Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Serum Micro-RNA-122 Level as a Simple Noninvasive Marker of MAFLD Severity
Authors Hegazy MA, Abd ALgwad I, Abuel Fadl S, Sayed Hassan M, Ahmed Rashed L, Hussein MA
Received 9 December 2020
Accepted for publication 19 March 2021
Published 19 May 2021 Volume 2021:14 Pages 2247—2254
DOI https://doi.org/10.2147/DMSO.S291595
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Mona A Hegazy,1 Ibrahim Abd ALgwad,1 Soheir Abuel Fadl,1 Mohamed Sayed Hassan,1 Laila Ahmed Rashed,2 Maha A Hussein1
1Internal Medicine Department, Kasr Al-Aini Hospitals, Cairo University, Cairo, Egypt; 2Biochemistry Department, Kasr Al-Aini Hospitals, Cairo University, Cairo, Egypt
Correspondence: Mona A Hegazy
Internal Medicine Department, Kasr Al-Aini Hospitals, Cairo University, Cairo, Egypt
Email [email protected]
Background: Metabolic-associated fatty liver disease (MAFLD) is a common disease worldwide. Micro-RNA-122 is known to be the most abundant micro-RNA expressed in the liver.
Objective: To evaluate the association of micro-RNA-122 and the degree of steatosis and fibrosis in obese patients with MAFLD.
Methods: The study included 120 obese Egyptian patients with MAFLD, which were diagnosed and classified according to ultra-sonographic liver findings. All patients enrolled in the study were subjected to thorough clinical examination and laboratory investigations (serum micro-RNA-122 levels by PCR, lipid profile, liver biochemistry, and functions). Fibro-scan was used to assess the level of fibrosis.
Results: There was a significant increase in levels of micro-RNA-122 in obese patients with MAFLD compared to controls (p< 0.001). Micro-RNA-122 level was lower in patients with mild liver steatosis than patients with moderate or severe steatosis (p< 0.001). It was lower in patients with a mild degree of fibrosis than those with mild or moderate fibrosis (p< 0.001). Micro-RNA-122 was significantly positively correlated with low-density cholesterol and triglycerides level, and liver enzymes, and negatively correlated to high-density cholesterol (p< 0.001).
Conclusion: Serum micro-RNA-122 could be a useful predictor of assessing MAFLD severity regarding level of steatosis or fibrosis.
Keywords: MAFLD, micro RNA, steatosis and fibrosis
Introduction
MAFLD is considered a new nomenclature for metabolic-associated fatty liver disease as an alternative name of NAFLD.1–4 Nonalcoholic fatty liver disease (NAFLD) includes a wide range of conditions from simple steatosis to (NASH) nonalcoholic steatohepatitis, which can lead to fibrosis, cirrhosis, and even hepatocellular carcinoma. These diseases have a high morbidity and mortality rate.5,6 Fibrosis is the major determinant of hepatic and extrahepatic complications of MAFLD and MAFLD identifies fibrosis better than NAFLD.7,8 So it is important to diagnose MAFLD with its different stages.
Obesity is considered as a major problem in the whole world. In Egypt, its prevalence reaches about 35.3% in 2015.9 Obesity is considered one of the major risk factors for MAFLD. There is a strong association of MAFLD with the metabolic syndrome.10,11 Micro-RNAs are small, endogenous, noncoding RNAs of about 21–22 nucleotides with essential genes that regulate functions in living organisms as normal metabolism. They have an important role in metabolic diseases.11 Micro-RNA-122 is found in the liver, it regulates many metabolic processes, as oxidation, synthesis fatty acid, and cholesterol biosynthesis.13–15
Several studies detect extracellular micro-RNAs in body fluids, serum, and plasma. They were considered to be good biomarkers to predict liver diseases and different kinds of cancers.16
Objectives
To assess the level of serum micro-RNA-122 in obese patients and its relation to the degree of steatosis in patients with MAFLD diagnosed by ultrasonography and degree of fibrosis detected by fibro-scan.
Study Design and Participants
A prospective cross-sectional case control study which was conducted at Kasr El Ainy hospital from January 2019 to September 2019. It included 120 Egyptian obese patients with metabolic fatty liver disease (MAFLD). They were divided according to ultra-sonographic findings into three groups with different degrees of steatosis (40 mild, 40 moderate, and 40 severe steatosis). Liver elasticity “fibrosis” was measured by fibro-scan. Sixty obese subjects who were age- and sex-matched with normal liver texture by ultrasonography were enrolled in the study as control.
Data Collection
Informed consent was obtained from all participants before inclusion. The study satisfied the requirements of Revised Helsinki Declaration of biomedical ethics, approved by Research Ethics Committee, Faculty of Medicine, Cairo University.
Clinical data was collected including patient demographics and anthropometric measurements (age, gender, and body mass index “BMI”). Lipid profile (HDL-cholesterol, LDL-cholesterol and triglycerides), liver enzymes (alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase serum albumin, prothrombin time, prothrombin concentration, gamma-glutamyl transpeptidase (GGT), and bilirubin were measured. Virology screen for hepatitis B and C (HCVAb and HBVsAg) were done. Serum micro-RNA-122 levels were estimated by real-time polymerase chain reaction (PCR) using the TaqMan®MicroRNA Assays.17 The 2−∆∆ct method was used to calculate relative changes in micro-RNA-122 expression determined from real-time quantitative PCR.
We excluded all patients who consume more than 20 g of alcohol, patients with diabetes, positive hepatitis C virus antibody results or hepatitis B virus surface antigen, autoimmune, cholestatic or drug-induced liver disease.
Assessment of Hepatic Steatosis by US
We performed ultrasound for all the participants of the study using the same machine operator (MH) utilizing a high-resolution multifrequency B-mode scanner (SDD-5500; Aloka, Tokyo, Japan) 2.5–5.0 MHz transducer. According to the ultrasound results we classified the degree of MAFLD into mild, moderate, and severe. Mild when there was a diffuse hyperechoic echo texture, moderate when this echogenicity of the liver increased compared to the kidneys but still associated with good visual images of the intrahepatic vessels, and severe when there was vascular blurring and deep attenuation (increase in the echogenicity of the liver which leads to poor penetration so that the visual images of both the posterior segment of the liver and the hepatic vessels were very poor).18 We used this classification of MAFLD instead of liver biopsy as liver ultrasonography correlates well with the MAFLD activity score of liver biopsy. Therefore, stage 1 steatosis by liver biopsy correlates with mild steatosis by US, while stage 3 steatosis by liver biopsy correlates with severe steatosis by US.
The damage to the liver was evaluated according to the NAFLD activity score (NAS). NAS is the sum of steatosis degree (scale from 0–3); scale from 0–2 is hepatocellular ballooning and scale from 0–3 is lobular inflammation. Therefore, if NAS <3 is not NASH, borderline NASH ranges from 3–4, while definite NASH ≥5. As US conforms with stage 3 steatosis by liver biopsy, if NAS is ≥3, and with either the presence or absence of inflammation and ballooning, the patient might be 3 only (borderline NASH) or above 3, reaching 4–6 (NASH).19
Fibroscan Examination
This was done by an expert radiologist blind to the liver ultrasonographic results with Echosen's Fibroscan 502 (Paris, France). The patient was lying in the supine position with his right arm abducted and the forearm under the head. The right side of the body was bare to examine the liver and was slightly elevated. The probe, covered with gel, was placed on the intercostal space over the right liver lobe. The operator selected a part of the liver of about at least 6-cm thick without any vessels, using A-mode images of the fibroscan device. The depth of the measurement ranged from 25–45 mm.
Ten validated measurements were performed in each patient. The success rate was calculated by dividing the number of validated measurements by the total number of measurements. Procedures with 10 validated measurements and a success rate of at least 60% were considered reliable. The values of hepatic fibrosis using fibroscan measurement were expressed in kilopascal (kPa). To determine the amount of hepatic steatosis, the controlled attenuation parameter (CAP) test was used, and the results were reported in decibel/meter (dB/m). CAP cut-off values indicated liver steatosis and ranged from S0, indicating no steatosis (S), to S3, indicating severe steatosis. CAP values indicating S ≥1 ranged from 237.0–259.0 dB/m, from 259.0–291.0 dB/m for S ≥2, and from 291.0–400.0 dB/m for S ≥3.
Cut-off values for fibrosis diagnosis were <5.5 for fibrosis stage F0 (no fibrosis), 5.5–8.0 for F1 (mild fibrosis), 8.0–10.0 for F2 (moderate fibrosis), 11.0–16.0 for F3 (severe fibrosis), and >16.0 for F4 (cirrhosis). F grading system is the output of fibroscan equipment by itself quantitatively by 0–4.
Statistical Analysis
We coded and entered the data using the SPSS (Statistical Package for the Social Sciences) version 25. Data was summarized using mean, standard deviation, median, minimum and maximum, or median and interquartile range in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Kruskal–Wallis and Mann–Whitney tests. For comparing categorical data, Chi square (χ2) test was done. Correlations between variables were done utilizing Spearman correlation coefficient. We considered p-values less than 0.05 as statistically significant.
Results
The summaries of demographic and clinical assessments data of 120 obese Egyptian patients are in Tables 1 and 2. The study was conducted at Kasr El Ainy hospital on 120 obese Egyptian patients who came to the hospital for routine checkup and were discovered to have MAFLD. They were divided according to ultra-sonographic findings into three groups with different degrees of steatosis (40 mild, 40 moderate, and 40 with severe steatosis).
 |
Table 1 Comparison between the Three Groups regarding the Degree of Steatosis and the Control Group |
 |
Table 2 Comparison between the Three Groups regarding the Degree of Fibrosis the Control Group |
Micro-RNA-122 and Degree of Steatosis
There were significant differences between patients with MAFLD and a control group regarding BMI, lipid profile (LDL, HDL, triglycerides) AST, ALT, alkaline phosphatase, GGT, bilirubin, and albumin. Serum micro-RNA-122 levels were lower in MAFLD patients with mild steatosis than those with moderate and severe steatosis with a p-value ˂0.001 (Table 1 and Figure 1).
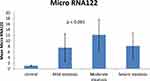 |
Figure 1 Level of micro-RNA-122 in mild, moderate, and severe degrees of steatosis and the control group. |
Micro-RNA-122 and Degree of Fibrosis
By fibroscan serum levels of micro-RNA-122 were significantly lower in patients with a severe degree of fibrosis than in patients with moderate and mild degrees of fibrosis with p-values ˂0.001 (Table 2 and Figure 2).
 |
Figure 2 Level of micro-RNA-122 in mild, moderate, and severe degrees of fibrosis and the control group. |
Correlation between Level of Micro-RNA-122 and All Other Variables in MAFLD Patients
Serum micro-RNA-122 was significantly positively correlated with BMI, LDL (Figures 3 and 4), TG, ALT, AST, and GGT with a p-value <0.001. On the contrary, it was significantly negatively correlated with HDL with a p-value <0.001.
 |
Figure 3 Correlation of micro-RNA-122 and LDL in different grades of steatosis and control. |
 |
Figure 4 Correlation of micro-RNA-122 and LDL in different grades of fibrosis and control. |
Combination Analysis of Level Micro-RNA-122 Regarding Steatosis and Fibrosis Degree
We identify the patients with moderate steatosis (score of 2) and fibrosis ≤F2 (score of 2), that is a total of 4, and compare them with scores less than 4. There was a difference in the mean level of micro-RNA-122 in those with scores less than 4 (mean=9.64) and those with a score of 4 (mean=8.91) but with no statistical significance as p-value was 0.466.
Discussion
MAFLD is highly prevalent in developed countries and is considered to be the most common cause of chronic liver disease.20 Worldwide over the last years the incidence of MAFLD has markedly increased. In developed countries, the incidence can be as high as 60% to over 90% among obese subjects.10 It is known that gut and adipose tissue produce circulating pro-inflammatory cytokines, adipokines, and endotoxins, which lead to free fatty acids accumulation and insulin resistance, which may induce hepatocellular damage by oxidative stress.21 Also, the disturbance in the metabolism of lipids can have an important role in the pathogenesis of NASH.22
Liver biopsy is used for staging of NAFLD patients, however it has many drawbacks, such as patient discomfort, sampling error, the difference in histopathologic results, and the high cost,23 so we need another alternative for the evaluation of NAFLD.24
Fibroscan can be used to evaluate liver elasticity in relation to fibrosis by an ultrasound-based vibration-controlled transient elastography device. It has satisfactory results for detection of fibrosis and diagnosis of MAFLD.25 Several studies evaluated the use of fibroscan in patients with MAFLD.26 There was a good correlation between the liver stiffness and the degree of hepatic fibrosis in multiple liver diseases, including NAFLD.27
In this study there were significant increases in BMI, ALT, AST, GGT, LDL-cholesterol, and TG in MAFLD patients compared to control, with a p-value ˂0.001, and significant decrease in HDL-cholesterol in MAFLD patients compared to control, with p-value ˂0.001. These results are in agreement with previous studies of Hu et al, Huang et al, and Pirola et al.28–30
We found that serum levels of micro-RNA-122 were significantly higher in MAFLD patients than age- and sex-matched overweight participants with normal liver, and these results go hand-in-hand with studies of Cermelli et al and Panera et al.31,32
A significant positive correlation was found between serum micro-RNA-122 levels and ALT, AST, and GGT) with p-value ˂0.001. This correlation was in agreement with previous studies of Pirola et al,30 and Auguet et al,33 who found a significant strong correlation between micro-RNA-122 and liver enzymes (AST and ALT) with a p-value ˂0.001. This association might be explained by the fact that micro-RNA-122 and liver enzymes are released in the setting of active liver injury. Pirola et al30 suggested that micro-RNA-122 increases ALT level by activating the translation at multiple sites of the coding gene. On the other hand the study of Miyaaki et al34 revealed that there were no significant correlations observed between liver enzymes (ALT and AST) and the expression levels of serum micro-RNA-122. Serum micro-RNA-122 was significant positively correlated with BMI, LDL, TG, and negatively correlated with HDL, this expresses the role of micro-RNA-122 which is the most abundant microRNA in the liver in controlling many metabolic pathways, including fatty acid synthesis and oxidation and cholesterol biosynthesis.35 Previous studies had correlated the level of serum micro-RNA-122 and degree of steatosis in NAFLD patients, and concluded that its level was lower in patients with mild steatosis than those with more severe steatosis diagnosed according to liver ultrasound grading.31,32 We found that serum micro-RNA-122 levels were significantly lower among MAFLD patients with a mild degree of steatosis (Mean±SD=7.52±5.07) compared to those with moderate (12.13±5.53) and severe degrees of steatosis (8.17±4.55) with a p-value ˂0.05. Serum micro-RNA-122 levels were inversely correlated with fibrosis degree, being lower in patients with a severe degree of fibrosis than those with moderate and mild degrees of fibrosis. This is in agreement with studies done in 2015 which documented that serum levels of micro-RNA-122 were higher in patients with mild fibrosis compared to those with severe fibrosis, and confirmed the under-expression of micro-RNA-122 in advanced fibrosis.36 According to Pirola et al's study in 2015, liver micro-RNA-122 expression was 10-fold (p<0.03) downregulated in NASH patients with variable degrees of fibrosis compared with simple steatosis. 30 The cause for this variation in micro-RNA-122 levels in different stages of MAFLD may represent the progressive loss of hepatocytes in worsening liver injury. Hepatocytes are the main source of micro-RNA-122, progression of liver fibrosis will lead to replacement of hepatocytes with extracellular matrix, therefore hepatic micro-RNA-122 levels may be decreased with a severe degree of fibrosis.31 These results indicate that micro-RNA-122 serum levels may be used as a good biomarker to predict liver fibrosis in MAFLD patients.
The controversy in our study that the serum level of micro-RNA-122 was higher in patients with moderate steatosis than those with mild and severe steatosis could be explained by the more sub-classification of liver steatosis into three categories (mild, moderate, and severe) that we followed in the present study, while most of the previous studies categorized patients according to the degree of steatosis into two groups (patients with mild (˂33%) and severe (˃33%) degrees of steatosis).
As fibrosis increased, the level of micro-RNA-122 decreased to reach the lowest level in F3 and F3–F4 stage and liver cirrhosis. In this study none of the MAFLD patients with mild steatosis or moderate steatosis had advanced fibrosis or liver cirrhosis (F3 and F4), 85% of patients with mild steatosis and 70% of patients with moderate steatosis had F0–F1. In severe steatosis, 5% of patients had F0–F1, 50% of patients were F2 or F2–F3 and 45% of patients were F3 or F3–F4. Therefore, in patients with moderate steatosis there were high fat content but no advanced fibrosis, so the level of micro-RNA-122 was higher compared to patients with severe steatosis who had higher fat content but more advanced fibrosis.
As micro-RNA-122 was positively correlated with BMI and serum lipid profile; it might interact with environmental and lifestyle factors, or act independently to affect the susceptibility to fat accumulation in the liver. The link between micro-RNA-122 expression level and development or progression of fatty liver disease needs further studies to develop therapies to prevent and control NASH.
In conclusion, a strong association was found between serum micro-RNA-122 and MAFLD progression diagnosed by ultrasound examination in the presence of obesity. The serum micro-RNA-122 level can be a useful prognostic marker not only for predicting the amount of fat accumulating in liver cells (degree of steatosis), but also following up of its level can early predict progression to liver fibrosis.The contribution of micro-RNA-122 to the pathogenesis of MAFLD and its role for treatment or even prevention of MAFLD needs further studies with large numbers of patients and liver biopsies, which were considered limitations of this study.
Acknowledgements
We would like to acknowledge our great Kasr Al Ainy Hospital, and its workers, nurses, and staff members, for all the support and help in this study and throughout our careers.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
2. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
3. Mendez-Sanchez N, Arrese M, Gadano A, et al. The latin American Association for the Study of the liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6(1):65–72. doi:10.1016/S2468-1253(20)30340-X
4. Wai-Sun WV, Lai-Hung WG, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clinic Gastroenterol Hepatol. 2020.
5. Demir M, Lang S, Steffen HM. Nonalcoholic fatty liver disease: current status and future directions. J Dig Dis. 2015;16(10):541–557. doi:10.1111/1751-2980.12291
6. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221–235. doi:10.1055/s-0035-1562943
7. Eduardo VG, Luis CB, Vincent WW, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443–457.e17. doi:10.1053/j.gastro.2018.04.034
8. Su L, Jiaofeng H, Mingfang W, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi:10.1111/liv.14548
9. Afshin A, Forouzanfar MH, Reitsma MB, et al.; The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi:10.1056/NEJMoa1614362.
10. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi:10.1002/hep.28431
11. Kim HJ, Kim HJ, Lee KE, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169. doi:10.1001/archinte.164.19.2169
12. Yang YM, Seo SY, Kim TH, et al. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid. Hepatology. 2012;56:2209–2220. doi:10.1002/hep.25912
13. Ali B, Henning G, Jacob G, et al. The epigenetic drug discovery landscape for metabolic associated fatty liver disease. Trends Genet. 2020;36(6):429–441. doi:10.1016/j.tig.2020.03.003
14. Mohamed E, Luca V, Stefano R. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68(2):268–279. doi:10.1016/j.jhep.2017.09.003
15. Schrauder MG, Strick R, Schulz-Wendtland R, et al. Circulating micro-RNAs as potential blood-based markers for early stage breast cancer detection. PLoS One. 2012;7:e29770. doi:10.1371/journal.pone.0029770
16. Chen C, Ridzon D, Broomer A, et al. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res. 2005;33(20):e179. doi:10.1093/nar/gni178
17. Rumack CM. Diagnostic ultrasound. In: Rumack CM, editor. General Adult Ultrasound. St Louis: Mosby; 1998:110–112.
18. Hegazy M, Mostafa A. Liver ultrasound scanning in the detection of hepatic steatosis and fibrosis in NASH patients. Egypt J Intern Med. 2012;24:27–31.
19. Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost two fold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–944. doi:10.1111/jgh.13264
20. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–1048. doi:10.1016/j.metabol.2015.12.012
21. Aguilar C, Sirvent J, Guiu-Jurado E. Altered fatty acid metabolism-related gene expression in liver from morbidly obese women with non-alcoholic fatty liver disease. Int J Mol Sci. 2014;15:22173–22187. doi:10.3390/ijms151222173
22. Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–485. doi:10.3748/wjg.v20.i2.475
23. Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375(9724):1419–1420. doi:10.1016/S0140-6736(09)62195-4
24. Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871. doi:10.1038/ajg.2012.331
25. Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199–208. doi:10.1002/hep.24624
26. Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi:10.1136/gut.2005.069153
27. Hu J, Xu Y, Hao J, et al. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364–371. doi:10.1007/s13238-012-2036-3
28. Huang J, Iqbal J, Saha PK, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147. doi:10.1002/hep.21632
29. Pirola CJ, Fernandez Gianotti T, Castano GO, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64(5):800–812. doi:10.1136/gutjnl-2014-306996
30. Cermelli S, Ruggieri A, Marrero JA, et al. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6(8):e23937. doi:10.1371/journal.pone.0023937
31. Panera N, Gnani D, Crudele A, et al. MicroRNAs as controlled systems and controllers in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(41):15079–15086. doi:10.3748/wjg.v20.i41.15079
32. Auguet T, Aragonès G, Berlanga A, et al. miR33a/miR33b* and miR122 as possible contributors to hepatic lipid metabolism in obese women with nonalcoholic fatty liver disease. Int J Mol Sci. 2016;17:1620. doi:10.3390/ijms17101620
33. Miyaaki H, Ichikawa T, Kamo Y, et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014;34:e302–e307. doi:10.1111/liv.12429
34. Salvoza NC, Klinzing DC, GopezCervantes J, et al. Association of circulating serum miR-34a and miR-122 with dyslipidemia among patients with non-alcoholic fatty liver disease. PLoS One. 2016;11(4):e0153497. doi:10.1371/journal.pone.0153497
35. Gerhard G, DiStefano J. Micro RNAs in the development of non-alcoholic fatty liver disease. World J Hepatol. 2015;7(2):226–234. doi:10.4254/wjh.v7.i2.226
36. Halasz T, Horvath G, Par G, et al. miR-122 negatively correlates with liver fibrosis as detected by histology and fibroscan. World J Gastroenterol. 2015;21:7814–7823. doi:10.3748/wjg.v21.i25.7814
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
