Back to Journals » Infection and Drug Resistance » Volume 17
Sepsis and Hepatapostema Secondary to Chromobacterium Violaceum Infection on Lower Limb Skin: A Case Report
Authors Li K , Han D , Alhaskawi A , Liu T, Wang X, Yang W, Lu H, Fang X
Received 1 November 2023
Accepted for publication 8 March 2024
Published 14 March 2024 Volume 2024:17 Pages 1003—1010
DOI https://doi.org/10.2147/IDR.S445366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Kun Li,1 Dongsheng Han,2 Ahmad Alhaskawi,3 Tingting Liu,1 Xiaojuan Wang,4 Wu Yang,1 Hui Lu,3 Xueling Fang1
1Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Laboratory Medicine, the First Affiliated Hospital, Zhejiang University school of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Orthopaedics, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 4Department of Clinical Pharmacy, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Xueling Fang, Department of Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79, Qingchun Road, Shangcheng District, Hangzhou, Zhejiang, People’s Republic of China, Fax +86 571-87236838, Email [email protected] Hui Lu, Department of Orthopaedics, The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79, Qingchun Road, Shangcheng District, Hangzhou, Zhejiang, People’s Republic of China, Fax +86 571-87236121, Email [email protected]
Background: Chromobacterium violaceum (C. violaceum) is a Gram-negative bacterium capable of causing severe infections in both humans and specific animals. Despite its infrequency, C. violaceum infections exhibit a notably high mortality rate. The timely and precise detection of this pathogen stands as a critical factor in achieving effective diagnosis and treatment. Traditional diagnostic approaches possess limitations, particularly in terms of their time-consuming nature and the range of pathogens they can identify. Metagenomic next-generation sequencing (mNGS) testing has emerged as a highly promising diagnostic tool for infectious diseases.
Methods: Within this case report, we present a patient who developed a C. violaceum infection subsequent to a lower limb infection, leading to the progression of sepsis, a liver abscess, septic shock, multi-organ dysfunction, and altered mental status. Samples of the patient’s blood and tissue from the lower limb skin are collected, and the infection is swiftly diagnosed through mNGS, allowing for the immediate initiation of suitable treatment.
Results: The mNGS results revealed the patient’s infection with C. violaceum. Subsequent conventional bacterial culture results were concordant with the mNGS findings. Following comprehensive management measures, including prompt and effective anti-infective treatment, the patient achieved cure and was successfully discharged.
Conclusion: This case underscores the significance of employing advanced diagnostic methodologies like mNGS for the early detection of uncommon pathogens such as C. violaceum. The expedited diagnosis and timely intervention hold the potential to substantially enhance patient outcomes in cases of severe infections instigated by this bacterium.
Keywords: chromobacterium violaceum, sepsis, infection, hepatapostema, liver, liver abscess, metagenomic next-generation sequencing
Introduction
Chromobacterium violaceum (C. violaceum), a gram-negative facultative anaerobic bacillus, thrives in the soil and stagnant waters of tropical and subtropical climates.1 The discovery of C. violaceum dates back to 1881.2 In 1905, Woolley isolated it from a diseased water buffalo in the Philippines and elucidated its pathogenicity that same year.3 The inaugural documented case of human infection emerged in 1927. Since then, around 200 cases have been reported globally4,5 with a majority in Southeast Asia and only a handful in China. Distinct regions exhibit unique clinical features.4–9 Notably, C. violaceum infections have an alarmingly high mortality rate, especially among individuals with sepsis, with reported mortality rates ranging from 57% to 80%.10,11
Metagenomic next-generation sequencing (mNGS), an impartial pathogen detection approach, has become an appealing strategy. Several studies have affirmed the diagnostic advantages of infectious diseases through mNGS.12,13
We present a rare case of sepsis and a hepatic abscess resulting from a C. violaceum infection in a lower limb. The patient’s survival hinged on the early diagnosis facilitated by mNGS and subsequent effective treatment.
Case Presentation
On August 19, 2022, a 62-year-old male patient was admitted to the Intensive Care Unit (ICU) of Zhejiang University School of Medicine’s First Affiliated Hospital. His admission was prompted by a 7-day ulceration on his left lower limb and 1-day disturbance of consciousness. The patient, identified as a diabetic farmer, had a medical history of type 2 diabetes and had been using the medications dapagliflozin, pioglitazone, and metformin over an extended period.
The sequence of events leading to his admission began with the patient noticing a 10 cm pustule above his left ankle joint seven days prior (Figure 1A and B). Attempting a home remedy, he used a toothpick to drain the pustule. Subsequently, he developed a fever with a peak temperature of 40°C. As his self-administered antipyretic medications proved ineffective, he sought medical attention at Shaoxing People’s Hospital in the Shangyu District.
Diagnostic evaluations unveiled significant laboratory results. His white blood cell count (WBC) was measured at 9.5 × 109/L, with neutrophils (N%) accounting for 84.9%. Inflammation markers were elevated, with C-reactive protein (CRP) levels reaching 296.4 mg/L. Elevated liver enzymes were also noted, with alanine transaminase (ALT) at 88 U/L. Creatinine levels were found to be 111.1 μmol/L, glucose was elevated at 22.8 mmol/l, and cardiac markers creatine kinase isoenzyme (CK-MB) and troponin I were 2.91 ng/mL and 0.03 ng/mL, respectively.
Imaging studies provided further insights into the patient’s condition. Chest CT scans revealed a minor infection in the right upper lung lobe, accompanied by a small accumulation of fluid within both pleural cavities. Abdominal CT scans unveiled multiple low-density nodules within the liver. Additionally, a head and neck CT angiography (CTA) detected multiple calcified plaques within the left internal carotid artery’s cavernous sinus segment, which led to mild luminal narrowing. Given these findings, the primary diagnoses included lower limb soft tissue infection and sepsis.
The initial course of treatment involved the administration of imipenem/cilastatin, alongside other supportive measures, in an attempt to manage the patient’s worsening condition. Despite these interventions, the patient’s health continued to decline. On August 19, 2022, he slipped into a coma with a Glasgow Coma Scale (GCS) score of 3+1+1, accompanied by low blood pressure and oliguria. Subsequently, he was transferred to the ICU of the hospital for more intensive care.
Upon admission, a physical examination revealed a GCS score of 1+1+1, a body temperature of 36.8°C, a pulse rate of 131 beats/minute, and a respiratory rate of 26 breaths/minute. His blood pressure of 119/94 mmHg was being supported with the use of epinephrine. Pupil examination indicated equal size and roundness, albeit with a sluggish light reflex. Notably, an ulceration measuring approximately 10 cm above the left ankle joint was observed, alongside an oval-shaped necrotic area roughly 5 cm in diameter on the skin (Figure 1A and B). Further physical examinations did not yield any remarkable findings.
Laboratory findings upon admission unveiled a WBC count of 2.24 × 109/L, with neutrophils at 1.91 × 109/L. Additional results included a B-type natriuretic peptide precursor quantification of 6938 pg/mL, activated partial thromboplastin time of 46.0 seconds, prothrombin time of 16.3 seconds, and a D-dimer level of 27,701 ug/L. Notable liver enzyme levels included ALT at 744 U/L and AST at 3885 U/L. Other values included total bilirubin (TBIL) of 13.9 μmol/L, direct bilirubin of 12.6 μmol/L, high-sensitivity C-reactive protein at 429.59 mg/L, and procalcitonin (PCT) exceeding 100.00 ng/mL. The patient’s glycosylated hemoglobin A1c was measured at 10.4%. Arterial lactate was found to be 2.0 mmol/L, pH stood at 7.20, while the partial pressure of carbon dioxide (PaCO2) and pappenheimer oxygen (PaO2) were 28.8 mmHg and 121.0 mmHg, respectively. The tendency changes in the laboratory results and reference range were summarized in Table 1.
 |
Table 1 Biological Parameters of the Patient |
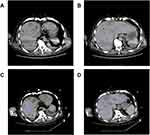
Further imaging and diagnostic procedures were conducted. An abdominal color-Doppler ultrasound indicated possible abscess formation in the right liver due to heterogeneous echoes. The abdominal CT scan unveiled numerous low-density lesions within the liver, accompanied by a minor amount of fluid in the abdominal cavity, suggestive of cholecystitis (Figure 2). The chest CT scan depicted interstitial changes in both lungs, accompanied by pleural effusion on both sides and partial lung collapse. A head CT scan demonstrated a slightly reduced density patch-like shadow in the left frontal and vertex regions.
Upon admission, the patient’s Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated to be 27, while the Sequential Organ Failure Assessment (SOFA) score was 18. The primary diagnoses included septic shock, lower limb skin infection, hepatic abscess, and altered consciousness. The patient’s altered state of consciousness was attributed to hypoperfusion of the left cerebral hemisphere due to septic shock-induced effects. This conclusion was drawn from a result of a CTA showing chronic occlusion in the left internal carotid artery.
For treatment, a combination of Levofloxacin (500 mg QD) and Meropenem (1g Q8H) was administered to combat the infection. Various medical interventions were employed, including fluid resuscitation, continuous norepinephrine infusion to maintain mean arterial pressure above 80 mmHg, administration of acetylcysteine and Levosimendan, along with Dobutamine to enhance myocardial function. Low molecular weight heparin (LMWH) was used for anticoagulation, and interventions were targeted at liver support, blood sugar control, and addressing hypoalbuminemia. Mechanical ventilation was applied through endotracheal intubation to alleviate respiratory distress. Continuous renal replacement therapy (CRRT) was utilized for electrolyte balance and acid-base regulation, along with nutritional support.
Importantly, ultrasound-guided percutaneous drainage of the hepatic abscess resulted in obtaining a pale brown purulent fluid for bacterial analysis. To ascertain the pathogen causing the limb infection, a blood sample was subjected to Next-Generation Sequencing (NGS) testing,13 which identified C. violaceum on August 21, 2022 (Figure 3B). Treatment for the C. violaceum infection continued with Levofloxacin and Meropenem.
On August 24, 2022, necrosis in the left calf, accompanied by local oozing and a foul odor, prompted surgical debridement. The procedure involved the thorough removal of necrotic tissue, followed by the application of a fasciocutaneous flap and repair of the exposed bone surface with antibiotic bone cement (Figure 1C). Continuous vacuum sealing drainage (VSD) was employed as part of the treatment.
Tissue samples were collected for NGS testing, confirming the presence of C. violaceum (Figure 3C). The subsequent conventional bacterial culture results aligned with the findings of mNGS (Figure 3A). Susceptibility testing demonstrated sensitivity to both Levofloxacin and Meropenem.
The patient’s condition exhibited gradual improvement post-treatment. By the sixth day of admission (August 24, 2022), vasopressors were discontinued, lactate levels normalized, and septic shock resolved. Respiratory function significantly improved, with an oxygenation index surpassing 300. Meanwhile, body temperature and inflammatory markers decreased (procalcitonin 3.12 ng/mL, high-sensitivity C-reactive protein 88.22 mg/L on August 25). The patient regained consciousness and was able to follow medical instructions. Renal function improved notably, with a 24-hour urine output of approximately 3750 mL on August 22, leading to discontinuation of CRRT. On August 25, the patient successfully underwent tracheal tube removal and weaning off the ventilator post-respiratory exercises. At this point, his APACHE II score stood at 11, and the SOFA score was 3.
On September 2, 2022, the patient was transferred from the ICU to the general ward of the infection division. Continued improvement in his condition led to his discharge from the hospital on September 22, 2022, with plans for outpatient follow-up care.
Discussion
C. violaceum infections in humans are indeed rare occurrences. Risk factors associated with such infections include exposure to contaminated environments and chronic immunosuppressive conditions like diabetes mellitus, glucose-6-phosphate dehydrogenase (G6PD) deficiency, and chronic granulomatous disease (CGD).14,15 Moreover, individuals with compromised immune systems are more susceptible to these infections.15 The clinical spectrum of C. violaceum infections is quite broad, with common symptoms encompassing soft tissue and organ infections, liver abscesses, meningitis, and hemolytic-uremic syndrome. Notably, disseminated infections, which include sepsis, septic shock, multi-organ dysfunction syndrome, and neurological complications, often lead to fatal outcomes.16 It’s worth mentioning that C. violaceum infection is frequently associated with chronic granulomatous disease (CGD), neutrophil dysfunction, and Glucose-6-Phosphate Dehydrogenase deficiency (G6PDD).15
Our case involves a 62-year-old male with a history of diabetes who initially presented with skin ulceration on the lower leg, which subsequently progressed to sepsis, liver abscesses, septic shock, multi-organ dysfunction, and altered consciousness. Importantly, the patient had no history of diarrhea, suggesting that the infection likely resulted from exposure of the wound to soil and stagnant water. The localized infection on the lower extremity eventually spread to the bloodstream, disseminating to the liver.
Diagnosing C. violaceum infections can be challenging due to their rarity and similarity to other bacterial infections. Laboratory diagnosis typically involves isolating and identifying the bacteria from clinical specimens, where it appears as violet or purple colonies on agar medium. Biochemical tests, such as oxidase and catalase testing, can further confirm the identification. Clinically, most cases are confirmed by culturing the bacteria from secretions, body fluids, and blood. However, confirming non-pigmented strains can be extremely difficult, despite their pathogenicity being unrelated to pigment production.17 C. violaceum is generally considered to be of low virulence, but highly virulent strains possess high levels of superoxide dismutase and catalase, which might protect them from human phagocytic attacks, resulting in severe and often fatal human infections.18
Early diagnosis, timely intervention, and appropriate treatment are paramount in improving the prognosis of C. violaceum infections. Metagenomic testing, based on high-throughput sequencing technologies like Illumina and the Beijing Genomics Institute (BGI), has emerged as a crucial tool. Metagenomic next-generation tests analyze all genetic material in a sample, including host and pathogen DNA or RNA. By comparing the obtained sequences to existing nucleotide sequences of microbes in databases, suspicious pathogenic microorganisms can be identified.12 Currently, the most common NGS applications in diagnostic microbiology laboratories include targeted NGS with various enrichment methods, such as amplification or probe hybridization, and mNGS.12
To date, there have been relatively few reported cases, with only a small proportion diagnosed by mNGS.7 In our case, determining the source of fever and sepsis following the patient’s admission with lung infection and pleural effusion on chest CT necessitated differentiation. By obtaining blood samples, pus, and tissue samples from the infected area of the lower limb for routine bacterial culture and mNGS, we were able to detect C. violaceum by mNGS in both the blood and local tissue samples. This led us to consider the lower limb skin infection as the potential source of sepsis and liver abscess in this case. The early and accurate diagnosis of C. violaceum infection through mNGS allowed us to promptly administer a combination of intravenous levofloxacin and meropenem, ultimately leading to a better prognosis for the patient.
Due to the rarity of this infection, there is limited data available regarding the antibiotic susceptibility pattern of this microorganism. It is known that C. violaceum exhibits resistance mechanisms to many antibiotics, including multidrug efflux pumps,1 and is generally resistant to penicillins and cephalosporins.14 Empirical treatment often involves antibiotic combinations based on susceptibility testing results, such as aminoglycosides (amikacin, gentamicin), fluoroquinolones, trimethoprim-sulfamethoxazole, and meropenem. The goal of presenting this case is to provide clinicians with further insights into C. violaceum infection, facilitating early recognition and diagnosis. Ultimately, the aim is to reduce mortality rates associated with this infection through timely comprehensive treatment and critical care support.
Conclusion
C. violaceum is a rare yet potentially severe pathogen that can result in serious infections in humans. This case reiterates that C. violaceum infections can be effectively treated. Timely identification, precise diagnosis, and comprehensive treatment are pivotal for enhancing the prognosis. Next-generation sequencing may offer greater sensitivity in pathogen detection, facilitating early diagnosis and more focused antimicrobial therapy. Furthermore, it is imperative to bolster public health measures for infection prevention and control to mitigate the impact of this uncommon bacterium on human health.
Ethical Approval
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Consent Statement
The patient provided written informed consent to allow the case details and any accompanying images to be published.
Acknowledgments
The study was supported by various colleagues from the hospital’s Department of Critical Care Medicine and Department of Orthopedics.
Author Contributions
KL, TL, XW participated in patient treatment, data collection, and article writing; XF participated in the formulation of the patient’s treatment plan, supervised writing of manuscript; HL, AA administered surgical therapy for patient, prepared the images. DH detected sample and participated in NGS-related data processing. WY prepared the CT images. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received external funding from the National Natural Science Foundation of China (82003660), the Nature Science Foundation of Zhejiang province (LYY21H300004), and the Zhejiang Province Medical and Health Science and Technology Program (2019RC170). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Justo GZ, Duran N. Action and function of Chromobacterium violaceum in health and disease: violacein as a promising metabolite to counteract gastroenterological diseases. Best Pract Res Clin Gastroenterol. 2017;31(6):649–656. doi:10.1016/j.bpg.2017.10.002
2. Ray P, Sharma J, Marak RS, et al. Chromobacterium violaceum septicaemia from north India. Indian J Med Res. 2004;120(6):523–526.
3. Parajuli NP, Bhetwal A, Ghimire S, et al. Bacteremia caused by a rare pathogen - Chromobacterium violaceum: a case report from Nepal. Int J Gen Med. 2016;9:441–446. doi:10.2147/IJGM.S125183
4. Iwamoto K, Yamamoto M, Yamamoto A, et al. Meningitis caused by Chromobacterium haemolyticum suspected to be derived from a canal in Japan: a case report. J Med Case Rep. 2023;17(1):171. doi:10.1186/s13256-023-03913-1
5. Alisjahbana B, Debora J, Susandi E, Darmawan G. Chromobacterium violaceum: a Review of an Unexpected Scourge. Int J Gen Med. 2021;14:3259–3270. doi:10.2147/IJGM.S272193
6. Zhang P, Li J, Zhang Y-Z, Li X-N. Chromobacterium violaceum infection on lower limb skin: a case report. Medicine. 2021;100(6):e24696. doi:10.1097/MD.0000000000024696
7. Lang L, Wang M, Huang X, et al. Successful treatment of a patient with recurrent infection of Chromobacterium violaceum. BMC Infect Dis. 2021;21(1):484. doi:10.1186/s12879-021-06216-2
8. Chang CY, Lee YT, Liu KS, Wang YL, Tsao SM. Chromobacterium violaceum infection in Taiwan: a case report and literature review. J Microbiol Immunol Infect. 2007;40(3):272–275.
9. Chen CH, Lin LC, Liu CE, Young TG. Chromobacterium violaceum bacteremia: a case report. J Microbiol Immunol Infect. 2003;36(2):141–144.
10. Lee J, Kim JS, Nahm CH, et al. Two cases of Chromobacterium violaceum infection after injury in a subtropical region. J Clin Microbiol. 1999;37(6):2068–2070. doi:10.1128/JCM.37.6.2068-2070.1999
11. Karthik R, Pancharatnam P, Balaji V. Fatal Chromobacterium violaceum septicemia in a South Indian adult. J Infect Dev Ctries. 2012;6(10):751–755. doi:10.3855/jidc.1866
12. Li N, Cai Q, Miao Q, Song Z, Fang Y, Hu B. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5(1):2000792. doi:10.1002/smtd.202000792
13. Han D, Yu F, Zhang D, et al. The real-world clinical impact of plasma mNGS Testing: an Observational Study. Microbiol Spectr. 2023;11(2):e0398322. doi:10.1128/spectrum.03983-22
14. Batista JH, da Silva Neto JF. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol. 2017;8:2213. doi:10.3389/fmicb.2017.02213
15. Yang CH, Li YH. Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc. 2011;74(10):435–441. doi:10.1016/j.jcma.2011.08.013
16. Kothari V, Sharma S, Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med. 2017;10(8):744–752. doi:10.1016/j.apjtm.2017.07.022
17. Diaz Perez JA, Garcia J, Rodriguez Villamizar LA. Sepsis by Chromobacterium violaceum: first case report from Colombia. Braz J Infect Dis. 2007;11(4):441–442. doi:10.1590/s1413-86702007000400016
18. Kaniyarakkal V, Orvankundil S, Lalitha SK, Thazhethekandi R, Thottathil J. Chromobacterium violaceum septicaemia and urinary tract infection: case reports from a Tertiary Care Hospital in South India. Case Rep Infect Dis. 2016;2016:6795743. doi:10.1155/2016/6795743
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.