Back to Journals » Clinical Interventions in Aging » Volume 15
Shelterin Complex at Telomeres: Implications in Ageing
Authors Mir SM, Samavarchi Tehrani S , Goodarzi G, Jamalpoor Z , Asadi J, Khelghati N, Qujeq D , Maniati M
Received 1 April 2020
Accepted for publication 18 May 2020
Published 3 June 2020 Volume 2020:15 Pages 827—839
DOI https://doi.org/10.2147/CIA.S256425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Seyed Mostafa Mir,1– 3 Sadra Samavarchi Tehrani,4,5 Golnaz Goodarzi,4,5 Zahra Jamalpoor,1 Jahanbakhsh Asadi,6 Nafiseh Khelghati,7 Durdi Qujeq,2,3 Mahmood Maniati8
1Trauma Research Center, AJA University of Medical Sciences, Tehran, Iran; 2Student Research Committee, Babol University of Medical Sciences, Babol, Iran; 3Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran; 4Department of Clinical Biochemistry, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran; 5Student Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran; 6Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan, Iran; 7Department of Clinical Biochemistry, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran; 8School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Correspondence: Zahra Jamalpoor
Trauma Research Center, AJA University of Medical Sciences, Tehran, Iran
Tel +989126011941
Email [email protected]
Abstract: Different factors influence the development and control of ageing. It is well known that progressive telomere shorting is one of the molecular mechanisms underlying ageing. The shelterin complex consists of six telomere-specific proteins which are involved in the protection of chromosome ends. More particularly, this vital complex protects the telomeres from degradation, prevents from activation of unwanted repair systems, regulates the activity of telomerase, and has a crucial role in cellular senescent and ageing-related pathologies. This review explores the organization and function of telomeric DNA along with the mechanism of telomeres during ageing, followed by a discussion of the critical role of shelterin components and their changes during ageing.
Keywords: ageing, sheltering complex, telomeres
Introduction
Telomeres are nucleoprotein structures at the end of eukaryotic chromosomes, which consist of DNA repeating sequences (TTAGGG) and individual proteins. These structures maintain genome integrity by capping the chromosome terminus.1,2 Telomeres are associated with specific proteins composed of six-subunits called either shelterin. Telomeres associated with shelterin complex play an essential role in chromosome protection and regulation of telomere length. In each round of replication, due to the “end replication problem”, telomeres become slightly shorter.3–5 Telomeres progressively shorten during ageing both in in vitro and in vivo conditions. When the telomere reaches a critical length, this leads to cell cycle arrest, cellular senescence, and apoptosis. Therefore, telomere length can regulate the lifespan of the cells. On the other hand, some mutations in telomere-binding proteins, render cells to escape from telomere shortening and become immortal.6 However, It is now documented that senescent cells secrete factors that influence age-associated diseases while remaining viable but not dividing for long periods.7 Accumulation of senescent cells during ageing reflects a gradual increase in different types of damage in different tissues.8,9 Therefore, senescent cells often exhibit high levels of various forms of damage accumulation overtime in an old organism, including DNA damage and oxidative modifications.10 One of the most significant markers of ageing in both microorganisms and senescent cells are short telomeres.11 In addition, dysfunctional/damaged telomeres result in senescence in postmitotic cells.12 Therefore, the present review attempts to explore the organization and function of telomeric DNA, the mechanisms through which telomeres influence ageing, and the critical role of shelterin components therein.
Organization and Function of Telomeric DNA
Mammalian telomeric DNA with a length of 5–15 kb in human is composed of TTAGGG repeats, the 3` end of which demonstrates a G rich mono-strand overhang stretched out beyond its counterpart.13 The G-rich single-stranded overhang can fold back and invade the double-stranded region of the telomere.14 Telomeres in mammals and many other organisms form t-loops. T-loop is formed by invasion 3` overhang into telomeric DNA.15,16 Also, evidence suggest that 3` overhang pairs with the C-rich strand and displaces the G-rich strand into a D loop.17,18 Besides, t-loop hides the 3`end from telomerase and DNA repair activities and prevents from false activation of DNA repair types of machinery such as ataxia telangiectasia mutated (ATM) and Rad3-related protein (ATR) signalling pathways.19–21 Telomeres are made up of G-quadruplex (G4).22 Studies providing novel insights into G4 and G4-related protein interactions have revealed a potential involvement of G4s in essential processes such as initiation of DNA replication and telomere maintenance.23 Also, it has been suggested that G4s have regulatory roles in oncogene promoter regions, transcription, and translation.24
Telomerase is a ribonucleoprotein (RNP) enzyme, with two essential subunits: telomerase reverse transcriptase (TERT) and telomerase RNA (TR). Telomerase recognizes the 3`-OH at the end of chromosomes and extends telomeres by using associated RNA molecules as a template.25 Recent studies have indicated that telomeric DNAs are not transcriptionally silenced and telomere position effect phenomenon occurs when a normally euchromatic gene is inserted into the telomere of a eukaryotic chromosome.26,27 Despite these findings, it has been revealed that telomeric DNA is transcripted by RNA polymerase II from telomere-repetitive nucleotide region, found on the 3` end of the chromosome, to the long non-coding RNA termed telomeric repeat-containing RNA (TERRA).28,29 Accumulated data indicate that TERRA transcription has a pivotal role in the regulation of heterochromatin formation at telomeres and in the protection of DNA from deterioration or fusion with neighbouring chromosomes.29,30 Also, TERRA is considered a scaffold molecule is promoting the recruitment of proteins and enzymatic activities at the 3` end of the chromosome.31,32 TERRA forms DNA-RNA hybrid structures at telomeres, known as R-loops.33 TERRA influenced heterochromatin formation at telomeres by interacting with several heterochromatin-associated proteins such as methyl-CpG-binding protein (MeCP2) and heterochromatin protein 1 (HP1), and heterochromatic histone modifications, especially H3 K9me3.34–36 Also, as highlighted above, TERRA is a critical regulatory factor in controlling the telomeric length and telomerase activity in telomeres through interaction with various protein complexes such as shelterin and CST as a trimeric complex including Ctc1, Stn1, Ten128,32,37 and predominantly with the hnrpA1. Indeed human hnRNP A1 can disrupt the higher-order structure of human telomeric DNA38,39 and TERRA act as a scaffold in the telomere-neighbouring region and inhibits hnRNPA1 localization at the telomere.40
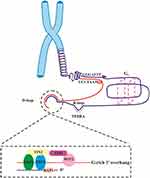
In human cells, the shelterin complex is composed of six proteins, including TRF1, TRF2, POT1, RAP1, TIN2 and TPP141 (Figure 1). CST is a trimeric protein complex consisting of CTC1, STN1/OBFC1, and TEN1. Both Shelterin and CST complexes are involved in capping, protecting and regulating telomeres via prevention of DNA repair machinery from detecting double-strand DNA breaks and induction of folding telomeric DNA homologous end joining (NHEJ), protects the telomeres from ATM activation, controls TERRA transcription, and facilitates unwinding of t-loop to allow telomerase access to the telomeric DNA.42 Furthermore, it has been shown that RAP1 cooperates with TRF2 in repressing aberrant homologous recombination (HR) at telomeres.43 Hence, TRF2/RAP1 heterodimer has an important role in protecting telomeres.
TPP1/POT1 heterodimer complex performs an important protective activity at telomeres by inhibiting ATR kinase and promoting telomerase activity at telomeres.44 TIN2, as the main core of the shelterin complex, has a pivotal role in the suppressing of ATM and ATR signalling pathways through interactions with POT1/TPP1 heterodimer and in stabilizing TRF2 at the telomeric DNA.45–47 As mentioned earlier, the CST complex is important for telomeric DNA maintenance in a multifaceted manner. Indeed, the CST complex promotes efficient replication of telomeric DNA, and thus it has a positive role in maintaining telomere stability. CST depletion leads to delaying C strand synthesis and enhancing the rates of fragile telomeres, which results in the maintenance of extended G-overhangs.48 The CST complex performs these actions by regulating DNA polymerase alpha-primase (polα-primase).49 Besides, this complex contributes to genome maintenance by controlling the access of telomerase to the chromosome terminus, which is a process closely associated with the shelterin complex.
Mechanism of Telomere Shortening During Ageing
Telomeres are subject to shortening during each cell division due to end the replication problem.50 Telomerase enzyme, as reverse transcriptase, solves this problem by adding the TTAGGG sequence to the 3`-end of the chromosomes and maintains telomere length and cellular immortality.51 Telomerase is expressed in embryonic stem cells, and its expression is silenced in most adult somatic tissues. However, this is not sufficient to maintain the telomere length.52–54 Excessive shortening of chromosomes results in the loss of essential genes in their end.55 Indeed, when the length of telomeres becomes critically short, the secondary protective structure does not occur; thus the cells enter replicative senescence.13 Meanwhile, shelterin complex can act as a critical regulator for the activity of telomerase. It serves as a negative regulator of telomerase since it is attached to telomeres in a manner dependent on their length (Table 1).56
 |
Table 1 The Role of the Components of Shelterin Complex in Telomeres and Telomerase Regulation |
Excessive telomere shortening due to a defect in the function of telomerase or any components of the shelterin activates the DNA damage response (DDR) at chromosome ends by ATM/ATR where DDR system enables the NHEJ,57 leading to end-to-end chromosomal fusion, increased HR, and genomic instability. On the other hand, activation of DDR system stimulates the tumour suppressors p53 and p21, which in turn induces cell cycle checkpoint activation to take time for DNA repair, apoptosis or senescence in the cell, finally causing ageing.54
Telomeric Repeat Binding Factors
The telomeric repeat binding factors 1 and 2 (TRF1 and TRF2) are components of the shelterin complex which directly connected to double-stranded telomeric 5ʹ-TTAGGG-3ʹ repeat and have a similar domain structure. These proteins are often in the form of dimer binding to repeat sequences 5ʹ-TTAGGG-3ʹ of telomeric DNA. On the other hand, TRF1 and TRF2 are connected to TIN2 and RAP1, respectively.51,54 It has been shown that TRF1 and TRF2 participate in DNA double-strand break repair at non-telomeric regions.58 Both TRF1 and TRF2 regulate telomerase activity, but neither plays a role in regulating telomerase gene expression.59 TRF1 suppresses telomerase action on telomere region, while TRF2 activates a telomeric degradation without any effect on telomerase.60 Thus, increasing the expression of TRF1 and TRF2 acts as a negative regulator for telomere length, and also an inhibitor for telomerase enzyme.61
TRF1
It has been shown that accessibility of TRF1 is essential for the maintenance of telomere, and downregulation of TRF1 can activate the ATR kinase and consequently induce fragile site phenotypes at the telomere. TRF1 is a telomeric protector against repair systems, and its availability is crucial for telomere function. Therefore, telomere length is associated with ageing, and the role of telomere length regulators such as TRF1 and TRF2 is so crucial in this regard. On the other hand, TRF1 localizes not only telomeres but also to mitotic spindles and has a decisive regulatory role in microtubule polymerization in vitro.61,62
A study assessing the integrity of telomeres and the stability of telomere protection during ageing in endothelial cells (EC) showed that telomere length decreased by 62%. This telomere dysfunction is accompanied by a 33% decrease in TRF1 mRNA expression and a 24% reduction in the TRF1 protein.63 Thus, decreased telomere length in ageing cells causes negative feedback and inhibits TRF1 gene expression.64,65 Furthermore, telomere length does not affect the amount of the shelterin subunits.66 However, a study conducted on the neonatal human dermal fibroblasts showed that the protein level of the TRF2 decrease significantly during ageing.67
The critical role TRF1 plays in telomere biology, and disease has remained unclear. TRF1 depletion in mouse embryonic fibroblasts (MEFs) did not change telomere length. On the other hand, P53 is the protector of the genome which exerts its tumour suppression activity by regulating a large number of downstream targets involved in cell cycle arrest, DNA repair, and cellular senescence.68 Instead, it has been reported to lead to rapid induction of p53/RB-dependent cellular senescence simultaneous with the accumulation of full damage foci at telomeric DNA.68,69 This persistent DNA damage activates phosphorylation of the ATM/ATR kinases, and their downstream effectors, the kinases CHK1 and CHK2, resulting in cell cycle arrest.69 Cells deficient in TRF1 show abundant end-to-end telomere fusions involving both chromosomes and sister chromatids.70 These mice die and show reduced skin thickness and skin stratification, as well as severe skin hyperpigmentation. Newborn mice also have focal dysplasia in the epithelia of the palate, the non-glandular stomach, oesophagus, tongue and skin. These pathologies are accompanied by activation of a persistent DDR at telomeres, which induces activation of the p53/p21 and p16 pathways, resulting in an in vivo cell cycle arrest. The latter produces dramatic alterations in the properties of the epithelial stem cells. Thus, morphological development of the hair follicles and the sebaceous glands is completely impaired.68,70
TRF2
TRF2 suppresses an ATM-dependent DDR at chromosome ends and inhibits end-to-end chromosome fusions and classical NHEJ by folding telomeric DNA into a t-loop.42 Moreover, TRF2 and RAP1 are essential to prevent t-loop cleavage by repressing the activation of PARP1 (poly ADP-ribose polymerase 1) to protect telomeres from HR-mediated repair in mammals.43 Investigation of the effect of TRF2 deletion showed that end-to-end chromosome fusion strongly depends on the binding of 53BP1 to damaged chromosome. In the absence of 53BP1, this fusion can be rescued.71 Also, in hepatocytes, the inhibition of the NHEJ pathway and DDR by the elimination of 53BP1 does not have a considerable impact on phenotypes.72 In contrast, in epidermal cells, TRF2 depletion stimulates a rapid DDR that leads to inhibition of cell proliferation, and cell death.73 This distinct different outcome is achieved due to the diverse nature of tissues.73
A new mouse model with excessive short telomeres has been generated by overexpressing the TRF2 telomere-binding protein, known as K5-TRF2 mice.74 It was reported that in the presence of telomerase and regular enzyme activity, telomere length could be short, leading to precocious ageing and increased cancer.75 In K5-TRF2 mice, by increasing the TRF2 expression, short telomeres are possible only in the presence of a nuclease called XPF. With the removal of XPF nucleases, it has been observed that telomeres begin to expand even with TRF2. It is proposed that this enzyme rapidly degrades telomeres by TRF2 overexpression.74–76 Therefore, telomere-binding proteins have a direct role during ageing, which may be independent of telomerase, and they may serve as a new tool to understand the consequences of critical telomere shortening as having a crucial effect on human life and ageing pathologies.
TIN2
As an adaptor protein, the TRF1-interacting protein 2 (TIN2) plays a linking role at the shelterin complex. Also, this vital protein can bind TRF1 and TPP1-POT1 complex, which construct the bridge among different shelterin components.54 Furthermore, attachment of TIN2 to TRF1 induces changes in TRF1 conformation and stabilizes the telomeric structure, and these changes inhibit telomerase access to telomeres.77,78 Therefore, TIN2 is an inhibitor of the telomerase enzyme, and the absence of TIN2 is useful for telomere accessibility and elongation.79,80
On the other hand, it has been illustrated that shelterin components play critical roles in the activation of DDR mediators. For instance, two of the most essential DDR regulators are ATM and ATR, the activation of which is caused by depletion of TIN2.45,81,82 Interestingly, TIN2 can act independently of TPP1. That is, a truncated form of TIN2 lacking the TPP1 interaction can compel telomerase-dependent telomere extension.79 Recently, several studies have been done on the biological correlation of TIN2 with other shelterin components during ageing79,83 In this regard, mutations in TIN2 are associated with premature ageing phenotypes.84 It was shown that heterozygous TIN2-R282H mutation in telomerase-positive human cells via a knock-in approach does not interfere with the spatial structure of other shelterin components on telomeres. However, TIN2-R282H mutation either activates the telomeric DNA damage signalling or presents different aspects of telomere instability related to telomerase activity. These observations indicate that TIN2 has a direct role in mediating telomere length induced by telomerase, apart from its position in telomere protection.80 Accumulating evidence has shown that TIN2 has a role in mitochondria.84,85 Meanwhile, mitochondrial metabolism has been demonstrated to regulate ageing.86,87 Therefore, further investigation of the correlation between TIN2, mitochondrial metabolism, and ageing is a hot topic. Regarding different studies, we think that new research is needed to verify the direct effect of TIN2 in mitochondrial metabolism, DNA damage response, and ageing.
POT1
Increasing data have shown that one of the critical issues in the field of telomere length control is to manage how diversely proteins bound to the duplex telomeric DNA regulate telomerase.88,89 Protection of telomeres 1 (POT1) has illustrated this vital challenge. POT1 as a critical component of the shelterin complex is directly connected to 3′ single-stranded G-overhang. Also, POT1 can bind to TRF190 via protein-protein interactions. G-quadruplex formation inhibits telomere elongation; however, POT1 by displacing/replacing a g-quadruplex structure promotes telomerase activity.91 Besides, POT1 regulates telomere length and telomere capping, and it has a vital role in the regulation of telomerase activity on telomeres. Furthermore, several functions of this protein include protection of chromosome ends from recombination, catastrophic chromosome instability, and abnormal chromosome segregation.92 Many studies have been conducted on the association between the function of POT1 with senescence.93,94 For instance, depletion of POT1 in mouse embryonic fibroblasts (MEFs) and chicken cells was found to lead to a detrimental DNA damage response in telomeres resulting in telomere dysfunction-induced foci (TIFs).92 According to one study, the loading of POT1 to telomeric single strand DNA (ssDNA) regulates telomere elongation. In addition, it was shown that PTOP, as a new telomere protein, interacts with both TIN2 and POT1. This novel protein binds to the POT1 and recruits it to telomeres. Therefore, suppression of PTOP inhibits the localization of POT1 to telomeres.95 In humans, the shelterin complex has a single POT1 protein, while in mice the genome has two POT1 orthologs, namely Pot1a and Pot1b, each has its function at telomeres. For instance, Pot1a is involved in the inhibition of the DNA damage response at telomeres, while Pot1b is required in maintaining telomere terminus structure.96,97 Hockemeyer and colleagues observed that POT1a and POT1b were predominantly not necessary for the suppression of telomere fusions, and they reported that human telomerase requires one POT1 protein, as opposed to mouse telomeres which have two.97 Meanwhile, POT1a loss through tumour suppressor P53 leads to ageing induction.98,99 It was also found that POT1 is associated with repressing the ATR pathway, and deletion of POT1a or POT1b did not change telomere length.97,100 Recently, increasing evidence has indicated that many components of shelterin complex such as Pot1b control hematopoietic stem cell (HSC) activity and survival during ageing.101 Furthermore, Pot1a knockdown raises DNA damage response and represses self-renewal in mouse HSC.102 Moreover, overexpression of Pot1a inhibits DNA damage response, maintains self-renewal activity, and rejuvenates aged HSCs.103 Yu et al demonstrated that at the time of DNA double-strand breaks, POT1 arrives at DNA damage sites.58 It suppresses the efficiency of non-homologous end-joining (NHEJ), fixes DNA double-strand breaks, and promotes NHEJ fidelity.58 Also, POT1 overexpression represses the protein stability of Lig3, which is the principal regulator of alternative NHEJ (alt-NHEJ), hence inhibits the efficiency of alt-NHEJ.58
TPP1
One of the six components of the shelterin complex is TPP1, which is called TINT1, PTOP or PIP1. This adaptor protein not only binds TIN2 and POT1, but mainly interacts with POT1.54,104 Concerning the role of TPP1 in linking TIN2 and other components of shelterin complex with POT1, any mutation leads to structural alteration in TPP1 for recruiting of the shelterin complex.105,106 One of the most critical functions of TPP1 is regulation of telomerase recruitment to telomeres via the interaction with telomerase reverse transcriptase (TERT), as a catalytic part of the telomerase, for telomere maintenance.106–108 Besides, through mediators of DNA damage response such as ATM and ATR, TPP1 plays a pivotal role in growth arrest.109 Therefore, POT1 and TPP1 bind as a heterodimer to ss (single-strand) telomere DNA to inhibit DNA damage responses from capped telomeres.14,110 On the other hand, it was shown that mouse cells lacking TPP1 exhibit an increase in chromosomal fusions with non-homologous chromosomes.111 Since this protein is a binding subunit, it has been shown that the conditional elimination of TPP1 in MEFs causes a simultaneous removal of POT1a and POT1b from telomeres. In contrast, the other subunits of the shelterin components are not altered in the telomeres.104 Regarding the vital role of TPP1 and POT1 in telomere end protection via repression of DNA damage response and also chromosome fusion, it was illustrated that depletion of TPP1 elicits a robust DNA damage response at telomeres, which is mediated by ATR and results in an increase of ss telomeric DNA.44,104 Also, TPP1-depleted cells seem to recapitulate the telomere dysfunction phenotypes observed in Pot1a/b double knockout cells, in agreement with the proposed role for TPP1 in recruiting POT1a and POT1b to chromosome ends.104 As a nicotinamide dinucleotide (NAD+)-dependent deacetylases, Sirtuin 1 is a protein encoded by the SIRT1 gene in humans, and it is involved in different cellular events including DNA repair and ageing.112 Notably, SIRT1 suppresses age-related mesenchymal stem cells (MSCs) senescence by mechanisms that include enhanced TPP1 expression, increased telomerase activity, and reduced DNA damage.113 Furthermore, according to a study using genome engineering of human embryonic stem cells by knockdown and overexpression of shelterin subunit in human tumour cells, TPP1 recruits telomerase to telomeres via a region termed the TEL-patch.114 The TEL-patch of TPP1 is genetically essential for telomere elongation and thus, long-term cell viability.114 Meanwhile, TPP1 provides a necessary step of telomerase activation as well as feedback regulation of telomerase by telomere length, which is necessary to determine the appropriate telomere length set point in human embryonic stem cells.114 Hence, it has been revealed that TPP1 has multiple vital roles in telomere elongation and stem cell telomere length homeostasis.106,115 Recently, it was found that in Tpp1-deficient MEFs and mice with Tpp1 deletion, induction of telomere damage foci and cell cycle arrest.116 Moreover, similar to TRF1 deficiency, TPP1-null mice died, which in turn indicating severe skin hyperpigmentation, as well as defective hair follicle morphogenesis.116 These phenotypes are rescued via p53 suppression, demonstrating that p53 is a central effector of proliferative defects related to TPP1 deletion. Unpredictably, TPP1 deletion leads to decreased TERT binding to telomeres and enhanced telomere shortening in MEFs and mice.116 Overall, according to different reports in the case of TPP1 function, and its relationships with other components of shelterin complex including, TIN2 and POT1, this protein plays a linking role in appropriate telomere maintenance. Also, as mentioned above, via recruitment of telomerase, TPP1 has a vital role in the control of telomere length.
RAP1
Repressor/activator protein 1 or RAP1 is another part of the shelterin complex that can only connect to telomeres by binding to TRF2.51,117 The highly conserved RAP1 is encoded by TERF2IP gene, which is the only component of the shelterin complex that is not vital in mice. Hence, in contrast to other shelterin subunits, RAP1 does not have a protective role in telomeres. One of the most essential contradictions about this protein is that some studies have indicated that RAP1 plays a dual role at the telomere. Several studies demonstrated that at telomere, RAP1 was implicated in preventing non-homologous end joining, whereas other studies reported that RAP1 was involved in preventing homologous recombination at telomere.118–122 One the other hand, it was shown that TRF2 recruited RAP1 to telomeric repeats, and that the status of RAP is dependent on TRF2 in mouse cells.123 RAP1 has many other functions in both the nucleus and cytoplasm, including transcriptional repression of telomere-proximal genes, and transcriptional activation of hundreds of mRNA-encoding genes, including highly transcribed ribosomal proteins and glycolytic enzyme-encoding genes.124 It has been revealed the mice that were deleted RAP1 are viable but have shorter telomeres and developed skin hyperpigmentation at adulthood.125 Thus, RAP1 is not necessary for mouse viability and telomere capping, whereas it is implicated in the protection of telomere recombination, fragility and length.125 Also, RAP1 plays a crucial role in the regulation of gene expression due to adjacency with subtelomeric regions of chromosomes.118,126,127 RAP1 mainly activates gene expression, but sometimes acts as an inhibitor (notably at telomeres and the silent mating loci).128,129 In healthy cells, RAP1 only covers the telomeric and subtelomeric region of the chromosome. Still, in senescent cells, due to critically short telomeres, RAP1 is transmitted to promoters of natural and new RAP1 targets at senescence (NRTS).127,130 By connecting to the new promoter, RAP1 causes the displacement of histone. Eventually, this displacement leads to site-specific histone losses. Furthermore, RAP1 is transmitted to the promoters of histone genes and inhibits their transcription. As a result of cells that are senesced, RAP1 increases with decreasing histone levels, which in turn results in more RAP1 occupancy at NRTS at senescence131,132 (Figure 2).
 |
Figure 2 Schematic representations show the mechanism of ageing by telomere shortening, and RAP1 effects on histone, as well as alterations of gene expression involved in ageing. |
One of the non-telomeric activities of RAP1 is its regulatory role in the NF-kB signalling pathway.133 It has been indicated that increased expression of RAP1 in senescence cells induces NF-κB pathway, shortens the telomere and starts ageing, and that releasing the RAP1 from telomeres to other regions contributes to the activation of NF-kB.125,134,135 Mechanistically, despite its inability to bind directly to DNA, Rap1 can exclusively modulate NF-kB-dependent transcription in mammals.133,136 Meanwhile, mammalian Rap1 interacts with IKKs, and depends on their interaction with IKKs for their ability to activate NF-Kb.136 Rap1 is essential for IKK-mediated P65 phosphorylation, but not IkBα.137 Also, NF-κB signalling pathway seems to be one of the primary mediators of ageing that is activated by genotoxic, oxidative, and inflammatory stresses.138 On the other hand, NF-κB pathway modulates expression levels of cytokines, growth factors, and genes which are involved in controlling the senescence.139 Also, the transcriptional activity of NF-κB is increased in most of the diseases associated with age. In mouse models, inhibition of NF-κB leads to delayed onset of age-related symptoms and pathologies138 (Figure 3). Taken together, accumulating evidence has shown that RAP1 not only plays a crucial role in protecting the telomere ends from the attacks via the different DNA repair mechanisms but also has multiple functions in specific signalling pathways and metabolism. Given the controversy over the particular purpose of RAP1, further investigation is necessary to evaluate this protein comprehensively. For instance, the role of RAP1 in signalling pathways involved senescent cells could be a line of inquiry.
 |
Figure 3 Schematic representation shows the mechanism of regulatory RAP1 through NF-κB pathway, which leads to the induction of cellular senescence. |
Conclusion
Regulation of shelterin biology during ageing is still poorly understood, even though shelterin-related complexes are involved in ageing-related pathologies. Each of these proteins has a unique role during ageing. TRF1 and TRF2 are two components of the shelterin complex which regulate the telomerase activity. TIN2 is an inhibitor of the telomerase enzyme. POT1 is another member of the shelterin complex which regulates telomere length and telomere capping. Moreover, it has a role in the regulation of telomerase activity on telomeres. TPP1 provides an essential step in telomerase activation and the feedback regulation of telomerase by telomere length. RAP1 is another member of the shelterin complex that can only connect to the telomeres by binding to the TRF2. RAP1 is also one of the NF-κB pathway regulators. Excessive telomere shortening due to a defect in the components of the shelterin complex activates ageing. Understanding the role and mechanism of action of every protein in this complex concerning ageing may assist in the design of drugs to delay ageing.
Acknowledgments
The authors would like to thank Ali Sadeghinia for a critical reading of the text and his helpful comments on the manuscript.
Disclosure
The authors report that there are no conflicts of interest in this work.
References
1. Aksenova AY, Mirkin SM. At the beginning of the end and in the middle of the beginning: structure and maintenance of telomeric DNA repeats and interstitial telomeric sequences. Genes. 2019;10(2):118. doi:10.3390/genes10020118
2. Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996;31(4):443–448. doi:10.1016/0531-5565(96)00005-8
3. Maestroni L, Matmati S, Coulon S. Solving the telomere replication problem. Genes. 2017;8(2):55. doi:10.3390/genes8020055
4. Olovnikov AM. A theory of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41(1):181–190. doi:10.1016/0022-5193(73)90198-7
5. Watson JD. Origin of concatemeric T7DNA. Nat New Biol. 1972;239(94):197–201. doi:10.1038/newbio239197a0
6. Lidzbarsky G, Gutman D, Shekhidem HA, Sharvit L, Atzmon G. Genomic instabilities, cellular senescence, and aging: in vitro, in vivo and aging-like human syndromes. Front Med. 2018;5.
7. Tchkonia T, Zhu Y, Van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. doi:10.1172/JCI64098
8. Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: relevance for clearance of senescent cells. Aging Cell. 2019;18(1):e12841. doi:10.1111/acel.12841
9. Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi:10.1016/j.cell.2005.02.003
10. Coppé J-P, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:12. doi:10.1371/journal.pbio.0060098
11. Campisi J, Di Fagagna FDA. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi:10.1038/nrm2233
12. von Zglinicki T, Wan T, Miwa S. Senescence in post-mitotic cells: a driver of aging? Antioxid Redox Signal. 2020. doi:10.1089/ars.2020.8048
13. Zhu Y, Liu X, Ding X, Wang F, Geng X. Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology. 2019;20(1):1–16. doi:10.1007/s10522-018-9769-1
14. Saretzki G. Telomeres, Telomerase and Aging. Biochemistry and Cell Biology of Aging: Part I Biomedical Science. Springer; 2018:221–308.
15. Muñoz‐Jordán JL, Cross GA, de Lange T, Griffith JD. t‐loops at trypanosome telomeres. EMBO J. 2001;20(3):579–588. doi:10.1093/emboj/20.3.579
16. Stansel RM, de Lange T, Griffith JD. T‐loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 2001;20(19):5532–5540. doi:10.1093/emboj/20.19.5532
17. Cimino-Reale G, Pascale E, Battiloro E, Starace G, Verna R, D’Ambrosio E. The length of telomeric G-rich strand 3′-overhang measured by oligonucleotide ligation assay. Nucleic Acids Res. 2001;29(7):e35–e. doi:10.1093/nar/29.7.e35
18. de Lange T. Shelterin-mediated telomere protection. Annu Rev Genet. 2018;52(1):223–247. doi:10.1146/annurev-genet-032918-021921
19. Arnoult N, Karlseder J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat Struct Mol Biol. 2015;22(11):859. doi:10.1038/nsmb.3092
20. Yarmohamadi A, Asadi J, Gharaei R, Mir M, Khoshnazar AK. Valproic acid, a histone deacetylase inhibitor, enhances radiosensitivity in breast cancer cell line. J Rad Cancer Res. 2018;9(2):86. doi:10.4103/jrcr.jrcr_37_17
21. Karimian A, Mir SM, Parsian H, et al. Crosstalk between Phosphoinositide 3-kinase/Akt signaling pathway with DNA damage response and oxidative stress in cancer. J Cell Biochem. 2019;120(6):10248–10272. doi:10.1002/jcb.28309
22. Wang Y, Yang J, Wild AT, et al. G-quadruplex DNA drives genomic instability and represents a targetable molecular abnormality in ATRX-deficient malignant glioma. Nat Commun. 2019;10(1):943. doi:10.1038/s41467-019-08905-8
23. Hänsel-Hertsch R, Di Antonio M, Balasubramanian S. DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat Rev Mol Cell Biol. 2017;18(5):279. doi:10.1038/nrm.2017.3
24. De Magis A, Manzo SG, Russo M, et al. DNA damage and genome instability by G-quadruplex ligands are mediated by R loops in human cancer cells. Proc Natl Acad Sci. 2019;116(3):816–825. doi:10.1073/pnas.1810409116
25. Wu RA, Upton HE, Vogan JM, Collins K. Telomerase mechanism of telomere synthesis. Annu Rev Biochem. 2017;86(1):439–460. doi:10.1146/annurev-biochem-061516-045019
26. Doheny JG, Mottus R, Grigliatti TA. Telomeric position effect—a third silencing mechanism in eukaryotes. PLoS One. 2008;3(12):e3864. doi:10.1371/journal.pone.0003864
27. Aparicio OM, Gottschling DE. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8(10):1133–1146. doi:10.1101/gad.8.10.1133
28. Cusanelli E, Chartrand P. Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity. Front Genet. 2015;6:143. doi:10.3389/fgene.2015.00143
29. Farnung BO, Brun CM, Arora R, Lorenzi LE, Azzalin CM. Telomerase efficiently elongates highly transcribing telomeres in human cancer cells. PLoS One. 2012;7:4. doi:10.1371/journal.pone.0035714
30. Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35(4):403–413. doi:10.1016/j.molcel.2009.06.025
31. Porro A, Feuerhahn S, Reichenbach P, Lingner J. Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol Cell Biol. 2010;30(20):4808–4817. doi:10.1128/MCB.00460-10
32. Flynn RL, Centore RC, O’Sullivan RJ, et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature. 2011;471(7339):532–536. doi:10.1038/nature09772
33. Graf M, Bonetti D, Lockhart A, et al. Telomere length determines TERRA and R-loop regulation through the cell cycle. Cell. 2017;170(1):72–85. e14. doi:10.1016/j.cell.2017.06.006
34. Bettin N, Oss Pegorar C, Cusanelli E. The emerging roles of TERRA in telomere maintenance and genome stability. Cells. 2019;8(3):246. doi:10.3390/cells8030246
35. Deng Z, Campbell AE, Lieberman PM. TERRA, CpG methylation, and telomere heterochromatin: lessons from ICF syndrome cells. Cell Cycle. 2010;9(1):69–74. doi:10.4161/cc.9.1.10358
36. Nergadze SG, Farnung BO, Wischnewski H, et al. CpG-island promoters drive transcription of human telomeres. Rna. 2009;15(12):2186–2194. doi:10.1261/rna.1748309
37. Deng Z, Wang Z, Stong N, et al. A role for CTCF and cohesin in subtelomere chromatin organization, TERRA transcription, and telomere end protection. EMBO J. 2012;31(21):4165–4178. doi:10.1038/emboj.2012.266
38. Zhang Q-S, Manche L, Xu R-M, Krainer AR. hnRNP A1 associates with telomere ends and stimulates telomerase activity. RNA. 2006;12(6):1116–1128. doi:10.1261/rna.58806
39. Le PN, Maranon DG, Altina NH, Battaglia CL, Bailey SM. TERRA, hnRNP A1, and DNA-PKcs interactions at human telomeres. Front Oncol. 2013;3:91. doi:10.3389/fonc.2013.00091
40. Yamada T, Yoshimura H, Shimada R, et al. Spatiotemporal analysis with a genetically encoded fluorescent RNA probe reveals TERRA function around telomeres. Sci Rep. 2016;6(1):38910. doi:10.1038/srep38910
41. Schmutz I, de Lange T. Shelterin. Curr Biol. 2016;26(10):R397–R9. doi:10.1016/j.cub.2016.01.056
42. Benarroch-Popivker D, Pisano S, Mendez-Bermudez A, et al. TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection. Mol Cell. 2016;61(2):274–286. doi:10.1016/j.molcel.2015.12.009
43. Rai R, Chen Y, Lei M, Chang S. TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat Commun. 2016;7:10881. doi:10.1038/ncomms10881
44. Kibe T, Zimmermann M, de Lange T. TPP1 blocks an ATR-mediated resection mechanism at telomeres. Mol Cell. 2016;61(2):236–246. doi:10.1016/j.molcel.2015.12.016
45. Frescas D, de Lange T. A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice. Genes Dev. 2014;28(2):153–166. doi:10.1101/gad.233395.113
46. Hu C, Rai R, Huang C, et al. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res. 2017;27(12):1485. doi:10.1038/cr.2017.144
47. Rice C, Shastrula PK, Kossenkov AV, et al. Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nat Commun. 2017;8(1):14928. doi:10.1038/ncomms14928
48. Huang C, Dai X, Chai W. Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res. 2012;22(12):1681. doi:10.1038/cr.2012.132
49. Rice C, Skordalakes E. Structure and function of the telomeric CST complex. Comput Struct Biotechnol J. 2016;14:161–167. doi:10.1016/j.csbj.2016.04.002
50. Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225(4):951–960. doi:10.1016/0022-2836(92)90096-3
51. Shay JW. Telomeres and aging. Curr Opin Cell Biol. 2018;52:1–7. doi:10.1016/j.ceb.2017.12.001
52. Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22(5):654–667. doi:10.1101/gad.451008
53. Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8(4):299. doi:10.1038/nrg2047
54. Martínez P, Blasco MA. Role of shelterin in cancer and aging. Aging Cell. 2010;9(5):653–666. doi:10.1111/j.1474-9726.2010.00596.x
55. Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3(10):640. doi:10.1038/nchembio.2007.38
56. Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol. 2008;9(9):232. doi:10.1186/gb-2008-9-9-232
57. Mir SM, Samadian E, Alijanpour S, Khoshbin Khoshnazar A, Haghighatfard H, Sadeghi SH. Impact of Ionizing Radiation on the Expression of CDC25A Phosphatase (in vivo). Medical Laboratory Journal. 2016;10(5):22–26. doi:10.18869/acadpub.mlj.10.5.22
58. Yu Y, Tan R, Ren Q, et al. POT1 inhibits the efficiency but promotes the fidelity of nonhomologous end joining at non-telomeric DNA regions. Aging (Albany NY). 2017;9(12):2529. doi:10.18632/aging.101339
59. Smogorzewska A, van Steensel B, Bianchi A, et al. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20(5):1659–1668. doi:10.1128/MCB.20.5.1659-1668.2000
60. Ancelin K, Brunori M, Bauwens S, et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol. 2002;22(10):3474–3487. doi:10.1128/MCB.22.10.3474-3487.2002
61. Sfeir A, Kosiyatrakul ST, Hockemeyer D, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138(1):90–103. doi:10.1016/j.cell.2009.06.021
62. Nakamura M, Zhou XZ, Kishi S, Kosugi I, Tsutsui Y, Lu KP. A specific interaction between the telomeric protein Pin2/TRF1 and the mitotic spindle. Curr Biol. 2001;11(19):1512–1516. doi:10.1016/S0960-9822(01)00456-0
63. Hohensinner P, Kaun C, Buchberger E, et al. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochimica Et Biophysica Acta (BBA)-Molecular Cell Research. 2016;1863(2):360–367. doi:10.1016/j.bbamcr.2015.11.034
64. Van Steensel B, De Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385(6618):740–743. doi:10.1038/385740a0
65. Muñoz P, Blanco R, de Carcer G, et al. TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol Cell Biol. 2012;150(3):481–494. doi:10.1128/MCB.01339-08
66. Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus. 2011;2(2):119–135. doi:10.4161/nucl.2.2.15135
67. Swanson MJ, Baribault ME, Israel JN, Bae NS. Telomere protein RAP1 levels are affected by cellular aging and oxidative stress. Biomed Rep. 2016;5(2):181–187. doi:10.3892/br.2016.707
68. Martínez P, Thanasoula M, Muñoz P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23(17):2060–2075. doi:10.1101/gad.543509
69. Martínez P, Flores JM, Blasco MA. 53BP1 deficiency combined with telomere dysfunction activates ATR-dependent DNA damage response. J Cell Biol. 2012;197(2):283–300. doi:10.1083/jcb.201110124
70. Porreca RM, Herrera-Moyano E, Skourti E, et al. TRF1 averts chromatin remodelling, recombination and replication dependent-break induced replication at mouse telomeres. Elife. 2020;9:e49817. doi:10.7554/eLife.49817
71. Dimitrova N, Chen Y-CM, Spector DL, de Lange T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature. 2008;456(7221):524–528. doi:10.1038/nature07433
72. Denchi EL, Celli G, De Lange T. Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes Dev. 2006;20(19):2648–2653. doi:10.1101/gad.1453606
73. Martínez P, Ferrara‐Romeo I, Flores JM, Blasco MA. Essential role for the TRF 2 telomere protein in adult skin homeostasis. Aging Cell. 2014;13(4):656–668. doi:10.1111/acel.12221
74. Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet. 2005;37(10):1063–1071. doi:10.1038/ng1633
75. Blanco R, Muñoz P, Flores JM, Klatt P, Blasco MA. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 2007;21(2):206–220. doi:10.1101/gad.406207
76. Muñoz P, Blanco R, Blasco MA. Role of the TRF2 telomeric protein in cancer and aging. Cell Cycle. 2006;5(7):718–721. doi:10.4161/cc.5.7.2636
77. Okamoto K, Iwano T, Tachibana M, Shinkai Y. Distinct roles of TRF1 in the regulation of telomere structure and lengthening. J Biol Chem. 2008;283(35):23981–23988. doi:10.1074/jbc.M802395200
78. Ye Jeffrey Z-S, de Lange T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat Genet. 2016;6(1):618–623. doi:10.1038/ng1360
79. Kim S-H, Kaminker P, Campisi J. TIN2, a new regulator of telomere length in human cells. Nat Genet. 1999;23(4):405. doi:10.1038/70508
80. Frank AK, Tran DC, Qu RW, Stohr BA, Segal DJ, Xu L. The shelterin TIN2 subunit mediates recruitment of telomerase to telomeres. PLoS Genet. 2015;11(7):e1005410. doi:10.1371/journal.pgen.1005410
81. Samavarchi Tehrani S, Mahmoodzadeh Hosseini H, Yousefi T, et al. The crosstalk between trace elements with DNA damage response, repair, and oxidative stress in cancer. J Cell Biochem. 2019;120(2):1080–1105. doi:10.1002/jcb.27617
82. Tehrani SS, Karimian A, Parsian H, Majidinia M, Yousefi B. Multiple functions of long non‐coding RNAs in oxidative stress, DNA damage response and cancer progression. J Cell Biochem. 2018;119(1):223–236. doi:10.1002/jcb.26217
83. Ibáñez-Cabellos JS, Pérez-Machado G, Seco-Cervera M, Berenguer-Pascual E, García-Giménez JL, Pallardó FV. Acute telomerase components depletion triggers oxidative stress as an early event previous to telomeric shortening. Redox Biol. 2018;14:398–408. doi:10.1016/j.redox.2017.10.004
84. Chen L-Y, Zhang Y, Zhang Q, et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol Cell. 2012;47(6):839–850. doi:10.1016/j.molcel.2012.07.002
85. Billard P, Poncet DA. Replication stress at telomeric and mitochondrial dna: common origins and consequences on aging. Int J Mol Sci. 2019;20(19):4959. doi:10.3390/ijms20194959
86. Sullivan LB, Santos JH, Chandel NS. Mitochondria and telomeres: the promiscuous roles of TIN2. Mol Cell. 2012;47(6):823–824. doi:10.1016/j.molcel.2012.09.006
87. Lee JH, Jung M, Hong J, Kim MK, Chung IK. Loss of RNA-binding protein HuR facilitates cellular senescence through posttranscriptional regulation of TIN2 mRNA. Nucleic Acids Res. 2018;46(8):4271–4285. doi:10.1093/nar/gky223
88. Kuimov A. Polypeptide components of telomere nucleoprotein complex. Biochemistry (Moscow). 2004;69(2):117–129. doi:10.1023/B:BIRY.0000018941.81962.1c
89. Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73(1):177–208. doi:10.1146/annurev.biochem.73.071403.160049
90. Loayza D, De Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423(6943):1013. doi:10.1038/nature01688
91. Lee J, Okumus B, Kim D, Ha T. Extreme conformational diversity in human telomeric DNA. Proc Natl Acad Sci. 2005;102(52):18938–18943. doi:10.1073/pnas.0506144102
92. Baumann P, Price C. Pot1 and telomere maintenance. FEBS Lett. 2010;584(17):3779–3784. doi:10.1016/j.febslet.2010.05.024
93. Han X, Liu D, Zhang Y, et al. Akt regulates TPP 1 homodimerization and telomere protection. Aging Cell. 2013;12(6):1091–1099. doi:10.1111/acel.12137
94. Colgin LM, Baran K, Baumann P, Cech TR, Reddel RR. Human POT1 facilitates telomere elongation by telomerase. Curr Biol. 2003;13(11):942–946. doi:10.1016/S0960-9822(03)00339-7
95. Liu D, Safari A, O’Connor MS, et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol. 2004;6(7):673. doi:10.1038/ncb1142
96. Guo X, Deng Y, Lin Y, et al. Dysfunctional telomeres activate an ATM‐ATR‐dependent DNA damage response to suppress tumorigenesis. EMBO J. 2007;26(22):4709–4719. doi:10.1038/sj.emboj.7601893
97. Hockemeyer D, Daniels J-P, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126(1):63–77. doi:10.1016/j.cell.2006.04.044
98. Jones M, Bisht K, Savage SA, Nandakumar J, Keegan CE, Maillard I. The shelterin complex and hematopoiesis. J Clin Invest. 2016;126(5):1621–1629. doi:10.1172/JCI84547
99. Wu L, Multani AS, He H, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126(1):49–62. doi:10.1016/j.cell.2006.05.037
100. Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068. doi:10.1038/nature06065
101. Wang Y, Shen M-F, Chang S. Essential roles for Pot1b in HSC self-renewal and survival. Blood. 2011;118(23):6068–6077. doi:10.1182/blood-2011-06-361527
102. Hosokawa K, MacArthur BD, Ikushima YM, et al. The telomere binding protein Pot1 maintains haematopoietic stem cell activity with age. Nat Commun. 2017;8(1):1–15.
103. Hosokawa K, MacArthur BD, Ikushima YM, et al. The telomere binding protein Pot1 maintains haematopoietic stem cell activity with age. Nat Commun. 2017;8(1):804.
104. Kibe T, Osawa GA, Keegan CE, De Lange T. Telomere protection by TPP1 is mediated by POT1a and POT1b. Mol Cell Biol. 2010;30(4):1059–1066. doi:10.1128/MCB.01498-09
105. Patel T, Vasan R, Gupta D, Patel J, Trivedi M. Shelterin proteins and cancer. Asian Pac J Cancer Prev. 2015;16(8):3085–3090. doi:10.7314/APJCP.2015.16.8.3085
106. Zhong FL, Batista LF, Freund A, Pech MF, Venteicher AS, Artandi SE. TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell. 2012;150(3):481–494. doi:10.1016/j.cell.2012.07.012
107. Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492(7428):285.
108. Xin H, Liu D, Wan M, et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature. 2007;445(7127):559–562. doi:10.1038/nature05469
109. Zhang Y, Chen L-Y, Han X, et al. Phosphorylation of TPP1 regulates cell cycle-dependent telomerase recruitment. Proc Natl Acad Sci. 2013;110(14):5457–5462. doi:10.1073/pnas.1217733110
110. Chen C, Gu P, Wu J, et al. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat Commun. 2017;8(1):1–15. doi:10.1038/s41467-016-0009-6
111. Else T, Theisen BK, Wu Y, et al. Tpp1/Acd maintains genomic stability through a complex role in telomere protection. Chromosome Res. 2007;15(8):1001. doi:10.1007/s10577-007-1175-5
112. Toiber D, Sebastian C, Mostoslavsky R. Characterization of Nuclear Sirtuins: Molecular Mechanisms and Physiological Relevance. Histone Deacetylases: The Biology and Clinical Implication. Springer; 2011:189–224.
113. Chen H, Liu X, Zhu W, et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front Aging Neurosci. 2014;6:103. doi:10.3389/fnagi.2014.00103
114. Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492(7428):285–289.
115. Sexton AN, Regalado SG, Lai CS, et al. Genetic and molecular identification of three human TPP1 functions in telomerase action: recruitment, activation, and homeostasis set point regulation. Genes Dev. 2014;28(17):1885–1899. doi:10.1101/gad.246819.114
116. Tejera AM, d’Alcontres MS, Thanasoula M, et al. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell. 2010;18(5):775–789. doi:10.1016/j.devcel.2010.03.011
117. Bae NS, Baumann P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell. 2007;26(3):323–334. doi:10.1016/j.molcel.2007.03.023
118. Martinez P, Thanasoula M, Carlos AR, et al. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat Cell Biol. 2010;12(8):768.
119. Chen Y, Rai R, Zhou Z-R, et al. A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat Struct Mol Biol. 2011;18(2):213. doi:10.1038/nsmb.1974
120. Li B, Oestreich S, De Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101(5):471–483. doi:10.1016/S0092-8674(00)80858-2
121. Pardo B, Marcand S. Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J. 2005;24(17):3117–3127. doi:10.1038/sj.emboj.7600778
122. Sfeir A, Kabir S, van Overbeek M, Celli GB, de Lange T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327(5973):1657–1661. doi:10.1126/science.1185100
123. Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7(7):712. doi:10.1038/ncb1275
124. Johnson AN, Weil PA. Identification of a transcriptional activation domain in yeast repressor activator protein 1 (Rap1) using an altered DNA-binding specificity variant. J Biol Chem. 2017;292(14):5705–5723. doi:10.1074/jbc.M117.779181
125. Martinez P, Thanasoula M, Carlos AR, et al. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nat Cell Biol. 2010;12(8):768–780.
126. Donate LE, Blasco MA. Telomeres in cancer and aging. Philos Trans R Soc B. 2011;366(1561):76–84. doi:10.1098/rstb.2010.0291
127. Platt JM, Ryvkin P, Wanat JJ, et al. Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 2013;27(12):1406–1420. doi:10.1101/gad.218776.113
128. Ganapathi M, Palumbo MJ, Ansari SA, et al. Extensive role of the general regulatory factors, Abf1 and Rap1, in determining genome-wide chromatin structure in budding yeast. Nucleic Acids Res. 2010;39(6):2032–2044. doi:10.1093/nar/gkq1161
129. Yarragudi A, Miyake T, Li R, Morse RH. Comparison of ABF1 and RAP1 in chromatin opening and transactivator potentiation in the budding yeast Saccharomyces cerevisiae. Mol Cell Biol. 2004;24(20):9152–9164. doi:10.1128/MCB.24.20.9152-9164.2004
130. Lototska L, Yue JX, Li J, et al. Human RAP 1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020;21(4). doi:10.15252/embr.201949076.
131. Song S, Johnson FB. Epigenetic mechanisms impacting aging: a focus on histone levels and telomeres. Genes. 2018;9(4):201. doi:10.3390/genes9040201
132. Luo K, Vega-Palas MA, Grunstein M. Rap1–Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002;16(12):1528–1539. doi:10.1101/gad.988802
133. Zhang Y, Chiu S, Liang X, et al. Rap1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discovery. 2015;1(1):15007. doi:10.1038/cddiscovery.2015.7
134. Song S, Perez JV, Svitko W, et al. Rap1‐mediated nucleosome displacement can regulate gene expression in senescent cells without impacting the pace of senescence. Aging Cell. 2019;e13061.
135. Lian S, Meng L, Liu C, et al. PRL-3 activates NF-κB signaling pathway by interacting with RAP1. Biochem Biophys Res Commun. 2013;430(1):196–201. doi:10.1016/j.bbrc.2012.11.036
136. Teo H, Ghosh S, Luesch H, et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nat Cell Biol. 2010;12(8):758–767.
137. Teo H, Ghosh S, Luesch H, et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-κB-dependent gene expression. Nat Cell Biol. 2010;12(8):758.
138. Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in aging and disease. Aging Dis. 2011;2(6):449.
139. Pramanik KC, Makena MR, Bhowmick K, Pandey MK. Advancement of NF-κB signaling pathway: a novel target in pancreatic Cancer. Int J Mol Sci. 2018;19(12):3890. doi:10.3390/ijms19123890
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.