Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Severe Refractory Obsessive Compulsive Disorder and Depression: Should We Consider Stereotactic Neurosurgery?
Authors Zrinzo L
Received 16 December 2023
Accepted for publication 22 February 2024
Published 4 March 2024 Volume 2024:20 Pages 469—478
DOI https://doi.org/10.2147/NDT.S407210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Ludvic Zrinzo
Unit of Functional Neurosurgery, UCL Queen Square Institute of Neurology, London, UK
Correspondence: Ludvic Zrinzo, Functional Neurosurgery Unit, Department of Clinical & Motor Neurosciences, UCL Institute of Neurology, Second Floor, 33 Queen Square, London, WC1N 3BG, U.K, Email [email protected]
Abstract: Functional neurosurgery involves modulation of activity within neural circuits that drive pathological activity. Neurologists and neurosurgeons have worked closely together, advancing the field for over a century, such that neurosurgical procedures for movement disorders are now accepted as “standard of care”, benefiting hundreds of thousands of patients. As with movement disorders, some neuropsychiatric illnesses, including obsessive compulsive disorder and depression, can be framed as disorders of neural networks. Over the past two decades, evidence has accumulated that stereotactic neurosurgery can help some patients with mental disorders. Nevertheless, despite the availability of class I evidence for some interventions, there is a huge mismatch between the prevalence of severe refractory mental disorders and the number of referrals made to specialised functional neurosurgery services. This paper examines the historical trajectory of neurosurgery for movement and mental disorders. A review of neurosurgical techniques, including stereotactic radiofrequency ablation, gamma knife, deep brain stimulation, and magnetic resonance imaging guided focused ultrasound, explains the high degree of safety afforded by technological advances in the field. Evidence from clinical trials supporting functional neurosurgery for mental disorders, including obsessive compulsive disorder and depression, is presented. An improved understanding of modern functional neurosurgery should foster collaboration between psychiatry and neurosurgery, providing hope to patients whose symptoms are refractory to all other treatments.
Keywords: stereotactic neurosurgery, deep brain stimulation, obsessive compulsive disorder, major depression, stereotactic ablation, focused ultrasound
Introduction
Much of neurosurgical practice involves treatment of patients with focal pathologies that distort the structure of the central nervous system. This includes removal of blood clots, tumors, and herniated intervertebral discs. Conversely, functional neurosurgery involves modulation of activity within neural circuits that drive pathological activity, usually in the context of a network brain disorder. The prime example is the neurosurgical treatment of tremor, where modulation of abnormal neural activity can provide significant relief (Figure 1). Numerous randomized controlled trials have demonstrated the safety and efficacy of functional neurosurgical procedures in ameliorating the symptoms and quality of life of individuals living with tremor, Parkinson disease, and dystonia.1–4 Neurologists and neurosurgeons have worked together, advancing the field for over a century, such that neurosurgical procedures for movement disorders are now accepted as “standard of care”, benefiting hundreds of thousands of patients.
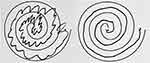 |
Figure 1 Stereotactic radiofrequency ablation thalamotomy for tremor. Hand drawn spiral before (left) and after (right). |
The success of functional neurosurgery in movement disorders has led several multidisciplinary groups around the world to explore whether a similar collaboration can advance the field of neuropsychiatry. As with movement disorders, some neuropsychiatric illnesses can be framed as disorders of neural networks. Over the past two decades, evidence has accumulated that stereotactic neurosurgery can help selected patients with mental disorders.5 Nevertheless, despite the availability of class I evidence for some interventions, there is a huge mismatch between the prevalence of severe refractory mental disorders and the number of referrals made to specialised functional neurosurgery services.6 There are varied reasons leading to this reality. Reviewing the historical trajectory of neurosurgery for movement and mental disorders provides some understanding of the current situation.
A Brief History of Functional Neurosurgery for Movement Disorders
Functional neurosurgery has its roots in the late nineteenth century and was born of collaboration between neurologists and neurosurgeons. John Hughlings Jackson, a neurologist, and Victor Horsley, the father of neurosurgery, promoted the theory of cortical localization of cerebral function. This led Horsley to perform resections of the motor cortex for the arrest of severe athetosis in the 1890s.7 That paresis or paralysis of a limb was preferable to the movement disorder is testament to these desperate times. By the middle of the 20th century, by combining serendipity and scientific exploration, it became clear that smaller, minimally invasive neurosurgical procedures were superior to large, open, and widely destructive ones.8–10 Accurate placement of small lesions within the basal ganglia and thalamus, deep within the brain, could ameliorate motor symptoms, without accompanying motor deficit. Again, this achievement relied on collaboration between a neurologist, Spiegel, and a neurosurgeon, Wycis. By fusing two technologies – ventriculography and the stereotactic technique – they introduced the concept of stereotactic functional neurosurgery to clinical practice in 1947.11
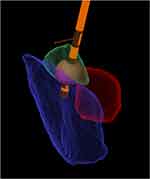
The stereotactic technique, initially described with non-human primates in 1906 by Horsley, allows precise navigation within the human brain and relies on fixation of a frame to the skull. Acquisition of images then allows spatial localization of structures visualized within the brain in relation to landmarks attached to the frame (fiducial markers). The principle is akin to laying a grid over a map, providing a reference from which accurate measurements, that inform neurosurgical plans, can be made (Figure 2). Several methods were used to create stereotactic lesions, including chemical ablation by injection procaine oil or alcohol, and cryotherapy with liquid nitrogen. However, radiofrequency ablation (RFA) is now the most common and enduring method. RFA involves passing a very high frequency electrical current through the tip of a probe, and coagulating immediately adjacent tissues, while monitoring and controlling temperature changes with a thermistor.12
Lars Leksell developed the gamma knife (GK) in the second half of the 20th century.13 This technique allowed incisionless stereotactic surgery by focusing numerous gamma rays on a small volume of brain tissue. The relatively low energy of each gamma source meant that no harm was done, except were the rays overlapped. The effects of GK therapy are gradual and delayed since radiation damage in the target region takes weeks or months to become evident.14
The introduction of dopamine revolutionized the medical treatment of Parkinson disease in the 1970s and enthusiasm for functional neurosurgery in movement disorders waned as a result.15 Nevertheless, it soon became clear that a significant proportion of patients had symptoms that were refractory to dopamine, could not tolerate medication, or developed complications, such as dyskinesia or motor fluctuations. This resulted in a renaissance in the field of functional neurosurgery for movement disorders, beginning in the 1980s, supported by significant technological advances.16
Chief among these technologies was magnetic resonance imaging (MRI). Specific MRI sequences can reveal neuroanatomy in exquisite detail, allowing visualization of anatomical targets relevant to functional neurosurgery. The ability to visualize the surgical target and to assess the veracity of the neurosurgical intervention vis-à-vis the intended target, allows unprecedented levels of accuracy, precision, and safety.16
The second development was the introduction of deep brain stimulation (DBS) by Alim Louis Benabid and Pierre Pollak, another neurosurgeon/neurologist team, and it had a huge impact on functional neurosurgery for movement disorders. They noted that continuous delivery of a small high frequency electrical current (above 130 Hz) had a similar effect to a lesion, but that these effects were reversible when the current was switched off.17 Titration of the electrical current allows for maximal beneficial effect, while minimizing associated side effects (Figure 3). Development of commercially available implantable hardware to deliver the electrical current introduces several challenges, including high cost, significant specialist time for programming, infection, and hardware malfunction. However, DBS provides a greater measure of safety than bilateral lesions for movement disorders and reduces the risk of severe balance, speech, and swallowing problems.18 DBS provides an opportunity to obtain electrical recordings from deep within the living human brain in an ethical fashion, leading to a greater understanding of human brain activity. Moreover, the ability to turn stimulation ON and OFF in a blinded fashion is a powerful tool in clinical trials (allowing sham stimulation). DBS became hugely popular, overtaking stereotactic lesion in the first few decades of the 21st century.19,20
The most recent technological wonder in functional neurosurgery is, once again, a fusion of different technologies. High Intensity MR-guided Focused Ultrasound (FUS) utilizes stereotactic principles to merge ultrasound and MR modalities.21,22 Numerous ultrasound sources deliver energy through the skull and are refocused to converge on a single spot within the brain. The energy within each ultrasound beam is too low to damage tissue but, where they overlap, enough energy is released to heat the tissues, causing protein coagulation and focal ablation. MRI is used both to visualize the anatomical target, and to confirm that heat is being delivered to the intended target using MR thermography. The ability to visualize the accuracy of targeting and the instantaneous effect has given FUS the edge over GK in functional neurosurgery.23 However, the enthusiasm for FUS has also driven resurgent interest in radiofrequency ablation, now bolstered by the greatly improved quality of MR images as compared to the initial RFA era.
As a result of all these advances, several surgical tools and approaches are now available to the functional neurosurgical team, including RFA, FUS, and DBS, each with its advantages and limitations. When employing a multidisciplinary approach, characterized by meticulous patient selection and lifelong follow up, patients can benefit from remarkable levels of safety and efficacy.24,25
A Brief History of Functional Neurosurgery for Psychiatric Disorders
As with movement disorders, neurosurgery for psychiatric disorders began with widely destructive procedures, in this case the infamous leucotomy or lobotomy procedure. Introduced by Egas Moniz in 1935, and popularized by Walter Freeman, neither of whom were trained neurosurgeons, thousands of people were subjected to this procedure.26 Freeman favored the “ice pick” method, driving its tip, freehand, through the roof of the orbit, and swiveling it back and forth to disconnect the frontal lobes. Although, surprisingly, some patients seemed to benefit, it soon became clear that side effects often outweighed any beneficial effects.
Some functional neurosurgeons followed the path taken with movement disorders, avoiding large areas of destruction, and making much smaller, stereotactic lesions within neural circuits known to drive pathological symptoms in psychiatric disorders. Procedures such as anterior cingulotomy and anterior capsulotomy appeared to provide significant relief from anxiety, obsessions, and low mood, without causing neurological deficits or negative effects on personality.27,28 Stereotactic GK capsulotomy for psychiatric disorders was introduced by Leksell as an minimally invasive procedure.29 However, an understandable backlash against lobotomy, combined with the introduction of effective antipsychotic agents, led to all forms of neurosurgery for psychiatric disorders falling out of favor in the latter years of the twentieth century.
More recently the renaissance of functional neurosurgery and introduction of DBS in movement disorders has spurred a flurry of interest in DBS for mental health disorders. Starting in 1999, case reports of DBS for mental disorders started to appear.30,31 This was soon followed by larger studies and randomized controlled trials with positive, encouraging results, especially in Gilles de la Tourette Syndrome and obsessive compulsive disorder (OCD).32–34
Gilles de la Tourette Syndrome
Gilles de la Tourette Syndrome (GTS) straddles the worlds of neurology and psychiatry. A childhood-onset disorder, the diagnosis is made when motor and phonic tics are present for over one year.35 Frequently, GTS is comorbid with obsessive–compulsive-behavior or -disorder, attention-deficit hyperactivity disorder, autism spectrum disorder, depression, and anxiety. Symptom severity often wanes beyond adolescence, but moderate or severe tics may persist. In some, severe tics are unresponsive to conventional treatment with behavioral therapy and/or medications with deleterious effects on physical and social function, employment, and quality of life. Self-injurious behaviors and/or violent tics can cause significant injury, and patients are at a four-fold increased risk of completed suicide. Patients with severe GTS require more effective therapies.
The first reported DBS procedures for GTS by Veerle Visser-Vandewalle in 1999, targeted the centromedian and parafascicular nuclei of the thalamus.31 The target was inspired by promising results after stereotactic lesions in this region, described by Hassler and Dieckmann in 1970. Several different anatomical targets have since been utilized for DBS in severe refractory GTS; however, the most popular are the thalamic region and the globus pallidus pars internus (GPi), with randomized trials reporting encouraging results at each target.36,37 A small randomized controlled trial (RCT) slightly favored GPi over thalamus, as did an international registry of DBS for GTS.38,39 Significant reduction in both motor and phonic tic scores were seen with stimulation at all three targets, that were sustained over time.
A randomized controlled trial of GPi stimulation published by our group in 2015 led to a significant improvement in tic severity, with an overall acceptable safety profile.36 This prompted a second RCT, that is currently recruiting patients, to explore whether GPi DBS is effective in reducing severe motor and vocal tics in a double-blind crossover setting.40 Investigation into the role of stereotactic ablation in GTS is far less advanced but open label data from case reports and small series suggest a potential role for anterior capsulotomy.41,42
Obsessive Compulsive Disorder
OCD is characterized by intrusive thoughts (obsessions) leading to repeated behaviors (compulsions) that are time consuming and lead to significant distress or functional impairment.43 OCD is a common mental disorder with a lifetime prevalence of 1–2%. Cognitive behavioral therapy and medication (such as selective serotonin reuptake inhibitors) are effective in most patients.43 However, around 10% of individuals are treatment refractory and remain severely impaired.44 Symptom severity is measured using the Yale-Brown Obsessive compulsive score (Y-BOCS), although changes on this scale do not always correlate well with quality-of-life scores.
Stereotactic anterior capsulotomy (ACAPS), with ablation of the anterior limb of the internal capsule, was first reported by Jean Talairach et al in 1949, as a means of limiting the extensive destruction of leucotomy procedures.27 Hugh Cairns and others proposed the anterior cingulotomy (ACING) as another means of limiting leucotomy side effects.45 Both procedures appeared effective at reducing obsessions and anxiety, while preserving personality.
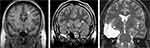
Radiofrequency ablation (RFA) is often used to perform stereotactic ACING procedures. However, stereotactic ACAPS can also be achieved with GK and FUS (Figure 4).46,47 A randomized controlled trial using GK-ACAPS vs sham in patients with severe refractory OCD revealed significant reduction in Y-BOCS in the treated arm.32 However, most data on clinical outcomes, for both ACAPS and ACING, derives from open label studies.
A recent systematic review of observational studies evaluated the outcome following ACING and ACAPS in patients with severe refractory OCD. At 12 months, Y-BOCS had fallen by 37% and 55%, respectively, with a full response at final long term follow up (defined as ≥35% reduction in Y-BOCS) of 41% and 54%, respectively.48
The first use of DBS for OCD was reported by Bart Nuttin in 1999.30 As with stereotactic ablation, several anatomical targets for DBS have emerged as effective in the treatment of OCD, with the most popular being the region of the ventral part of the anterior limb of the internal capsule (ALIC) and the anteromedial subthalamic nucleus (amSTN). Several randomized controlled trials, from different centers around the world, have demonstrated the safety and efficacy of DBS for OCD when comparing actual vs sham stimulation in the blinded phase of each study. Improvement in Y-BOCS has ranged from 18% to 53% when targeting the ALIC and from 25% to 44% when targeting the amSTN.33,34,49–51
A randomized controlled trial of DBS in OCD published by our group in 2019 compared ALIC with amSTN DBS in the same patients.33 DBS at each site produced a similar large effect on OCD symptoms with little additional gain following combined stimulation. Interestingly, amSTN but not ALIC DBS significantly improved cognitive flexibility, whereas ALIC DBS had a greater effect on mood. Theses differential improvements suggest that DBS at each site modulates distinct brain networks.
There have been no “head-to-head” studies comparing DBS with stereotactic ablation. However, our literature review compared the published results of DBS and ablation in the region of the ALIC.52 In an attempt to make reasonable comparisons, patients were stratified according to baseline severity. Patients suffering from severe OCD (Y-BOCS: 24–32) were more likely to respond if they underwent ACAPS rather than ALIC DBS. When examining patients with extreme OCD (Y-BOCS: ≥ 32), response rates were similar (∼50%) but patients were more likely to go into remission (Y-BOCS: ≤8) if they underwent ACAPS. When considering adverse events, weight gain was more likely in patients undergoing ACAPS, but 10% of ALIC DBS patients experienced problems with wound infection or hardware malfunction. These results were echoed by a more recent meta-analysis across different brain targets, where stereotactic ablation was found to have greater utility, with a greater percentage improvement in Y-BOCS, than DBS.53
Treatment Refractory Depression
Major depressive disorder (MDD) is one of the most common mental disorders, causing significant global disability, morbidity, and mortality.54 MDD has increasingly been regarded as a disorder of neural networks with cortical and subcortical structures, being implicated, including parts of the cingulate gyrus and orbitofrontal cortex.55 Most patients respond to a combination of psychotherapy and pharmacotherapy; in more severe cases, electroconvulsive therapy can provide relief. However, up to one-third of patients may not respond sufficiently, leading to a diagnosis of treatment-refractory depression (TRD).56
The evidence base for neurosurgery in TRD is less robust than that for OCD. Stereotactic ACAPS and ACING are the most common ablative procedures in this patient group. There are no randomized controlled trials of stereotactic ablation in TRD but their safety and efficacy are supported by level II evidence.57 In our systematic review of open label studies, 53% of patients responded to ACAPS or ACING (defined as ≥50% improvement in validated depression scores), of which 34% reached remission (defined as Montgomery–Åsberg Depression Rating Scale score ≤ 10 or Beck Depression Inventory ≤ 11).58 A significant number of individuals (26%) had a second procedure due to an unsatisfactory response to the first surgery. Validated scores improved by a mean of 44% after a second SRA procedure. When available before and after surgery, neurocognitive and personality testing were not significantly different at follow-up, with a trend towards improvement on some measures of executive function.
DBS for TRD has been proposed, with the most popular targets being the subcallosal cingulate gyrus, the ALIC, and the medial forebrain bundle. However, promising data from early open label studies have not been replicated in subsequent randomized controlled trials with little difference between sham vs real stimulation.59–61
Contemporary Neurosurgery for Mental Disorders
As with neurosurgery for movement disorders, it is important to note that, despite sometimes dramatic effects, neurosurgery does not result in a cure for mental disorders. In movement disorders, the best results are often obtained with a combination of neurosurgery and ongoing medical therapy. This is also true in the field of mental disorders where lifelong follow up is required and maximal benefit is achieved by ongoing behavioral and pharmacotherapy.62 A multidisciplinary approach, involving both psychiatrist and neurosurgeon, is essential during patient selection, perioperative care, and lifelong follow-up.57,63
Focused Ultrasound Capsulotomy in OCD and Depression
Focused ultrasound (FUS) is a powerful new tool in functional neurosurgery. The ability to perform incisionless stereotactic ablation allows comparison of “sham” to “real” FUS procedures, providing a means of performing double blind RCTs. One such trial of FUS for essential tremor provides a strong evidence base that allowed its rapid adoption.23 Open label trials of FUS for severe refractory OCD and depression have provided promising early results.47,64,65 RCTs of FUS capsulotomy for OCD and depression will likely follow.
DBS versus Ablation in Mental Disorders
Some perceive DBS to be superior to ablation in the context of mental disorders. This may be due to a combination of its perceived “reversibility” compared with the “finality” of an ablative procedure. However, DBS is much more expensive, demands specialist input to program the device, may require significant maintenance, and could cause severe symptom rebound should components fail or be removed because of infection. Importantly, there is little evidence that efficacy is higher, or that side effects are less common with DBS than with stereotactic ablation. The ability to switch DBS off or on in a blinded fashion may lead to the conclusion that randomized control trials are easier to conduct when compared to stereotactic ablation. However, “stun effects” from inserting the electrode and delay between stimulation and onset of clinical effect has confounded results. Additionally, although one might expect a significant placebo response after any neurosurgical intervention, this is likely to be even greater after DBS, especially given the frequent follow up visits required for “high-tech” adjustment of stimulation parameters. In summary, both surgical approaches have strengths and weaknesses, and further study of both is essential in a field that desperately needs novel and effective therapies.
Neurosurgery for Mental Disorders in the UK
DBS for mental disorders is not currently commissioned by the National Health Service (NHS) in the United Kingdom (UK), and such patients can only receive DBS as part of a clinical trial. However, stereotactic ablation is supported by the Royal College of Psychiatrists and commissioned by the NHS.66 It can only be performed in carefully selected patients within a framework of strong clinical governance and safeguarding.57 Mental Health legislation ensures that every patient is reviewed by the Mental Health Board of the Care Quality Commission.67 This process ensures that these vulnerable patients can provide consent, are fully informed of the potential risks and benefits, and have symptoms refractory to other therapies that are severe enough to warrant a neurosurgical intervention.
Although neurosurgery should only be considered when every other reasonable treatment has failed to provide relief, it should be considered in a timely fashion. Response or remission of symptoms following neurosurgery after 10 or 20 years of severe chronic symptoms still means that the individual has missed out on many of life’s opportunities. Failings within healthcare systems may prevent timely referral and these should be addressed and overcome.
Mismatch Between Incidence and Referral Patterns
Despite the availability of stereotactic ablation within the NHS, the number of patients with mental disorders being referred and treated by functional neurosurgery is paltry when compared to that for movement disorders.6 This is discordant with the much larger prevalence of patients suffering from refractory mental disorders. One reason may include conflation of modern functional neurosurgical techniques with lobotomy and the erroneous perception, among those who care for people with OCD and depression, that neurosurgery is highly destructive and carries high risks.
Another factor might be the artificial division between neurology and psychiatry in clinical practice. Psychiatrists often work in separate institutions and buildings, a historical relic of the “lunatic asylum” system. As a result, although neurologists are often co-located with neurosurgeons, fostering collaboration, psychiatrists and their patients remain distant from the reality of modern neurosurgery.68
Finally, ethical concerns for this vulnerable patient population are an important factor.69
Patients with severe refractory epilepsy are often referred for a neurosurgical opinion for their symptoms, even though the procedures they eventually undergo involve much larger regions of brain ablation (Figure 4). However, many patients with mental disorders do not get this opportunity. Could this be viewed as discrimination – conscious or otherwise – against patients who suffer from a mental disorder?
Conclusion
An improved understanding of modern functional neurosurgery by the psychiatric community should foster collaboration between these two disciplines. Indeed, given the available data, not considering functional neurosurgery in severely affected patients with refractory OCD may be of greater risk and harm to these individuals than the neurosurgical procedure. Although the data on neurosurgery for severe refractory depression is less robust, open label data suggest that 1 in 2 patients respond to cingulotomy or capsulotomy and 1 in 3 go into remission. Individual patients should be given the option of a neurosurgical opinion. Such a promising treatment deserves further scientific exploration.
Patients would benefit from informed psychiatrists making appropriate referrals to an experienced multidisciplinary functional neurosurgery team, within a framework of strong clinical governance and safeguarding. Moreover, referral of larger numbers of patients with OCD and depression for consideration of stereotactic neurosurgery would allow centers to design studies that would improve our knowledge of its role in the management of patients who have not responded to all other available treatments.
Abbreviations
ACAPS, anterior capsulotomy; ACING, anterior cingulotomy; ALIC, anterior limb of the internal capsule; amSTN, anteromedial subthalamic nucleus; DBS deep brain stimulation; FUS, Focused Ultrasound; GK, gamma knife; GPi, globus pallidus pars internus; GTS, Gilles de la Tourette Syndrome; MDD, Major depressive disorder; MRI, magnetic resonance imaging; NHS, National Health Service; OCD, obsessive compulsive disorder; RCT, randomized controlled trial; RFA, radiofrequency ablation; TRD, treatment-refractory depression; UK, United Kingdom; Y-BOCS, Yale-Brown Obsessive compulsive score.
Ethics Statement
Written informed consent was obtained for publication of the images in Figure 2.
Acknowledgments
This paper is based on the International Neuropsychiatry Association Lishman Lecture, delivered at the Royal College of Psychiatrists, London, UK, on September 16, 2022. The Functional Neurosurgery Unit is supported by the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre.
Disclosure
The author acts as a Consultant for Boston Scientific, Insightec, and Medtronic. The authors also reports Honoraria for educational activities from Medtronic, Boston Scientific, BrainLab, and InoMed. The author reports no other conflicts of interest in this work.
References
1. Odekerken VJJ, Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. doi:10.1016/s1474-4422(12)70264-8
2. Bie RM, Haan RJ, Nijssen PC, et al. Unilateral pallidotomy in Parkinson’s disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354(9191):1665–1669. doi:10.1016/s0140-6736(99)03556-4
3. Volkmann J, Mueller J, Deuschl G, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13(9):875–884. doi:10.1016/s1474-4422(14)70143-7
4. Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461–468. doi:10.1056/nejm200002173420703
5. Rissardo JP, Vora NM, Tariq I, Mujtaba A, Caprara ALF. Deep brain stimulation for the management of refractory neurological disorders: a comprehensive review. Medicina. 2023;59(11):1991. doi:10.3390/medicina59111991
6. Visser-Vandewalle V, Andrade P, Mosley PE, et al. Deep brain stimulation for obsessive–compulsive disorder: a crisis of access. Nat Med. 2022;28(8):1529–1532. doi:10.1038/s41591-022-01879-z
7. Horsley V. Brain-surgery. Br Med J. 1886;2(1345):670.
8. Hariz M, Lees AJ, Blomstedt Y, Blomstedt P. Serendipity and observations in functional neurosurgery: from James parkinson’s stroke to hamani’s & lozano’s flashbacks. Stereot Funct Neuros. 2022;100(4):201–209. doi:10.1159/000525794
9. Cooper IS. Surgical alleviation of parkinsonism: effects of occlusion of the anterior choroidal artery*. J Am Geriatr Soc. 1954;2(11):691–718. doi:10.1111/j.1532-5415.1954.tb02479.x
10. Gildenberg PL. Evolution of basal ganglia surgery for movement disorders. Stereot Func Neurosurg. 2006;84(4):131–135. doi:10.1159/000094844
11. Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic apparatus for operations on the human brain. Science. 1947;106(2754):349–350. doi:10.1126/science.106.2754.349
12. Organ LW. Electrophysiologic principles of radiofrequency lesion making.. Appl Neurophysiol. 1976;39(2):69–76. doi:10.1159/000102478
13. Leksell DG. Stereotactic radiosurgery: current status and future trends. Stereotact Funct Neurosurg. 1993;61(Suppl 1):1–5.
14. Witjas T, Carron R, Krack P, et al. A prospective single-blind study of Gamma Knife thalamotomy for tremor. Neurology. 2015;85(18):1562–1568. doi:10.1212/wnl.0000000000002087
15. Cotzias GC, Woert MHV, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276(7):374–379. doi:10.1056/nejm196702162760703
16. Zrinzo L. The role of imaging in the surgical treatment of movement disorders. Neuroimag Clin N Am. 2010;20(1):125–140. doi:10.1016/j.nic.2009.08.002
17. Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet Lond Engl. 2003;345(8942):91–95. doi:10.1016/s0140-6736(95)90062-4
18. Dowsey-Limousin P, Pollak P. Deep brain stimulation in the treatment of Parkinson’s disease: a review and update. Clin Neurosci Res. 2001;1(6):521–526. doi:10.1016/s1566-2772(01)00029-9
19. Gildenberg PL. Evolution of Neuromodulation. Stereot Func Neurosurg. 2005;83(2–3):71–79. doi:10.1159/000086865
20. Hariz MI. From functional neurosurgery to “interventional” neurology: survey of publications on thalamotomy, pallidotomy, and deep brain stimulation for Parkinson’s disease from 1966 to 2001. Mov Disord. 2003;18(8):845–853. doi:10.1002/mds.10470
21. Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47(8):1219–1236. doi:10.1088/0031-9155/47/8/301
22. Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858–861. doi:10.1002/ana.21801
23. Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi:10.1056/nejmoa1600159
24. Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review: clinical article. J Neurosurg. 2012;116(1):84–94. doi:10.3171/2011.8.jns101407
25. Rajabian A, Vinke S, Candelario-Mckeown J, et al. Accuracy, precision, and safety of stereotactic, frame-based, intraoperative MRI-guided and MRI-verified deep brain stimulation in 650 consecutive procedures. J Neurosurg. 2022:1–10. doi:10.3171/2022.8.jns22968
26. Caruso JP, Sheehan JP. Psychosurgery, ethics, and media: a history of walter freeman and the lobotomy. Neurosurg Focus. 2017;102(3):E6–8. doi:10.3171/2017.6.focus17257
27. Talairach J, Hecaen H, David M. Lobotomie préfrontale limitée par électrocoagulation des fibres thalamo-frontales à leur émergence du bras antérieur de la capsule interne. Rev Neurol. 1949;83(1):59.
28. Ballantine HT, Cassidy WL, Flanagan NB, Marino R. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26(5):488–495. doi:10.3171/jns.1967.26.5.0488
29. Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatr. 1983;46(9):797. doi:10.1136/jnnp.46.9.797
30. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354(9189):1526. doi:10.1016/s0140-6736(99)02376-4
31. Vandewalle V, Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724. doi:10.1016/s0140-6736(98)05964-9
32. Lopes AC, Greenberg BD, Canteras MM, et al. Gamma ventral capsulotomy for obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):1066–1076. doi:10.1001/jamapsychiatry.2014.1193
33. Tyagi H, Apergis-Schoute AM, Akram H, et al. A randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive-compulsive disorder: clinical and imaging evidence for dissociable effects. Biol Psychiatr. 2019;85(9):726–734. doi:10.1016/j.biopsych.2019.01.017
34. Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatr. 2016;21(9):1272–1280. doi:10.1038/mp.2015.124
35. Robertson MM, Eapen V, Singer HS, et al. Gilles de la Tourette syndrome. Nature Reviews Disease Primers. 2017;3:16097. doi:10.1038/nrdp.2016.97
36. Kefalopoulou Z, Zrinzo L, Jahanshahi M, et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trial. Lancet Neurol. 2015;14(6):595–605. doi:10.1016/s1474-4422(15)00008-3
37. Baldermann JC, Kuhn J, Schüller T, et al. Thalamic deep brain stimulation for Tourette Syndrome: a naturalistic trial with brief randomized, double-blinded sham-controlled periods. Brain Stimul. 2021;14(5):1059–1067. doi:10.1016/j.brs.2021.07.003
38. Welter ML, Mallet L, Houeto JL, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952–957. doi:10.1001/archneur.65.7.952
39. Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. Efficacy and safety of deep brain stimulation in tourette syndrome. JAMA Neurol. 2018;75(3):353. doi:10.1001/jamaneurol.2017.4317
40. Moosa A, Limousin P Deep brain stimulation in Tourette syndrome. Available from: https://www.isrctn.com/ISRCTN17008820.
41. Richieri R, Blackman G, Musil R, et al. Positive clinical effects of gamma knife capsulotomy in a patient with deep brain stimulation-refractory tourette syndrome and obsessive compulsive disorder. Clin Neurol Neurosurg. 2018;170:34–37. doi:10.1016/j.clineuro.2018.04.018
42. Sun B, Krahl SE, Zhan S, Shen J. Improved capsulotomy for refractory tourette’s syndrome. Stereot Funct Neuros. 2005;83(2–3):55–56. doi:10.1159/000086673
43. Veale D, Roberts A. Obsessive-compulsive disorder. BMJ. 2014;348:g2183.
44. Boschen MJ, Drummond LM. Community treatment of severe, refractory obsessive-compulsive disorder. Behav Res Ther. 2012;50(3):203–209. doi:10.1016/j.brat.2012.01.002
45. Whitty CWM, Duffield JE, Tov PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;1(6706):475–481.
46. Jung HH, Kim SJ, Roh D, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study. Mol Psychiatr. 2014;20(10):1205–1211. doi:10.1038/mp.2014.154
47. Davidson B, Hamani C, Rabin JS, et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two Phase I trials. Mol Psychiatry. 2020;25(9):1946–1957. doi:10.1038/s41380-020-0737-1
48. Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM, Sheth SA. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. J Neurosurg. 2016;124(1):77–89. doi:10.3171/2015.1.jns14681
49. Welter ML, Santos JFAD, Clair AH, et al. Deep brain stimulation of the subthalamic, accumbens, or caudate nuclei for patients with severe obsessive-compulsive disorder: a randomized crossover controlled study. Biol Psychiatr. 2020;90(10):e45–e47. doi:10.1016/j.biopsych.2020.07.013
50. Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121–2134. doi:10.1056/nejmoa0708514
51. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatr. 2010;67(10):1061–1068. doi:10.1001/archgenpsychiatry.2010.122
52. Pepper J, Hariz M, Zrinzo L. Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: a review of the literature. J Neurosurg. 2015;122(5):1028–1037. doi:10.3171/2014.11.jns132618
53. Kumar KK, Appelboom G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg. 2019;90:4. doi:10.1136/jnnp-2018-319318
54. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/s0140-6736(18)32279-7
55. Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi:10.1007/s00429-008-0189-x
56. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696–707. doi:10.1016/j.euroneuro.2007.03.009
57. Nuttin B, Wu H, Mayberg H, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg. 2014;85(9):1003–1008. doi:10.1136/jnnp-2013-306580
58. Münchenberg P, Joyce E, Matthews K, Christmas D, Zrinzo L. Stereotactic radiofrequency ablation for treatment-refractory depression: a systematic review and meta-analysis. Brain Sci. 2022;12(10):1379. doi:10.3390/brainsci12101379
59. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839–849. doi:10.1016/s2215-0366(17)30371-1
60. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatr. 2015;78(4):240–248. doi:10.1016/j.biopsych.2014.11.023
61. Coenen VA, Bewernick BH, Kayser S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;26(9):1–9. doi:10.1038/s41386-019-0369-9
62. Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychol Med. 2014;44(16):3515–3522. doi:10.1017/s0033291714000956
63. Hitti FL, Widge AS, Riva-Posse P, et al. Future directions in psychiatric neurosurgery: proceedings of the 2022 American Society for Stereotactic and Functional Neurosurgery meeting on surgical neuromodulation for psychiatric disorders. Brain Stimul. 2023;16(3):867–878. doi:10.1016/j.brs.2023.05.011
64. Kim M, Kim CH, Jung HH, Kim SJ, Chang JW. Treatment of Major depressive disorder via magnetic resonance–guided focused ultrasound surgery. Biol Psychiatr. 2018;83(1):e17–e18. doi:10.1016/j.biopsych.2017.05.008
65. Chang J, Jung HH, Kim SJ, et al. Bilateral thermal capsulotomy with magnetic resonance‐guided focused ultrasound for patients with treatment‐resistant depression: a proof‐of‐concept study. Bipolar Disord. 2020. doi:10.1111/bdi.12964
66. Royal College of Psychiatrists Committee on ECT and Related Treatments: statement on Neurosurgery for Mental Disorder (NMD), also known as Psychiatric Neurosurgery; 2017. Available from: https://www.rcpsych.ac.uk/docs/default-source/about-us/who-we-are/ectcommittee-vns-dbs-ablative-neurosurgery-statement-feb17.pdf?sfvrsn=eba0287a_2.
67. Mental Health Act 1983, Section 57. Available from: https://www.legislation.gov.uk/ukpga/1983/20/section/57.
68. Zeman A. Neurology is psychiatry--and vice versa. Pract Neurol. 2014;14(3):136–144. doi:10.1136/practneurol-2013-000761
69. Ford PJ, Kubu CS. Stimulating debate: ethics in a multidisciplinary functional neurosurgery committee. J Med Ethics. 2006;32(2):106–109. doi:10.1136/jme.200x.013151
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.