta; EDHS, Ethiopia mini demographic and health survey; HFA, Height for age; PAH, Pulmonary arterial hypertension; PDA, Patent ductus arteriosus; PS, Pulmonary stenosis; SAM-LVOO, Sub aortic membrane with Left ventricular outflow obstruction; SPHMMC, Saint Paul’s hospital millennium medical college; SD, Standard deviation; SPSS, Statistical package for social science; TAVPR, Total anomalous pulmonary venous return; TGA, Transposition of great arteries; TOF, Tetralogy of Fallot; VSD, Ventricular septal defect; WFA, Weight for age; WFH, Weight for height; WHO, World health organization; ZHA, Z score of height for age; ZWA, Z score of weight for age; ZWH, Z score of weight for height.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N. Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. IntJ Epidemiol. 2019;48(2):455–463. doi:10.1093/ije/dyz009
2. Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. doi:10.1161/CIRCULATIONAHA.113.008396.
3. Argent A, Balachandran R, Vaidyanathan B, Khan A, Kumar R. Management of undernutrition and failure to thrive in children with congenital heart disease in low- and middle-income countries. Cardiology in the Young. 2017;27(S6):S22–S30. doi:10.1017/S104795111700258X
4. Tsega T, Tesfaye T, Dessie A, Teshome T. Nutritional assessment and associated factors in children with congenital heart diseaseEthiopia. PLoS One. 2022;17(9):e0269518.
5. Ethiopian Public Health Institute Addis Ababa. Ethiopia Mini Demographic and Health Survey. Federal Democratic Republic of Ethiopia; 2019.
6. Van den Broeck J, Meulemans W, Eeckels R. Nutritional assessment: the problem of clinical-anthropometrical mismatch. Eur J Clin Nutr. 1994;48(1):60–65.
7. Green Corkins K. Nutrition-focused physical examination in pediatric patients. Nutr Clin Pract. 2015;30(2):203–209. doi:10.1177/0884533615572654
8. Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460–481. doi:10.1177/0148607113479972
9. Arodiwe I, Chinawa J, Ujunwa F, Adiele D, Ukoha M, Obidike E. Nutritional status of congenital heart disease (CHD) patients: burden and determinant of malnutrition at university of Nigeria teaching hospital Ituku - Ozalla, Enugu. Pak J Med Sci. 2015;31(5):1140–1145. doi:10.12669/pjms.315.6837.
10. Tchervenkov CI, Jacobs JP, Bernier P-L, et al. The improvement of care for paediatric and congenital cardiac disease across the world: a challenge for the World Society for Pediatric and Congenital Heart Surgery. Cardiol Young. 2008;18(Suppl S2):63–69. doi:10.1017/S1047951108002801
11. Woldesenbet R, Murugan R, Mulugeta F, et al. Nutritional status and associated factors among children with congenital heart disease in selected governmental hospitals and cardiac center,Addis Ababa Ethiopia. BMC Pediatr. 2021;21(538). doi:10.1186/s12887-021-03023-1
12. Assefa B, Tadele H. Severe acute malnutrition among unoperated Ethiopian children with congenital heart disease: a wake-up call to reverse the situation, a retrospective cross-sectional study. Ethiop J Health Sci. 2020;30(5):707–714. doi:10.4314/ejhs.v30i5.9.
13. Batte A, Lwabi P, Lubega S, et al. Wasting, underweight and stunting among children with congenital heart disease presenting at Mulago hospital. Uganda BMC Pediatr. 2017;17(10). doi:10.1186/s12887-017-0779-y
14. Okoromah CAN, Ekure EN, Lesi FEA, Okunowo WO, Tijani BO, Okeiyi JC. Prevalence, profile and predictors of malnutrition in children with congenital heart defects: a case-control observational study. Arch Dis Child. 2011;96(4):354–360. doi:10.1136/adc.2009.176644
15. Cernea S. Nutritional status and clinical outcomes of cardiac patients in acute settings. J Cardiovascul Emerg. 2018;4(1):5–7. doi:10.2478/jce-2018-0007
16. Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22(3):237–244. doi:10.1016/j.nut.2005.06.008
17. Varan B, ad T, Yilmaz G. Malnutrition and Growth Failure in Cyanotic and Acyanotic Congenital Heart Disease with and without Pulmonary Hypertension. Arch Dis Child. 1999;81:49–52.
18. Vaidyanathan B, Nair SB, Sundaram KR, et al.Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. 2008;45(7):541–546.
19. Lim CYS, Lim JKB, Moorakonda RB, et al. The impact of pre-operative nutritional status on outcomes following congenital heart surgery. Front Pediatr. 2019;7:429. doi:10.3389/fped.2019.00429.
20. Evangelista JK, Nigrin DJ, Erickson LC. Impact of anatomic closure on somatic growth among small. Asymptomatic. 2000;85:1472–1475.
21. Vaidyanathan B, Radhakrishnan R, Sarala DA, Sundaram KR, Kumar RK. What determines nutritional recovery in malnourished children after correction of congenital heart defects? Pediatrics. 2009;124(2):e294–9. doi:10.1542/peds.2009-0141.
22. Ratanachu-Ek S, Pongdara A.Nutritional status of pediatric patients with congenital heart disease: pre- and post cardiac surgery. J Med Assoc Thai. 2011;94(Suppl 3):S133–7.
23. Oyarzún I, Claveria C, Larios G, Le Roy Olivos C. Nutritional recovery after cardiac surgery in children with congenital heart disease. Rev Chil Pediatr. 2018;89(1):24–31. doi:10.4067/S0370-41062018000100024
24. Tamayo C, Manlhiot C, Patterson K, Lalani S, McCrindle BW. Longitudinal evaluation of the prevalence of overweight/obesity in children with congenital heart disease. Can J Cardiol. 2015;31(2):117–123. doi:10.1016/j.cjca.2014.08.024.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
