Back to Journals » Journal of Inflammation Research » Volume 17
Serum Homer1 is a Novel Biomarker for Predicting the Clinical Outcomes of Acute Ischemic Stroke Patients
Authors Lv W, Ruan Z, Zhang Q, Wei Y, Wu X, Dou YN, Chao W, Fei X, Fei Z
Received 3 December 2023
Accepted for publication 20 February 2024
Published 27 February 2024 Volume 2024:17 Pages 1337—1347
DOI https://doi.org/10.2147/JIR.S453018
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Weihao Lv,1,* Zhe Ruan,2,* Qianqian Zhang,3,* Yaxuan Wei,4,* Xiuquan Wu,1 Ya-Nan Dou,1 Wangshu Chao,1 Xiaowei Fei,1 Zhou Fei1
1Department of Neurosurgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, 710032, People’s Republic of China; 2Department of Neurology, Tangdu Hospital, Air Force Medical University, Xi’an, Shaanxi, 710032, People’s Republic of China; 3Department of Respiratory Medicine, Lanzhou University Second Hospital, Lanzhou, 730070, People’s Republic of China; 4Department of Neurology, Gansu Province Central Hospital, Lanzhou, 730070, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaowei Fei; Zhou Fei, Xijing Hospital, Air Force Medical University, No. 127, Changle West Road, Xincheng District, Shaanxi, 710032, People’s Republic of China, Tel +86-1882928538, Email [email protected]; [email protected]
Purpose: We aim to explore the relationship between Homer1 and the outcomes of AIS patients at 3 months.
Patients and Methods: This prospective cohort study was conducted from May 2022 to March 2023. In this study, we investigated the association between serum Homer1 levels by enzyme-linked immunosorbent assay at admission and functional outcomes of patients at 3 months after AIS.
Results: Overall, 89 AIS patients (48 good outcomes and 41 poor outcomes) and 83 healthy controls were included. The median serum Homer1 level of patients at admission with poor outcomes was significantly higher than that of patients with good outcomes (39.33 vs 33.15, P< 0.001). Serum Homer1 levels at admission were positively correlated with the severity of AIS (r = 0.488, P< 0.001). The optimal cutoff of serum Homer1 level as an indicator for an auxiliary diagnosis of 3 months functional outcomes was 35.07 pg/mL, with a sensitivity of 75.0% and a specificity of 92.7% (AUC 0.837; 95% CI [0.744– 0.907]; P< 0 0.001). The odds ratio of MRS > 2 predicted by the level of serum Homer1 after 3 months was 1.665 (1.306– 2.122; P< 0.001).
Conclusion: Serum concentrations of Homer1 have a high predictive value for neurobehavioral outcomes after acute ischemic stroke. Higher serum Homer1 levels (> 35.07 pg/mL) were positively associated with poor functional outcomes of patients 3 months post-stroke.
Keywords: Homer1, acute ischemic stroke, biomarker, outcomes
A Letter to the Editor has been published for this article.
Introduction
Acute ischemic stroke (AIS), with a high incidence rate, mortality, and disability rate, accounts for about 82% of all strokes1 and causes great harm to people’s health, families, and society.2 It is reported that the estimated prevalence, incidence, and mortality rate of stroke in China in 2020 were 2.6%, 505.2 per 100,000 person-years, and 343.4 per 100,000 person-years, respectively.3 Moreover, with the aging of the population and the increasing prevalence of risk factors such as diabetes, hypertension, and hyperlipidemia, China’s stroke burden is also increasing, which suggests that the strategy of stroke diagnosis and treatment needs to be further improved and strengthened, especially in China.4 Predicting the patients’ clinical outcomes accurately is still an unresolved issue, which is most concerning to patients and their families.
The National Institutes of Health Stroke Scale (NIHSS) score, a clinical assessment scale for stroke, helps in grading the severity of AIS and predicts the outcome for the patients.5,6 However, NIHSS has a low predictive value in some ischemic stroke patients with characteristics of arteriolar occlusion or perforating artery embolism.7,8 In addition, the NIHSS scale does not include neurological deficit symptoms of posterior circulation stroke, such as headache, diplopia, Horner’s syndrome, and gait abnormalities.9–11 Therefore, it is not rational to rely only on the NIHSS score at admission to assess the outcomes of patients. Additionally, neuroimaging can effectively determine the location and area size of cerebral infarction on admission, but some AIS patients have contraindications to magnetic resonance imaging (MRI). So, it is impossible to use MRI to evaluate the outcomes of all the patients. Blood-based biomarkers might provide additional information to prognostic factors readily available in clinical practice.
Homer1 protein, as a postsynaptic dense substance protein, is one of the subtypes of Homer family protein. There is the highest expression level of Homer1 in brain tissue, while other peripheral tissues such as the heart and skeletal muscles are relatively low.12,13 In animal experiments, numerous studies have found that Homer1 can exert multiple regulatory effects in different animal disease models. It was found that Homer1 was involved in the regulation of calcium signaling homeostasis.14 Homer1 also inhibits the phenotypic conversion of astrocytes, exerts anti-inflammatory effects, and alleviates damage in mice with intracerebral hemorrhage.15 Homer1 can improve the degree of brain injury in mice by regulating mitochondrial endoplasmic reticulum stress in ischemic brain injury.16 In addition, there is relatively little clinical research on Homer1. It has been reported that Homer1 plays a crucial role in the development of hepatocellular carcinoma (HCC) tumors and may be a potential diagnostic marker for HCC.17 Another study suggests that Homer1 has certain diagnostic significance in coronary heart disease.18 However, the relationship between Homer1 and the outcomes of AIS has not been studied. Based on previous work and the structural basis of the disrupted blood–brain barrier (BBB)19 following AIS, we speculate that Homer1 could be released into the cerebrovascular fluid and to a smaller extent in the peripheral blood serum after the attack in AIS, and Homer1 levels may be associated with patient’s outcomes. In this study, we aimed to study the relationship between the level of Homer1 in the peripheral serum of AIS patients at admission and the outcomes of the patients 3 months post-stroke.
Patients and Methods
Ethical Approval
Research involving human subjects complied with all relevant national regulations, and institutional policies and is by the tenets of the Helsinki Declaration, and has been approved by the Institutional Ethics Review Committee of Xijing Hospital (KY20232052-C-1). Informed consent was obtained from all individuals included in this study.
Study Population
A total of 144 patients diagnosed with AIS were prospectively enrolled in our study from the Brain Hospital of Xijing Hospital of Air Force Medical University from May 2022 to March 2023. Finally, after strict screening and follow-up by staff, complete data were available for only 89 patients. The inclusion criteria are as follows: (1) Acute onset, focal neurological deficits lasting >24 hours, admission <72 hours; (2) Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) results confirmed AIS. The exclusion criteria are as follows: (1) malignant tumors; (2) severe respiratory, hematological, or digestive system diseases; (3) pre-morbid modified Ranking Scale (mRS)>2; (4) Other diseases that may affect serum Homer1 levels, such as rheumatic immune disease, systemic, and local inflammation, severe heart disease, acute myocardial infarction, liver cirrhosis, severe kidney disease, and skeletal muscle lysis.
Collection of Data for Enrolled Clinical Patients
The definition of acute ischemic stroke complied with the World Health Organization criteria.20 Clinicians with strict training further classified the subtypes of AIS patients according to the definition used by Org 10,172 in the treatment of acute stroke (TOAST) trial.21 Meantime, the NIHSS score was used to score the patients’ neurological function defects. Demographic and clinical data were collected, including age, gender, NIHSS score, cholesterol, triglycerides, and homocysteine were also collected at admission. In this study, we strictly followed the inclusion and exclusion criteria and conducted strict follow-up. The NIHSS scores were made by staff who are strictly trained doctors blinded to the later laboratory measurements and data analysis process.
Outcomes Assessments
The main outcome measure was the MRS 3 months post-stroke, followed up by telephone. Staff attending follow-up were blinded to laboratory test results. This is a simplified overall functional assessment where 0 indicates no symptoms and 5 indicates severe disability.22 A score of 0 indicates no symptoms; 1 point represents mild symptoms but does not affect daily life; A score of 2 indicates mild disability but the ability to independently complete daily activities; A score of 3 indicates that moderate disability requires assistance to complete daily activities; A score of 4 indicates that severe disabilities require round-The-clock attendants; A score of 5 represents death; The higher the score, the poorer the patient’s functional status. Patients with an MRS score of 0–2 were defined as the group with good functional outcomes, and those with an MRS score of 3–5 were defined as the group with poor functional outcomes.
Collection of Serum Samples
A 10 mL blood sample was drawn from each patient’s antecubital vein within the immediate hours after admission, collected into a serum separator tube, and next step leaving the tube undisturbed in a vertical position overnight at 4°C or room temperature for up to 60 minutes, and then centrifuged at approximately 1000 g for 20 min. The serum was then transferred into plain polypropylene tubes and stored at −80°C pending for further processing.
ELISA Procedure
ELISA was performed in strict accordance with the manufacturer’s instructions. Briefly, we firstly performed gradient dilutions of protein standards, followed by further measurement of the absorbance of standards at each dilution gradient at an absorption wavelength of 450 nm, and establishment of a standard curve. Second, the absorbance of each serum sample after incubation was further measured to obtain the concentration value of Homer1 in each serum sample, which was measured in triplicate. These measurements were made by technicians blinded to the clinical outcomes at an independent laboratory. Human Homer Homolog1 ELISA Kit (Catalogue No: abx387846) was purchased from Abbexa in the UK.
Statistical Analyses
Continuous variables are summarized as medians with interquartile range (IQR), and categorical variables as absolute counts and percentages. Statistical significance for intergroup differences was assessed by Pearson’s χ2 for categorical variables and the Mann–Whitney U-test for continuous variables. Variables associated in the univariate analysis were entered into a forward stepwise logistic regression model to identify variables independently associated with predictors of stroke outcomes [odds ratios (OR) with their 95% confidence interval (CI) are given]. To calculate the sensitivity and specificity for biomarker cutoff values to predict the neurological function recovery of patients 3 months post-stroke, a receiver operator characteristic (ROC) curve was configured. The goodness-of-fit was assessed using the Hosmer–Lemeshow test. All data were analyzed using GraphPad version 9.0 (SPSS, Chicago, IL, USA), R software. All tests were two-sided and P<0.05 were considered to be statistically significant.
Results
Baseline Characteristics
Totally, 144 patients with AIS underwent consecutive screening from May 2022 to March 2023, and 100 patients met the inclusion criteria at baseline. At the 3-month follow-up, 89 patients were eventually enrolled in the study (Figure 1). The baseline clinical characteristics of 89 adult AIS patients and 83 healthy controls are shown in Table 1. In the investigation of risk factors for stroke, 73.0% of patients had hypertension, 34.8% had diabetes mellitus, 23.6% had Atrial fibrillation, 33.7% had a history of smoking, 33.7% had a history of stroke, 39.3% were accompanied by hyperlipidemia. On admission, 44.9% of patients had a slight neurologic deficit (NIHSS score < 8); 47.2%, a moderate neurologic deficit (NIHSS score from 8 to 20); and 7.9%, a severe neurologic deficit (NIHSS score > 20). According to the TOAST classification, 49.4% had a large artery atherosclerosis; 14.6%, a cardioembolism; 28.1%, a small vessel occlusion or lacunae; and 7.9%, a cryptogenic stroke or other types of embolism. Among all AIS patients, it was found that anterior circulation infarction (79.8%) accounted for the vast majority. In addition, 83 healthy controls were recruited to form a healthy control group. No differences were observed in the baseline characteristics, such as age or gender, between the AIS patients and the healthy controls.
 |
Table 1 Clinical and Demographic Characteristics of the AIS Patients and Healthy Controla |
 |
Figure 1 Study profile. |
Univariate Analysis of Serum Homer1 and Other Variables with Outcomes
Among the 83 healthy individuals included in this study, the concentration range of serum Homer1 was 11.05–22.60 pg/mL. Serum Homer1 levels at admission in the AIS patients were significantly higher than that of healthy controls [34.60 (6.85) vs 14.87 (4.4) pg/mL, P<0.001, Figure 2A]. In addition, serum Homer1 levels at admission in the good outcomes was lower than poor outcomes in the AIS groups [33.15(2.69) vs 39.33 (7.77) pg/mL, P<0.001, Figure 2B). Moreover, the NIHSS score in the poor outcomes group was significantly higher than that in the good outcomes group [10.50 (8.75) vs 6(5), P<0.001, Table 2]. No differences were observed in factors, such as age, gender, and other factors related to acute ischemic stroke (Table 2). Also, serum Homer1 levels at admission were positively correlated with the NIHSS score (r = 0.488, P<0.0001; Figure 3).
 |
Table 2 Comparison of Factors for Predicting 3-Month Post-Stroke Modified Rankin Score in 89 Patients with Strokea |
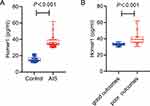 |
Figure 2 (A) Boxplots of Homer1 levels between AIS and control groups. (B) Boxplots of Homer1 levels for AIS patients with good and poor outcomes. Box=median + IQR; whisker=minimum to maximum. |
 |
Figure 3 Correlation between serum levels of Homer1 and the NIHSS score. |
In Figure 4, the area under the ROC curve of Homer1 is 0.837 (95% CI: 0.744–0.907, P<0.001). The area under the ROC curve of the NIHSS score is 0.759 (95% CI: 0.657–0.843, P<0.001). According to the ROC curve, the optimal cutoff serum Homer1 level as an indicator for an auxiliary diagnosis of 3 months functional outcomes was 35.07 pg/mL, with a sensitivity of 75.0% and a specificity of 92.7%. Similarly, according to the ROC curve of the NIHSS score, the optimal cutoff value as an auxiliary diagnostic index for 3 months functional outcomes is 8, the sensitivity is 66.67%, and the specificity is 73.17%. The area under the curve for Homer1 and NIHSS is 0.904 (95% CI: 0.843–0.966; P<0.001) for the combination of the two variables.
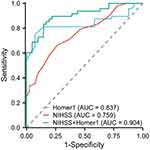 |
Figure 4 ROC for NIHSS, Homer1 and the combination of the latter for the prediction of 3 months AIS outcome. |
Logistic Regression Model for Predicting 3-Month Modified Rankin Score>2
To further identify independent predictors of neurological recovery post-stroke, we further performed multivariate logistic regression of two statistically significant factors in a univariate comparison. The results showed that NIHSS score (OR: 1.368, 95% CI: 1.130–1.655, P = 0.001) and serum Homer1 level (OR: 1.665, 95% CI: 1.306–2.122, P<0.001, Table 3) were significantly correlated with poor functional outcomes. As shown in Figure 5, to determine the prediction model we studied to calculate the prediction probability of each patient’s future outcomes events, the Hosmer and Lemeshow goodness-of-fit test was used to confirm that the calibration curve showed a good fit during internal validation (χ2 test = 3.626; P = 0.889).
 |
Table 3 Multivariate Logistic Regression Analysis of the Clinical Determinants of Functional Outcomes 3 Months Post-Stroke (n = 89)a |
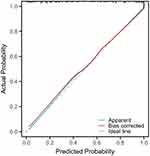 |
Figure 5 Calibration of prediction model. |
Discussion
In this prospective study, we firstly used a commercially available ELISA kit to detect serum Homer1 of AIS patients to evaluate the neurological outcomes of patients at 3 months post-stroke. It is indicated that serum Homer1 level is an independent predictor of outcomes in AIS patients, and a higher level of serum Homer1 at admission indicates poor outcomes. The optimal cutoff value of Homer1 for predicting outcomes is 35.07 pg/mL, with sensitivity and specificity of 75% and 92.7%, respectively.
In this study, the ROC showed that the diagnostic efficacy of Homer1 (AUC = 0.837) was higher than the diagnostic efficacy of NIHSS score (AUC = 0.759). At present, the NIHSS score has been widely applied in clinical practice and has played a significant role in the outcomes of patients. However, there are still certain limitations in evaluating stroke in the posterior circulation.23 Based on the statistical results of this clinical research, we speculated that the NIHSS score may not be sensitive enough to the symptom evaluation of some patients, possibly due to the high proportion of AIS patients with posterior circulation infarction (20.2%, Table 1) enrolled. At the same time, we further used the combined ROC curve to detect the diagnostic efficacy of the NIHSS score and serum Homer1, and the results (AUC = 0.904, Figure 4) showed higher diagnostic efficacy compared to the NIHSS score alone. Therefore, the combination of NIHSS score and Homer1 may predict the outcomes of AIS patients more accurately.
When acute ischemic stroke occurs, the core infarcted area and ischemic penumbra are formed in the ischemic hemisphere.24 With prolonged ischemia, the extent of the core infarct area gradually expands, accompanied by more severe neurological deficits.25 A large number of studies have shown that IL-10, IL-6, and TNFα can be used as biomarkers of brain injury when ischemic stroke occurs.26 However, compared with biomarkers such as IL-10, IL-6, and TNFα, serum Homer1 has higher tissue specificity and sensitivity, which is more accurate for the diagnosis and treatment of acute ischemic stroke. In animal experiments, the function of Homer1 has been extensively investigated. In different disease models, Homer1 plays a role by regulating the expression of various signaling pathway proteins.27 In clinical trials, Homer1 has been used as a biomarker in only a few cases, including hepatocellular carcinoma,17 as well as diagnostic studies of coronary heart disease.18 In addition, in this study, we found that the changes in Homer1 in the peripheral blood of patients with acute ischemic stroke, although there are other defects such as small sample sizes, provide a certain foundation for future research. Therefore, the clinical application of Homer1 still needs further exploration and discovery.
In this study, we strictly followed the treatment guidelines for suspected ischemic stroke patients. Immediately after admission, the patient underwent NIHSS scoring and emergency CT was used to rule out cerebral hemorrhage. In the second step, blood routine, coagulation function, electrolytes, and other general blood parameters will be urgently examined, and at the same time, we extracted blood samples from patients to detect serum Homer1. In the third step, if the patient meets the thrombolysis criteria, we would communicate with the patient and their family members to inform them of the risks, and then proceeded with IV-tPA treatment for the patient. If thrombolysis reperfusion failed, subsequent endovascular thrombectomy was performed. The time point for collecting Homer1 in this study was before the patient‘s treatment. Therefore, the serum Homer1 level at the time of patient admission is not significantly related to the patient’s treatment approach. Moreover, we did not collect peripheral serum from patients after treatment (regardless of successful reperfusion), so we are unable to determine the impact of reperfusion success and failure on serum Homer1 levels at this time. This is also a topic that needs further research and resolution in the next step of this study.
In summary, this simple test could help clinicians to assess the patient’s condition more accurately. Mechanistically, when the feeding artery is occluded or embolized, a series of pathological changes occur in the brain tissue. All nerve cells in the neurovascular unit undergo endoplasmic reticulum28 and mitochondrial stress29 due to ischemia and hypoxia, and ionic imbalance inside and outside the cell membrane leads to cytotoxic edema.30 At the same time, tight junctions between vascular endothelial cells are disrupted and vasogenic edema occurs.31 In addition, hypoxia leads to oxidative stress,29 and the release of inflammatory factors,32 resulting in further expansion of the injury. A series of pathological lesions eventually lead to neural cell disintegration and death, disruption of the BBB, and ultimately accompanied by increased Homer1 levels in peripheral blood.
The defect of our clinical research is that the sample size of patients included in this study is relatively small. Although our telephone follow-up strictly collects patient’s neurological recovery condition according to the MRS scoring criteria, there are still inevitable systematic deviations. Secondly, we did not consecutively collect peripheral blood from patients to detect temporal expression trends of serum Homer1. Also, neuroimaging data such as cerebral infarct size on DWI for AIS patients were not collected. Moreover, we did not count neurological recovery at 1 month, 6 months, and more long-term after stroke, so we could not describe the relationship between the outcomes of the patient’s condition and Homer1 at admission in detail. Finally, we used an enzyme-linked immunosorbent assay to detect the level of serum Homer1. Although we have tested the peripheral serum of each patient at least three times to obtain a relatively accurate concentration result, the ELISA test results are a semi-quantitative result of Homer1 calculated according to absorbance. Although the results of this study have great reference value for the use of serum Homer1 to predict AIS patients’ neurological recovery 3 months post-stroke, it is imperative to develop more precise tests.
Conclusion
This study reported a novel prognostic marker in AIS patients for the first time and proposed that there was a strong correlation between the level of serum Homer1 and the outcomes of AIS patients. The results showed that measuring the level of Homer1 in patients’ peripheral blood serum at the time of admission may be a potentially useful, relatively rapid, and minimally invasive method for predicting the recovery of neurological function in AIS patients 3 months post-stroke.
Ethical Approval
Research involving human subjects complied with all relevant national regulations, and institutional policies and is by the tenets of the Helsinki Declaration, and has been approved by the author’s Institutional Review Board (KY20232052-C-1).
Informed Consent
Informed consent was obtained from all individuals included in this study.
Acknowledgments
The authors thank the patients who participated in the study, the staff of the participating emergency departments, the research coordinators, and the laboratory technicians.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was funded by the National Natural Science Foundation of China (No. 81771239). The funding organization played no role in the design of the study, choice of enrolled patients, review and interpretation of data, preparation of the manuscript, or final approval of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mehra M, Vaduganathan M, Fu M, et al. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J. 2019;40(44):3593–3602. doi:10.1093/eurheartj/ehz427
2. Rodgers H, Bosomworth H, Krebs H, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394(10192):51–62. doi:10.1016/S0140-6736(19)31055-4
3. Tu WJ, Zhao Z, Yin P, et al. Estimated Burden of Stroke in China in 2020. JAMA Network Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
4. Tu WJ, Wang LD. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
5. Song S, Liang L, Fonarow G, et al. Comparison of Clinical Care and In-Hospital Outcomes of Asian American and White Patients With Acute Ischemic Stroke. JAMA neurol. 2019;76(4):430–439. doi:10.1001/jamaneurol.2018.4410
6. Brott T, Adams A, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989.
7. Braksick S, Wijdicks E. An NIHSS of 0 and a Very Disabling Stroke. Neurocritical Care. 2017;26(3):444–445. doi:10.1007/s12028-016-0339-6
8. Yaghi S, Willey J, Andrews H, Boehme A, Marshall R, Boden-Albala B. The Itemized NIHSS Scores Are Associated With Discharge Disposition in Patients With Minor Stroke. Neurohospitalist. 2016;6(3):102–106. doi:10.1177/1941874416641466
9. Martin-Schild S, Albright K, Tanksley J, et al. Zero on the NIHSS does not equal the absence of stroke. Ann Emergency Med. 2011;57(1):42–45. doi:10.1016/j.annemergmed.2010.06.564
10. Sato S, Toyoda K, Uehara T, et al. Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(24_part_2):2371–2377. doi:10.1212/01.wnl.0000304346.14354.0b
11. Libman R, Kwiatkowski T, Hansen M, Clarke W, Woolson R, Adams H. Differences between anterior and posterior circulation stroke in TOAST. Cerebrovascular Dis. 2001;11(4):311–316. doi:10.1159/000047659
12. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
13. Sjöstedt E, Zhong W, Fagerberg L, et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020;367(6482). doi:10.1126/science.aay5947
14. Yuan J, Kiselyov K, Shin D, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114(6):777–789. doi:10.1016/S0092-8674(03)00716-5
15. Fei X, Dou YN, Wang L, et al. Homer1 promotes the conversion of A1 astrocytes to A2 astrocytes and improves the recovery of transgenic mice after intracerebral hemorrhage. J Neuroinflammation. 2022;19(1):67. doi:10.1186/s12974-022-02428-8
16. Wei J, Wu X, Luo P, et al. Homer1a Attenuates Endoplasmic Reticulum Stress-Induced Mitochondrial Stress After Ischemic Reperfusion Injury by Inhibiting the PERK Pathway. Front Cell Neurosci. 2019;13:101. doi:10.3389/fncel.2019.00101
17. Luo P, Feng X, Jing W, et al. Clinical and Diagnostic Significance of Homer1 in hepatitis B virus-induced Hepatocellular Carcinoma. J Cancer. 2018;9(4):683–689. doi:10.7150/jca.22279
18. Jing X, Chen S, Jing W, Tan Q, Yu M, Tu J. Diagnostic potential of differentially expressed Homer1, IL-1β, and TNF-α in coronary artery disease. Int J Mol Sci. 2014;16(1):535–546. doi:10.3390/ijms16010535
19. Ren X, Hu H, Farooqi I, Simpkins J. Blood substitution therapy rescues the brain of mice from ischemic damage. Nat Commun. 2020;11(1):4078. doi:10.1038/s41467-020-17930-x
20. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin World Health Org. 1976;54(5):541–553.
21. Adams H, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.STR.24.1.35
22. Bamford J, Sandercock P, Warlow C, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20(6):828. doi:10.1161/01.STR.20.6.828
23. Sarikaya H, Arnold M, Engelter S, et al. Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke. 2011;42(9):2498–2502. doi:10.1161/STROKEAHA.110.607614
24. de Havenon A, Haynor DR, Tirschwell DL, et al. Association of Collateral Blood Vessels Detected by Arterial Spin Labeling Magnetic Resonance Imaging With Neurological Outcome After Ischemic Stroke. JAMA Neurol. 2017;74(4):453–458. doi:10.1001/jamaneurol.2016.4491
25. He F, Sullender CT, Zhu H, et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Sci Adv. 2020;6(21):eaba1933. doi:10.1126/sciadv.aba1933
26. Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin Diagn Lab Immunol. 1998;5(4):438–445. doi:10.1128/CDLI.5.4.438-445.1998
27. Luo P, Li X, Fei Z, Poon W. Scaffold protein Homer 1: implications for neurological diseases. Neurochem Int. 2012;61(5):731–738. doi:10.1016/j.neuint.2012.06.014
28. Louessard M, Bardou I, Lemarchand E, et al. Activation of cell surface GRP78 decreases endoplasmic reticulum stress and neuronal death. Cell Death Differ. 2017;24(9):1518–1529. doi:10.1038/cdd.2017.35
29. Petrovic-Djergovic D, Goonewardena S, Pinsky D. Inflammatory Disequilibrium in Stroke. Circulation Res. 2016;119(1):142–158. doi:10.1161/CIRCRESAHA.116.308022
30. Zhang J, Bhuiyan M, Zhang T, et al. Modulation of brain cation-Cl cotransport via the SPAK kinase inhibitor ZT-1a. Nat Commun. 2020;11(1):78. doi:10.1038/s41467-019-13851-6
31. Kitchen P, Salman M, Halsey A, et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell. 2020;181(4):784–99.e19. doi:10.1016/j.cell.2020.03.037
32. Griffin J, Zlokovic B, Mosnier L. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132(2):159–169. doi:10.1182/blood-2018-02-769026
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
