Back to Journals » Drug Design, Development and Therapy » Volume 18
RNA-Based Antipsoriatic Gene Therapy: An Updated Review Focusing on Evidence from Animal Models
Authors Lin ZC, Hung CF, Aljuffali IA, Lin MH, Fang JY
Received 1 November 2023
Accepted for publication 7 April 2024
Published 23 April 2024 Volume 2024:18 Pages 1277—1296
DOI https://doi.org/10.2147/DDDT.S447780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Zih-Chan Lin,1 Chi-Feng Hung,2– 4 Ibrahim A Aljuffali,5 Ming-Hsien Lin,6 Jia-You Fang7– 9
1Chronic Diseases and Health Promotion Research Center, Chang Gung University of Science and Technology, Puzi, Chiayi, Taiwan; 2School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan; 3Program in Pharmaceutical Biotechnology, Fu Jen Catholic University, New Taipei City, Taiwan; 4School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan; 5Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 6Department of Dermatology, Chi Mei Medical Center, Tainan, Taiwan; 7Pharmaceutics Laboratory, Graduate Institute of Natural Products, Chang Gung University, Kweishan, Taoyuan, Taiwan; 8Research Center for Food and Cosmetic Safety and Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Kweishan, Taoyuan, Taiwan; 9Department of Anesthesiology, Chang Gung Memorial Hospital, Kweishan, Taoyuan, Taiwan
Correspondence: Ming-Hsien Lin, Department of Dermatology, Chi Mei Medical Center, 901 Zhonghua Road, Yongkang, Tainan, Taiwan, Email [email protected] Jia-You Fang, Pharmaceutics Laboratory, Graduate Institute of Natural Products, Chang Gung University, 259 Wen-Hwa 1st Road, Kweishan, Taoyuan, 333, Taiwan, Email [email protected]
Abstract: Psoriasis presents as a complex genetic skin disorder, characterized by the interaction between infiltrated immune cells and keratinocytes. Substantial progress has been made in understanding the molecular mechanisms of both coding and non-coding genes, which has positively impacted clinical treatment approaches. Despite extensive research into the genetic aspects of psoriasis pathogenesis, fully grasping its epigenetic component remains a challenging endeavor. In response to the pressing demand for innovative treatments to alleviate inflammatory skin disorders, various novel strategies are under consideration. These include gene therapy employing antisense nucleotides, silencing RNA complexes, stem cell therapy, and antibody-based therapy. There is a pressing requirement for a psoriasis-like animal model that replicates human psoriasis to facilitate early preclinical evaluations of these novel treatments. The authors conduct a comprehensive review of various gene therapy in different psoriasis-like animal models utilized in psoriasis research. The animals included in the list underwent skin treatments such as imiquimod application, as well as genetic and biologic injections, and the results of these interventions are detailed. Animal models play a crucial role in translating drug discoveries from the laboratory to clinical practice, and these models aid in improving the reproducibility and clinical applicability of preclinical data. Numerous animal models with characteristics similar to those of human psoriasis have proven to be useful in understanding the development of psoriasis. In this review, the article focuses on RNA-based gene therapy exploration in different types of psoriasis-like animal models to improve the treatment of psoriasis.
Keywords: psoriasis, animal model, gene therapy, siRNA, miRNA
Introduction
Psoriasis vulgaris is the most common clinical manifestation of psoriasis, affecting approximately 90% of psoriasis patients. Psoriasis vulgaris is a long-lasting and noncommunicable condition to be characterized by pain, disfigurement, and disability. This disease impacts approximately 2% to 3% of the global population, manifesting as red, hardened, scaly patches on the skin, and can also affect the nails and joints.1 This disease can occur in any sex at any age.2 A recent study showed that instances of new-onset psoriasis or exacerbation of existing psoriasis were observed as cutaneous adverse events subsequent to COVID-19 vaccination. Therefore, psoriatic patients may necessitate frequent monitoring prior to and after receiving COVID-19 vaccination.3 Depending on the type and severity of psoriasis, the treatment modalities can be employed either separately or in conjunction with one another. However, a major concern with the currently available therapies is that most of them are associated with side effects when administered orally or intravenously.4 A diverse array of therapeutic approaches is commonly utilized in clinical practice for the management of psoriasis. A range of therapeutic techniques, including topical formulations such as gels and creams, phototherapy, systemic medications, and biologics, are utilized to manage moderate-to-severe psoriasis.5 Given the changes in skin characteristics resulting from psoriasis and the adverse effects associated with systemic oral and biological treatments, exploration of innovative approaches for psoriasis management is urgently needed. As a result, innovative treatment strategies that utilize gene therapy, such as antisense nucleotides, silencing RNA complexes, stem cell therapy, and antibody-based therapy, are being considered. While gene therapy is not currently available as a treatment for psoriasis, a growing body of research has explored the genetic underpinnings of psoriasis.6 Despite considerable investments in drug development, the rate of success for drugs in clinical trials remains relatively low. The use of animal models in preclinical research is pivotal in bridging the gap between the preclinical and clinical stages, but this process can be challenging due to the presence of flawed research. Choosing the validated and predictive animal model is essential in accurately addressing clinical questions.7
In the past three decades, animal models of psoriasis have become increasingly common in preclinical research, with each model offering distinct benefits and drawbacks. Certain models have demonstrated greater similarity to human disease, while others have yielded invaluable information on the underlying mechanisms of psoriasis pathogenesis.8 Gaining insights into the pathophysiology of psoriasis can be difficult in laboratory settings, as animals do not naturally develop this condition. However, different rodent models have been created through various methods, such as transgenesis, knockout, xenotransplantation, immunological reconstitution, drug induction, or spontaneous mutation, to shed light on specific aspects of psoriasis’s complex immune-mediated pathology.9 The use of animal models that simulate psoriasis has led to important discoveries about the mechanisms underlying chronic inflammatory disorders and has played a critical role in the development of many effective modern treatments.10–12 Through the use of animal models, particularly mice, researchers have gained valuable insights into psoriasis, including the effectiveness of various treatments and the intricate interplay among immune cells and inflammatory mediators. Consequently, the number of research papers focused on psoriasis-like animal models has steadily risen. Despite this trend, surprisingly, over the past five decades of utilizing animal models for such investigations, there has been no standardized experimental characterization of these models.9 Various preclinical animal model studies have contributed to several clinical advancements by elucidating the roles of specific immune cells and their factors, as well as unraveling the cellular and molecular mechanisms that underlie the interplay between immune cells and keratinocytes, which promote the development of psoriasiform skin inflammation.8
Widely Applied Mouse Models of Psoriasis
In recent decades, numerous research papers have explored various animal models for studying psoriasis. These models have significantly enhanced our understanding of the pathomechanisms underlying chronic inflammation in psoriasis. However, they have also brought to light several lingering concerns. With the emergence of new genetic, cellular, and molecular insights into human psoriasis pathogenesis, there has been a substantial increase in the development of innovative preclinical mouse models that mimic psoriasis.7 In our study, we categorized each psoriasis model into one of five distinct types: xenotransplantation models, transgenic and knockout models, IL-23-overexpressing models, and models involving the topical application of imiquimod (IMQ).
Xenotransplantation Models
Xenotransplantation models involve grafting psoriatic human skin onto immunodeficient mice. The transplanted human skin retains the characteristics of psoriasis, providing a platform to study the human immune response and psoriasis pathogenesis. These models closely resemble human psoriasis and are valuable for investigating immune responses and testing potential treatments. However, they can be more complex, time-consuming, and costly compared to other models.13,14
Transgenic and Knockout Mouse Models
Transgenic mice are genetically modified to express genes associated with psoriasis pathogenesis, while knockout mice lack specific genes relevant to psoriasis. These models facilitate the exploration of the roles of specific genes in psoriasis development and progression. They offer insights into the genetic underpinnings of psoriasis and are useful for studying molecular pathways. It is important to note that these models may not fully capture the complex, multifactorial nature of human psoriasis. Ha et al utilized IL-20RB-deficient mice (IL-20RB KO), which lack IL-20 receptors to investigate the role of these cytokine receptors in psoriasis.15
Spontaneous Mutation Models
Spontaneous mutations in mice initially served as the first animal models wherein specific genetic backgrounds and allelic mutations led to the development of a psoriasis-like dermatitis. These mutations naturally manifest in mice, resulting in a phenotype that mimics particular aspects of psoriasis. Notable examples of these spontaneous mutations include Asebia, chronic proliferative dermatitis, flaky tail, and flaky skin mice. However, their responses to antipsoriatic therapies like systemic etretinate or cyclosporin were inadequate. With the emergence of genetic and immunological manipulations, these spontaneous mutation models, often exhibiting intricate pathological changes across various organ systems, are progressively taking a backseat in psoriasis research.16
IL-23-Overexpressing Models
IL-23-overexpressing models involve engineering mice to have elevated levels of interleukin-23 (IL-23), a key cytokine associated with psoriasis pathogenesis. This leads to immune responses and skin inflammation that mimic aspects of psoriasis. These models focus on a specific pathway and cytokine implicated in psoriasis but may not fully represent the complex nature of the disease, which involves multiple cytokines and signaling pathways.17,18
Imiquimod (IMQ) Application Model
The psoriasis IMQ mouse model is commonly used to study psoriasis-like skin inflammation in mice. It relies on the topical application of IMQ, a toll-like receptor (TLR)7/8 agonist, to induce skin inflammation. The use of IMQ model offers a high level of reproducibility, swift disease development, and the ability to mimic human immune responses, thereby aiding the study of immunological aspects. This model can be readily scaled. The dose and duration can be adjusted with ease, and genetic modification is often unnecessary. It proves to be cost-effective and noninvasive, facilitating effective exploration of psoriasis mechanisms and potential treatments. Consequently, this model serves as a common starting point for investigations, prompting additional studies. While this model shares some similarities with human psoriasis, it also comes with limitations that researchers should be aware of. The IMQ model has drawbacks for thorough psoriasis research. It predominantly impacts the skin, excluding systemic involvement and restricting the examination of joint inflammation and systemic factors. The transitory nature of IMQ-induced inflammation obstructs the study of chronic psoriasis. Interindividual differences add complexity to result consistency. Disparities in immune response and the genetic uniformity of inbred mice hinder the applicability of findings to diverse human populations. The model’s simplicity does not align with the diverse nature of human psoriasis. Ethical issues arise regarding animal utilization in research. While the IMQ mouse model offers valuable insights into specific facets of psoriasis research, researchers should exercise caution when applying its findings to human psoriasis. This model is frequently employed as an initial step in research investigations.19,20
These alternative animal models serve as valuable resources for investigating various facets of psoriasis, whether it involves exploring the immune response, delivering into genetic factors, or dissecting specific cytokine pathways. Researchers typically select the model that best aligns with their research objectives and the particular dimension of psoriasis they intend to scrutinize.
The Pathogenesis and Genetics of Psoriasis
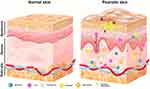
Psoriasis is an autoimmune-mediated inflammatory disorder involving T cells that usually manifests as erythemato-squamous plaques with well-defined borders that can cover a large area of the body.21 Autoimmune-mediated inflammation is responsible for the development of plaque-type psoriasis, which involves activated dermal dendritic cells that generate inflammatory cytokines, such as TNF and IL-23. The activated CD4+ and CD8+ T cells are part of the inflammatory cell population present in psoriatic skin lesions, and they play a significant role in driving disease progression. Specifically, they contribute to keratinocyte hyperproliferation by producing proinflammatory cytokines, including IFNγ, IL-17A/F, IL-22, and TNF-α.22 The recognition of epidermal autoantigens by Th17 cytokine-producing cells initiates epidermal hyperproliferation. This, in turn, prompts keratinocytes to produce chemokines that attract a range of leukocytes, including Th17 cells, dendritic cells, macrophages, and neutrophils. Simultaneously, keratinocytes generate antimicrobial peptides to activate innate immunity and various other inflammatory mediators, contributing to the amplification of the inflammatory response. This cascade leads to the maintenance of inflammation and the development of a psoriatic phenotype. Macrophages play a role in amplifying psoriasis inflammation and increasing the concentrations of Th1 cytokines. These findings emphasize the involvement of macrophages in the formation and persistence of psoriatic lesions.23 Circulating neutrophils migrate to psoriatic lesions, where they trigger processes like respiratory burst, degranulation, and the formation of NETs. These actions contribute to the immunopathogenesis of psoriasis, which encompasses an imbalance in T cell responses, keratinocyte proliferation, angiogenesis, and the development of autoantigens (Figure 1).24,25 The primary contributors to plaque psoriasis, the most common type of psoriasis, are cytokines such as TNF-α, IL-17, and IL-23, whereas pustular psoriasis, a less frequent form, is linked to an IL-36RN mutation. Various biologic therapies have been approved for the treatment of moderate to severe psoriasis by targeting these cytokines, including those that target TNF-α, IL-12/IL-23, IL-17, and IL-23/IL-39.26
Psoriasis is a complex disease influenced by genetic, environmental, and epigenetic factors that affect gene expression. Advances in genomics have led to the identification of more than 50 genetic markers associated with increased susceptibility to psoriasis in diverse ethnic populations through genome-wide association studies (GWAS) and genotyping platforms.27 Several of these genes linked to psoriasis susceptibility are found in close proximity to genes that regulate the adaptive and innate immune responses, as well as genes involved in the maintenance of skin barrier function. In current research, evidence has shown that psoriasis pathogenesis is affected by epigenetic modifications, such as histone modifications, promoter methylation, and the dysregulation of long noncoding RNAs (lncRNAs) and microRNAs (miRNAs). Various modifications in gene expression and signaling pathways, including abnormal differentiation and proliferation of keratinocytes, impaired communication between keratinocytes and inflammatory cells, neoangiogenesis, and chronic inflammation, are thought to contribute to the development of psoriasis. A study by Tsoi et al28 analyzed the expression of lncRNAs in psoriatic skin and identified 2942 previously annotated and 1080 novel lncRNAs in both involved and uninvolved areas of the skin. The analysis revealed that several lncRNAs, particularly those with varying expression levels, were coexpressed with immune-related genes. These newly identified lncRNAs were enriched in the epidermal differentiation complex and had distinct tissue-specific expression patterns and epigenetic profiles, suggesting their potential role in psoriasis pathogenesis. Therefore, the evidence from Tsoi et al’s study indicates that several lncRNAs may be involved in immune-mediated psoriasis development.28,29 Some circulating miRNAs in the blood may also serve as specific markers for psoriasis diagnosis, prognosis, and treatment response. In addition, GWAS have identified numerous single nucleotide polymorphisms (SNPs) that are associated with an increased risk of psoriasis, highlighting the involvement of the innate immune system in the pathogenesis of the disease. SNPs are genes involved in T-cell function and differentiation (such as ETS1, RUNX3, TNFRSF9, MBD2, and IRF4), type I interferon and cytokine signaling (such as ELMO1, TYK2, SOCS1, IFIH1/MDA5, RNF114, IRF4, RIG1/DDX58, and IFNLR1/IL28RA), and regulation of NF-κB-associated inflammatory signaling pathways (such as TNFAIP3, TNIP1, TYK2, REL, NFkBIA, CARD14, CARM1, UBE2L3, and FBXL19).30–32 Psoriasis is linked to genetic factors that involve the IL-23/IL-17 axis, and various genes implicated in this pathway include IL23R, IL12B, IL12RB, IL23A, IL23R, TYK2, STAT3, STAT5A/B, SOCS1, ETS1, TRAF3IP2, KLF4, and IF3.33–35
Epigenetic modifications, including changes in histone modifications, promoter methylation, and disruptions to lncRNAs and miRNAs, have been found to be involved in psoriasis pathogenesis. Several non-coding RNAs (ncRNAs) have been implicated in the pathogenesis of psoriasis. Here are some important ncRNAs and their roles in psoriasis:
miR-146a
miR-146a acts as a negative regulator of inflammation in psoriasis. It targets TNF receptor-associated factor 6 (TRAF6) and interleukin-1 receptor-associated kinase 1 (IRAK1), two key components of the NF-κB signaling pathway, thereby dampening the inflammatory response.36
miR-146b
miR-146b is another miRNA associated with psoriasis pathogenesis. It targets the TLR4 signaling pathway, downregulating pro-inflammatory cytokines and inhibiting keratinocyte hyperproliferation.37
miR-203
miR-203 is downregulated in psoriasis lesions. It plays a role in regulating keratinocyte differentiation, and its dysregulation contributes to the altered differentiation patterns seen in psoriasis.38
miR-21
miR-21 is upregulated in psoriatic lesions. It promotes inflammation and keratinocyte proliferation by targeting multiple genes that negatively regulate these processes, including programmed cell death 4 (PDCD4) and sprouty homolog 1 (SPRY1).39
miR-31
miR-31 is upregulated in psoriatic skin and contributes to the development of skin lesions by regulating keratinocyte proliferation and inflammation.40
Circular RNA ciRS-7 (Cdr1as)
Circular RNA ciRS-7 acts as a sponge for miR-7, which itself targets the mRNAs of several genes involved in inflammation. By sequestering miR-7, ciRS-7 indirectly promotes inflammation in psoriasis.41
HOX Transcript Antisense RNA (HOTAIR)
HOTAIR is a lncRNA that is upregulated in psoriatic skin. It contributes to inflammation by regulating pro-inflammatory cytokines, such as IL-6 and IL-8. HOTAIR also promotes keratinocyte proliferation and migration.42
LncRNA Maternally Expressed Gene 3 (MEG3)
MEG3 is downregulated in psoriasis lesions and is involved in modulating keratinocyte proliferation, inflammation, and immune response.43
Terminal Differentiation-Induced Noncoding RNA (TINCR)
TINCR is involved in keratinocyte differentiation and epidermal barrier formation. Dysregulation of TINCR can lead to abnormal differentiation patterns, a hallmark of psoriasis. This ncRNA plays various roles in regulating inflammatory responses, keratinocyte proliferation, differentiation, and epidermal barrier formation, all of which are crucial aspects of psoriasis pathogenesis.
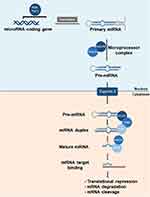
Understanding the functions of these ncRNAs provides insights into the molecular mechanisms underlying the disease and may offer potential targets for the development of new therapeutic strategies for psoriasis (Table 1).44 For developing the gene therapy targeting psoriasis, we should emphasize the role of miRNAs (miRNAs) that are essential in various cellular processes, including apoptosis, cell proliferation, morphogenesis, cell differentiation, metabolic regulation, and signal transduction. Their capacity to regulate multiple genes and the fact that a single gene can be regulated by different types of miRNAs are well established (Figure 2).45,46 Research on miRNAs and their potential role in psoriasis has primarily concentrated on the plaque-type form, with more than 250 miRNAs having dysregulated expression patterns identified in psoriatic skin.47 The miRNAs are known to play a crucial role in regulating the development of inflammatory cell subsets and controlling the intensity of inflammatory responses through the modulation of their target genes. These molecules can regulate several key processes involved in psoriasis pathogenesis, including keratinocyte differentiation, proliferation, and cytokine response, as well as T-cell activation, survival, and the interplay between immunocytes and keratinocytes by regulating the production of chemokines and cytokines.48–50 Multiple studies have suggested that certain miRNAs found in serum may be potential biomarkers for evaluating the severity of psoriasis and monitoring treatment response. Furthermore, recent research has shown that inhibiting miRNAs could be a promising therapeutic strategy for managing psoriasis.
 |
Table 1 List of Non-Coding RNAs in the Pathogenesis of Psoriasis |
Gene Therapy in Psoriasis
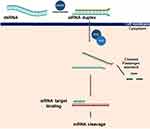
Despite substantial progress in medical research, a complete cure for psoriasis is lacking. Therefore, disease management is crucial for improving the quality of life for those with the condition. Although this can be a challenging task, it is essential to keep the disease under control and minimize its impact. New approaches for treating psoriasis are currently being explored, including gene therapy-based strategies such as antisense nucleotides, RNA interference (RNAi), stem cell therapy, and antibody-based therapy. Given that psoriasis is believed to be strongly influenced by genetics, utilizing gene therapy as a potential treatment option has emerged as a promising approach.51 Suppression of the T-cell-mediated immune response using cytokines is a potential strategy for reducing the severity of psoriasis. In a clinical study conducted on patients with severe psoriasis, the efficacy of IL-4, a cytokine with multiple functions, was evaluated. The study results indicated that IL-4 could effectively inhibit psoriasis in humans.52 One study conducted by Li et al53 investigated the efficacy of transdermal delivery of IL-4 plasmid for treating psoriasis in a K14-vascular endothelial growth factor (VEGF) transgenic mouse model. The study employed ultradeformable cationic liposomes to deliver 15 µg of plasmid DNA daily, and the results demonstrated that this method was successful in curing psoriasis. A study conducted by a research team investigated a new therapeutic strategy for treating psoriasis using an IL-4 expression plasmid delivered with dimethyl sulfoxide (DMSO) as a penetration enhancer.54 Their findings showed that the technique resulted in detectable levels of IL-4 in the skin and significant improvements in psoriasis symptoms in an animal model. Psoriasis is characterized by an overexpression of keratin 17 (K17), an intermediate filament protein that is specifically present in psoriatic lesions and has been implicated in the pathogenesis of the disease. To explore a novel treatment approach for psoriasis, Chang et al55 investigated the use of antisense oligodeoxynucleotides (ASODNs) and RNAi to downregulate the expression of K17, which has been suggested to play a role in the development of the disease. The effectiveness of topically applied K-17-specific antisense oligodeoxynucleotides (ASODNs) and liposome-encapsulated small interfering RNA (siRNA) in murine models with psoriasis was investigated. The study showed that this therapy led to a significant decrease in K-17 expression levels. This downregulation was found to inhibit the proliferation of keratinocytes and induce apoptosis.55 Studies have shown that nonviral somatic gene therapy directly targeting angiogenesis is a promising approach for the treatment of psoriasis, with high efficiency in halting the disease process.56 Numerous research studies have indicated that altering the activity of certain miRNAs can have a beneficial impact on the health of keratinocytes, potentially leading to therapeutic benefits in the treatment of psoriasis.57 One research by Xu et al58 revealed that miR-125b could potentially be a therapeutic target for psoriasis. MiR-125b was found to directly target fibroblast growth factor receptor 2 (FGFR2) and inhibit keratinocyte proliferation while promoting differentiation in primary human keratinocytes. One study59 reported that overexpression of miR-210 in CD4+ T cells resulted in an increase in the expression of proinflammatory cytokines such as IFN-γ and IL-17, as well as a decrease in the expression of regulatory cytokines such as IL-10 and TGF-β. According to research conducted by Guinea-Viniegra et al,57 animal models with psoriasis have shown promising results from anti-miR-21 therapy. Additionally, a study40 found that TGF-β1 upregulates miR-31 expression, and reducing miR-31 levels in primary human keratinocytes led to a decrease in IL-1β and IL-8 expression. Based on the findings of previous studies,57,60 promoting the expression of miR-125b and inhibiting miR-31 or miR-210 are promising therapeutic strategies for the treatment of psoriasis, which can be supported by experimental data that provide valuable insights into the underlying mechanisms of the disease. Another potential strategy in treating psoriasis involves the use of RNAi molecules, which have become a promising class of therapeutics due to their ability to silence genes by targeting the mRNA transcripts. This approach has gained interest in recent years due to the potential to design RNAi molecules for many genes (Figure 3).61 One study conducted by Desmet et al62 found that a siRNA-based combination therapy targeting the DEFB4, TSLP, and KRT17 genes resulted in downregulation of the expression of these key genes involved in psoriasis, along with decreased differentiation and keratinization markers, indicating potential efficacy in treating psoriasis. One strategy for treating psoriasis involves targeting multiple genes related to the disease at the level of epidermal keratinocytes, which holds promise for potential therapeutic benefits.
One recent study conducted by Mandal et al63 explored the use of ionic liquids to deliver NFKBIZ siRNA into the skin for the treatment of psoriasis. The results showed that the treatment was effective in suppressing aberrant gene expression and downregulating psoriasis-related signals such as TNF-α and IL-17A, demonstrating its potential therapeutic efficacy in psoriasis. Lee et al49 used the ablative lasers to assist in the delivery of nanocarriers that can be effective for targeting IL-6 siRNA to the skin and attenuating psoriasiform dermatitis. There are numerous therapies available to manage psoriasis, none of them offer a permanent cure. Psoriasis treatments have been significantly transformed by advancements in biologics and gene therapy for a possible purpose of prolonged control of psoriasis mitigation.
MicroRNA Therapeutics in the Psoriasis-Like Animal Models
The miRNAs are a type of RNA molecule that do not code for proteins and typically consist of approximately 22 nucleotides.64,65 One notable finding in psoriasis research is the association between altered miRNA expression and the disease, which was reported as early as 2007. To date, over 250 miRNAs have been identified to have differential expression in psoriatic skin or blood, according to various studies.66 MiRNAs have the potential to regulate various cellular processes in skin cells, including proliferation, differentiation, apoptosis, and cytokine production, as well as the activation and function of T-cell subsets.40,67–69 Recent research has also shown that certain miRNAs present in the blood of psoriasis patients may serve as biomarkers for diagnosing, monitoring, and treating the disease, as they correlate with Psoriasis Area Severity Index (PASI) scores.70,71 Additionally, genetic variations in miRNAs have been shown to contribute to susceptibility to psoriasis.72 Thus, miRNA is an important target for the treatment of psoriasis.
Many studies have used IMQ to investigate different miRNA mechanisms and therapeutic effects in psoriasis. van der Fits et al conducted a study using normal mice that were not genetically modified.73 These researchers treated the mice with IMQ, which is a ligand for TLR7 and TLR8, for six consecutive days. This method allowed them to create a temporary model of psoriasis-like disease. Researchers have used the IMQ model to survey miR-126,74 miR-145-5p,75 miR-146a/b,76,77 miR-148a,78 miR-149,79 miR-155,80 miR-193b-3p,81 miR-205-5p,82 miR-210,69,83 miR-21-3p/IL-22,84 miR-215-5p,85 miR-31,40 miR-340,86 and miR-let-7b87 in mice (Table 2).
 |
Table 2 Compilation of Previous Studies Investigating miRNA Therapeutics Discovery in Animal Models of Psoriasis |
One study40 recently reported that miR-31 targets protein phosphatase 6 (PP6) and is highly expressed in psoriatic skin. The role of PP6 in preserving the balance of the skin is widely acknowledged, and the broad range of functions of serine/threonine phosphatases further emphasizes its importance.88,89 A breeding technique was utilized to generate mice with loxP-flanked PP6 alleles (known as Pp6fl/fl) in combination with keratin 5-Cre (K5) mice, which enabled the selective deletion of Pp6 from keratinocytes. The resulting K5.Pp6fl/fl mice at 16 weeks of age exhibited skin lesions characterized by scaly plaques on the faces, ears, upper backs, and tails. Further analysis revealed that these mice showed several epidermal abnormalities, such as acanthosis, hyperkeratosis, parakeratosis, microabscesses on the surface of the thickened epidermis, and significant infiltration of immune cells into the dermis. To explore the therapeutic potential of arginase inhibition for treating psoriasis, Yan et al40 induced skin inflammation similar to the clinical manifestation of psoriasis by exposing cynomolgus monkeys and C57BL/6 mice to IMQ on their back shoulders.
Xu et al58 conducted a study to explore whether TGF-β1 can regulate miR-31 expression in vivo. To do so, these researchers examined miR-31 expression in the skin of mice that had been genetically modified to overexpress TGF-β1 (referred to as K5.TGF-β1 transgenic mice).56 The K5.TGF-β1 transgenic mice exhibited an epidermis-specific increase in TGF-β1 expression and displayed a psoriasis-like skin phenotype.90–92 The results showed that etanercept successfully reduced psoriasis-like symptoms and lowered miR-31 expression by fourfold compared to that of the saline-treated mice. The study also utilized in situ hybridization analysis, which revealed an overexpression of miR-31 in the hyperplastic epidermis of K5.TGF-β1 transgenic mice relative to wild-type mice. Moreover, treatment with etanercept resulted in decreases in both epidermal hyperplasia and miR-31 expression. The authors proposed that the rise in miR-31 expression in keratinocytes, induced by TGF-β1, could be responsible for the infiltration of inflammatory cells observed in K5.TGF-β1 mice by potentially promoting the secretion of chemoattractants. The researchers believe that the increased expression of miR-31 could have important implications, especially considering the vital roles of TGF-β1 and IL-1β in the differentiation of Th17 cells, which are associated with the development of psoriasis. The induction of miR-31 by TGF-β1 could trigger the production of IL-1β, leading to the exacerbation of Th17-mediated inflammation, which is a characteristic feature of psoriasis.93
Yan et al40 reported that miR-31, which is stimulated by NF-κB, plays a vital role in the excessive growth of the epidermis in psoriasis. To investigate this further, the authors used a keratin 5-Cre transgenic mouse model to develop miR-31fl/fl/K5-Cre mice. Notably, K5-Cre transgenic mice were initially established by Mao et al in 2003.94 Further investigations conducted on live subjects revealed that mice deficient in IL-17A displayed a notable decrease in protein phosphatase 6 catalytic subunit (ppp6c) levels in the epidermis compared to IL-17A-sufficient mice after one week of IMQ treatment.95 These findings suggest that the activation of NF-κB induced by inflammatory cytokines suppresses ppp6c expression through miR-31 induction in keratinocytes. Moreover, the researchers identified ppp6c as a critical target of miR-31 in this process.
In their study, Guinea-Viniegra et al57 described the development of locked nucleic acid-modified anti-miR-21 compounds that specifically target miR-21. These compounds were evaluated for their efficacy in psoriasis-like mouse models and a mouse xenotransplantation model using psoriatic tissue obtained from patients. The study results indicated that inhibiting miR-21 could reduce psoriatic pathology, highlighting a potential therapeutic strategy. The study included two groups of mice, one with a genetic modification (DKO* mice JunBf/f; c-Junf/f K5-CreERT) and the other group without the modification (JunBf/f; c-Junf/f). To induce a psoriasis-like phenotype, researchers treated both groups with tamoxifen.96 In this research, a severe combined immunodeficiency (SCID) mouse xenotransplantation model was also established. Patients diagnosed with psoriasis vulgaris and with a PASI score exceeding 15 provided lesional skin biopsies of 400 mm depth, which were obtained using a dermatome. After 1 cm2 biopsies were obtained, the samples were grafted onto SCID mice and covered with Xeroform and adhesive plaster. The grafts were monitored for three weeks to confirm their stability before initiating the treatment (Table 2).
SiRNA Therapeutics in the Psoriasis-Like Animal Models
RNAi is an innate gene regulatory mechanism that helps to regulate cellular genes and safeguard against foreign genetic materials. This mechanism selectively decreases gene expression at the post-transcriptional level by inducing sequence-specific knockdown. RNAi employs cellular pathways that process double-stranded RNA molecules, which can originate from either endogenous or foreign DNA sources. These pathways convert these molecules into short double-stranded RNA molecules that are typically 21–23 nucleotides in length.97 The RNA-induced silencing complex is responsible for integrating siRNA molecules, typically 21–23 nucleotides in length and generated from double-stranded RNA molecules, which are essential in regulating gene expression. The siRNA molecules can integrate into the RNA-induced silencing complex and cleave specific target mRNA molecules through perfect complementarity between one of the two siRNA strands and the target sequence.98 Both synthetic siRNA duplexes and DNA-encoded short hairpin RNAs (shRNAs) have been shown to effectively target predetermined mRNA targets in laboratory animals by being efficiently processed by the cellular RNAi machinery.99–103 The use of RNAi tools is prevalent in animal studies and shows potential for therapeutic applications in humans through the use of small RNA effectors.104
Researchers have recently adopted a new technique for targeting newly identified genes of interest in mouse models of psoriasis by using topical application of siRNA. This approach, which is time-efficient and can be utilized in acute or chronic psoriasis models, has gained popularity among researchers. While purchasing sufficient siRNA can be expensive, it remains a more cost-effective alternative to creating a new line of mice through genetic engineering. Various studies assessed IL-6,30 c-Rel,105 IFI27,106 K17,107 Pcsk9,108 PLA2G4B,109 TNIP1,110 and Trim21-siRNA111 in an IMQ mouse model to survey the effect of potential siRNA therapeutics in psoriasis (Table 3).
 |
Table 3 Compilation of Previous Studies Investigating siRNA Therapeutics Discovery in Animal Models of Psoriasis |
According to research, existing treatments for psoriasis, such as TNF-α-specific antagonists, bind directly to TNF-α to reduce its levels. However, the overexpression of TNF-α in psoriatic skin lesions is primarily attributed to the activation of MAPK-activated protein kinase 2 (MK2), which regulates post-transcriptional mechanisms.114,115 As a result, targeting TNF-α protein alone may not be an effective strategy for treating psoriasis. It remains unclear whether targeting TNF-α mRNA instead of the protein would be a more effective approach. Jakobsen et al112 explored the potential of using DNA-encoded small RNA effectors to target TNF-α mRNA via RNAi. This group conducted an experiment using shRNA-encoding lentiviral vectors in a psoriasis xenograft transplantation model. The results showed a decrease in the psoriasis phenotype, as indicated by improved clinical scores, reduced epidermal thickness, and lowered levels of TNF-α mRNA in the treated skin. The authors of the study obtained skin biopsies from psoriasis patients containing both epidermis and dermis using a keratome, and the samples were used to generate the psoriasis xenograft transplantation model. The authors of the study divided each keratome skin biopsy into multiple grafts, where each graft had dimensions of 1.5×1.5 × 0.05 cm. These grafts were then transplanted onto 6- to 8-week-old C.B-17 SCID mice, as previously described.94 The study revealed that the utilization of small RNAs that target the 3′ UTR of the TNF-α gene via lentiviral delivery can initiate an efficient RNAi response in human psoriatic skin. Consequently, this technique may be a therapeutic strategy for treating psoriasis, as it successfully relieved the psoriatic phenotype in a xenograft transplantation model.112
Bracke et al113 conducted a study to investigate the effectiveness of DEFB4-siRNA-containing SECosomes applied topically in targeting hBD-2 using a bioengineered skin-humanized mouse model for psoriasis. The authors found that treatment with SECosomes containing DEFB4-siRNA resulted in a significant improvement in the psoriatic phenotype, as evidenced by the normalization of skin architecture, decrease in the number and size of dermal blood vessels, and restoration of transglutaminase activity, filaggrin expression, and stratum corneum appearance. These results suggest that SECosome technology in combination with a skin-humanized mouse model for psoriasis can be a valuable preclinical tool for identifying potential therapeutic targets for the disease (Table 3).
Other Gene Therapeutics and Psoriasis-Like Animal Models
The delivery of vaccines and therapeutics through topical gene transfer to the skin presents a promising strategy due to its noninvasive and painless nature. Li et al53 indicated that the topical delivery of murine IL-4 (mIL-4) was effective in treating psoriasis-like symptoms in the K14-VEGF transgenic mouse model using IL-4 delivered through ultradeformable cationic liposomes. This finding highlights the potential of topical gene transfer as a promising avenue for the treatment of various dermatological conditions in humans. In another study,116 the K14-VEGF transgenic mouse model was utilized as a tool to leverage the advantages of the IMQ model and maintain its efficacy. This investigation found that the K14-VEGF mouse model induced with IMQ had significantly more severe skin inflammation than the IMQ-induced wild-type mouse model. The authors of the study examined the stability of inflammation on Days 8, 10, and 13 and observed that it remained consistent throughout the observation period. This study aimed to improve the current model and indicated that the K14-VEGF mouse induced with IMQ has the potential to be a valuable tool for psoriasis research (Table 4).
 |
Table 4 List of Other Applications Discovery in Animal Models of Psoriasis |
Jin et al120 employed a guinea pig model with propranolol-induced psoriasiform skin lesions. Female guinea pigs weighing 250–400 g were subjected to a propranolol-induced psoriasis-like skin lesion model. The treatment involved the topical application of 5% propranolol in an emulsifying ointment on the dorsal surfaces of their auricles twice daily for three weeks. This model is known to exhibit similar features to those observed in human psoriasis, including hyperkeratosis, parakeratosis, and acanthosis.
Studies in the past have provided detailed descriptions of the engineering of K5tTA and TetosTie2 mice, as well as the generation of double transgenic KC-Tie2 mice through genotyping. Psoriasiform skin inflammation, increased levels of IL-23 and IL-17A cytokines, proinflammatory monocytosis, and neutrophilia have been observed in the K5tTA and TetosTie2 double transgenic KC-Tie2 mouse model. Notably, these symptoms precede the formation of thrombi in the carotid artery.119 Li et al121 conducted a study in which they treated KC-Tie2 mice with antibodies targeting IL-23, IL-17A, or IL-17RA and reported outcomes similar to the clinical efficacy observed in patients with psoriasis. According to the study, the administration of IL-23, IL-17A, or IL-17A receptor (IL-17RA) antibodies to KC-Tie2 mice demonstrated similar clinical efficacy observed in psoriasis patients, resulting in a delay in occlusive thrombus formation and reduced acanthosis. Notably, while skin inflammation was reduced, there was a decrease in splenic neutrophils, while the levels of monocytes and T cells remained unchanged. The researchers showed that inhibiting the function of IL-23 or IL-17A in a pre-existing mouse model of psoriasis can reduce skin inflammation, decrease the number of circulating neutrophils, and prolong thrombosis clotting times. The study results suggest that targeting cytokines that cause psoriatic inflammation could alleviate cardiovascular comorbidities.
In addition to investigating monogenic skin disorders, there is an increasing focus on elucidating the molecular genetics that underlie inflammatory skin diseases, such as eczema and psoriasis, to explore novel therapeutic approaches aimed at targeting these mutations.122 Novel therapeutic strategies aimed at targeting mutations underlying psoriasis are being actively researched. CARD14 gene mutations, which cause the upregulation of inflammatory cytokines, have been identified in multiple subtypes of psoriasis.123 Psoriasis involves various genetic factors, including the NLRP3 inflammasome, which can trigger a heightened inflammatory response and resistance to glucocorticoid treatment. In mouse models, it has been demonstrated that codelivering Cas9 targeting NLRP3 with dexamethasone can alleviate symptoms of psoriasis, such as skin edema, mast cell infiltration, and overall inflammatory activity, to a greater extent than Cas9‒NLRP3 or dexamethasone treatments alone.118,124
The precise pathogenic mechanism by which rare autosomal mutations in CARD14 contribute to psoriasis susceptibility in humans remains unknown, despite their established association. Mellett et a117 recently conducted a study using a mouse model with a gain-of-function Card14ΔE138 mutation from psoriasis patients. Their findings indicated that CARD14 hyperactivation alone can initiate the immunopathogenic pathways responsible for psoriasis via the IL-17/IL-23 axis. Card14ΔE138 transgenic mice exhibit various histopathological features and gene expression patterns similar to human psoriasis. When treating Card14ΔE138 transgenic mice, which exhibit a psoriatic phenotype similar to humans, with an IL-23p19 neutralizing antibody, the expression of antimicrobial peptides and proinflammatory cytokines associated with psoriasis could be reduced. This study suggests that the Card14ΔE138 transgenic mouse model is clinically relevant for psoriasis research (Table 4).
Expert Opinion
The future of psoriasis models seems promising, and novel genetic techniques that enable the deletion, depletion, or tracking of tagged cells in vivo are providing fresh ways to identify the molecular and cellular pathways that connect inflammation initiated in the skin to distant organ damage. As more models are developed based on genetic loci identified through GWASs, a deeper understanding of specific gene mutations and their effects is anticipated. New mouse models can be created using a combination of advanced sequence analysis and systems biology approaches, such as bulk RNA sequencing (RNASeq), spatial RNASeq, and single-cell RNASeq, along with emerging techniques such as cytometry by time of flight (CyTOF) and omics approaches. These methods show promise for identifying new targets and facilitating the development of new models. It is expected that forthcoming animal models for chronic psoriasiform inflammation will improve the understanding of chronic inflammatory responses, inflammatory circuits, and immune regulatory networks for investigation of the key events that initiate intricate immunological pathways. Investigations focusing on refining chronic inflammatory reactions, inflammatory loops, and immune cybernetics in future animal models of chronic psoriasiform inflammation are expected to provide important insights into the underlying mechanisms of chronic inflammation and associated diseases.
We summarize several key points to highlight in this review: 1. We consolidate recent research on antipsoriatic gene therapy using various psoriasis-like animal models. 2. Emerging treatments for psoriasis encompass gene therapy, presenting promising avenues with microRNA, lncRNA, and RNA interference complexes. 3. Targeting key psoriasis genes directly with designed miRNAs in psoriatic animal models proves valuable for the development of miRNA-based therapies for psoriasis. 4. The potential of siRNA to modify posttranscriptional pathways and target dysregulated genes has garnered attention in treating psoriatic animal models.
Conclusion
At present, gene therapy is not a viable option for treating psoriasis, but there has been a growing focus on researching the underlying genetic factors behind the condition. Despite substantial investments in drug development, the success rate of new drugs in clinical trials remains disappointingly low. Employed for moderate to severe psoriasis, monoclonal antibodies offer temporary relief without genetic alteration. Gene therapy and monoclonal antibodies represent distinct approaches to psoriasis treatment. Gene therapy shows potential for addressing the root causes and delivering long-lasting effects, although it remains experimental and raises safety and ethical concerns. The choice depends on psoriasis severity, patient preferences, and treatment availability. In the future, some patients may benefit from a combination of both approaches as personalized medicine evolves.125
The results obtained from animal models play a crucial role in closing the gap between preclinical research and clinical trials. The challenges and limitations of animal models are presently being discussed,9,126 with particular attention given to their validation for a specific purpose. Moreover, there are established guidelines that assist in choosing, developing, and implementing animal models for research purposes. Furthermore, the utilization of humanized mouse models and preclinical assessment of clinical features are suggested to enhance clinical translation.
The manipulation of genes within the same target tissue may have varying effects on different skin compartments, reflecting the diverse pathophysiology of psoriasis (Figure 4). For example, a comparison of the transcriptional profiles of five mouse models (namely, IMQ, K14-amphiregulin, K5.TGF-b, K5.Tie2 and K5.Stat3C) with those of human psoriasis showed similarities in the epidermis but differences in immune-related profiles.127 To eliminate genetic variations in humans, animals with a uniform genetic background are genetically modified with targeted mutations in individual genes. In addition to genetic modifications, the impact of epigenetic regulation and interactions between genetic susceptibility loci should be considered in the study of psoriasis. Therefore, the genetic background plays a crucial role in this process. Although the animal models of psoriasis offer certain benefits, they have limitations and may not entirely replicate human conditions. The use of immunodeficient animals with engrafted human skin and immune cells has become increasingly popular due to its potential benefits, but it also presents certain limitations. Although engrafting human skin and immune cells onto immunodeficient animals offers advantages, such as eliminating genetic variations in humans, it also has drawbacks. According to James Krueger, a psoriasis researcher at Rockefeller University in New York, the integration of cell biology, expression profiling, genetics, and therapeutics has led to a comprehensive understanding of the condition. Professor Krueger believes that this translational approach from bedside to bench could also be applied to other autoimmune diseases and cancer, as the pieces of the puzzle fit together seamlessly.128
Abbreviation
ASODN, antisense oligonucleotide; CARD14, caspase recruitment domain family member 14; CARM1, Coactivator-associated arginine methyltransferase 1; Cas9, CRISPR associated protein 9; Cdr1as, Cerebellar degeneration-related protein 1 antisense RNA; DDX58, DEAD (Asp-Glu-Ala-Asp) box polypeptide 58; DEFB4, Defensin Beta 4A; ELMO1, Engulfment And Cell Motility 1; ETS1, ETS Proto-Oncogene 1; FBXL19, F-box and leucine-rich repeat protein 19; FGFR2, fibroblast growth factor receptor 2; GWAS, genome-wide association studies; hBD-2, Human beta-defensin-2; HOTAIR, HOX Transcript Antisense RNA; IF3, initiation factor 3; IFIH1, IFIH1 interferon induced with helicase C domain 1; IFN, Interferon; IFNLR1, Interferon Lambda Receptor 1; IL, interleukin; IRAK1, interleukin-1 receptor-associated kinase 1; IRF4, IRF4 interferon regulatory factor 4; KLF4, Krüppel-like factor 4; KRT17, Keratin 17; LncRNA, long non-coding RNAs; MBD2, Methylated DNA binding domain protein 2; MDA5, melanoma differentiation-associated protein 5; MEG3, Maternally Expressed 3; miRNA, microRNA; NETs, neutrophil extracellular traps; NFkBIA, nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; PDCD4, programmed cell death 4; Pp6fl/fl, loxP-flanked PP6 alleles; PPP6C, protein phosphatase 6 catalytic subunit; REL, REL Proto-Oncogene; RIG1, retinoic acid-inducible gene I; RNA, ribonucleic acid; RNF114, ring finger protein 114; RUNX3, RUNX family transcription factor 3; SECosomes, surfactant-ethanol-cholesterol-osomes; siRNA, small interfering RNA; SNPs, single-Nucleotide Polymorphisms; SOCS1, SOCS1 suppressor of cytokine signaling 1; SPRY1, sprouty homolog 1; STAT, Signal transducer and activator of transcription; TGF-β, Transforming growth factor beta; TINCR, Terminal differentiation-induced noncoding RNA; TLR, Toll-Like Receptors; TNF, tumor necrosis factor; TNFAIP3, Tumor necrosis factor, alpha-induced protein 3; TNFRSF9, TNF Receptor Superfamily Member 9; TRAF6, TNF receptor-associated factor 6; TSLP, thymic stromal lymphopoietin; TYK2, Tyrosine kinase 2; UBE2L3, Ubiquitin Conjugating Enzyme E2 L3;VEGF, vascular endothelial growth factor.
Funding
The authors are grateful for the financial support from Ministry of Science and Technology of Taiwan (MOST-110-2320-B-182-011-MY3), Chang Gung University of Science and Technology (ZRRPF6N0011) and Chi Mei Medical Center (110-CM-FJU-06). The authors also express their gratitude for the graphic design expertise provided by Jia-Yin Lin.
Disclosure
The authors declare no competing interests in this work.
References
1. Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi:10.1046/j.1365-2230.2001.00832.x
2. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23(6):775–799. doi:10.1007/s40257-022-00721-z
3. Yu J, Zhao Q, Wang X, et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J Autoimmun. 2022;133:102916. doi:10.1016/j.jaut.2022.102916
4. Bakshi H, Nagpal M, Singh M, et al. Treatment of psoriasis: a comprehensive review of entire therapies. Curr Drug Saf. 2020;15(2):82–104. doi:10.2174/1574886315666200128095958
5. Sawarkar SP, Yadav V. Novel drug delivery strategies and gene therapy regimen as a promising perspective for management of psoriasis. Indian J Dermatol Venereol Leprol. 2021;87(3):333–340. doi:10.25259/IJDVL_470_19
6. Denayer T, Stöhr T, Van Roy M. Animal models in translational medicine: validation and prediction. New Horiz Transl Med. 2014;2(1):
7. Gangwar RS, Gudjonsson JE, Ward NL. Mouse models of psoriasis: a comprehensive review. J Invest Dermatol. 2022;142(3):884–897. doi:10.1016/j.jid.2021.06.019
8. Schön MP, Manzke V, Erpenbeck L. Animal models of psoriasis‒highlights and drawbacks. J Allergy Clin Immunol. 2021;147(2):439–455. doi:10.1016/j.jaci.2020.04.034
9. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-7
10. Schön MP, Erpenbeck L. The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front Immunol. 2018;15(9):1323. doi:10.3389/fimmu.2018.01323
11. Conrad C, Gilliet M. Psoriasis: from pathogenesis to targeted therapies. Clin Rev Allergy Immunol. 2018;54(1):102–113. doi:10.1007/s12016-018-8668-1
12. Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 2021;184(1):14–24. doi:10.1111/bjd.19380
13. Fraki JE, Briggaman RA, Lazarus GS. Uninvolved skin from psoriatic patients develops signs of involved psoriatic skin after being grafted onto nude mice. Science. 1982;215(4533):685–687. doi:10.1126/science.7036342
14. Krueger GG, Chambers DA, Shelby J. Involved and uninvolved skin from psoriatic subjects: are they equally diseased? Assessment by skin transplanted to congenitally athymic (nude) mice. J Clin Invest. 1981;68(6):1548–1557. doi:10.1172/JCI110409
15. Ha HL, Wang H, Claudio E, et al. IL-20-receptor signaling delimits IL-17 production in psoriatic inflammation. J Invest Dermatol. 2020;140(1):143–151. doi:10.1016/j.jid.2019.06.127
16. Yadav K, Singh D, Singh MR, et al. Preclinical study models of psoriasis: state-of-The-art techniques for testing pharmaceutical products in animal and nonanimal models. Int Immunopharmacol. 2023;117:109945. doi:10.1016/j.intimp.2023.109945
17. Jiang W, Zhu FG, Bhagat L, et al. A Toll-like receptor 7, 8, and 9 antagonist inhibits Th1 and Th17 responses and inflammasome activation in a model of IL-23-induced psoriasis. J Invest Dermatol. 2013;133(7):1777–1784. doi:10.1038/jid.2013.57
18. Shi Z, Wu X, Rocha CS, et al. Short-term Western diet intake promotes IL-23-mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141(7):1780–1791. doi:10.1016/j.jid.2020.11.032
19. Vinardell MP. Methodological shortcomings in the reports of the imiquimod psoriatic model. Exp Dermatol. 2022;31(3):299–303. doi:10.1111/exd.14479
20. Badanthadka M, Dsouza L. Imiquimod-induced psoriasis mice model: a promising tool for psoriasis research? Res J Pharm Technol. 2020;13:7.
21. Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(20):7488. doi:10.3390/ijms21207488
22. Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–1613. doi:10.4049/jimmunol.1800013
23. Kamata M, Tada Y. Dendritic cells and macrophages in the pathogenesis of psoriasis. Front Immunol. 2022;13:941071. doi:10.3389/fimmu.2022.941071
24. Chiang CC, Cheng WJ, Korinek M, et al. Neutrophils in psoriasis. Front Immunol. 2019;10:2376. doi:10.3389/fimmu.2019.02376
25. Singh R, Koppu S, Perche PO, et al. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. 2021;22(23):12793. doi:10.3390/ijms222312793
26. Nedoszytko B, Szczerkowska-Dobosz A, Stawczyk-Macieja M, et al. Pathogenesis of psoriasis in the ”omic” era. Part II. Genetic, genomic and epigenetic changes in psoriasis. Postepy Dermatol Alergol. 2020;37:3.
27. Ray-Jones H, Eyre S, Barton A, et al. One SNP at a time: moving beyond GWAS in psoriasis. J Invest Dermatol. 2016;136(3):567–573. doi:10.1016/j.jid.2015.11.025
28. Tsoi LC, Iyer MK, Stuart PE, et al. Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol. 2015;16(1):24. doi:10.1186/s13059-014-0570-4
29. Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2(7):e610. doi:10.1371/journal.pone.0000610
30. Lee KY, Leung KS, Tang NLS, et al. Discovering genetic factors for psoriasis through exhaustively searching for significant second order SNP-SNP interactions. Sci Rep. 2018;8(1):15186. doi:10.1038/s41598-018-33493-w
31. Ghafouri-Fard S, Eghtedarian R, Taheri M, et al. The eminent roles of ncRNAs in the pathogenesis of psoriasis. Noncoding RNA Res. 2020;5:3.
32. Schön MP. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol. 2019;10:1764. doi:10.3389/fimmu.2019.01764
33. Zhang P, Zhao M, Liang G, et al. Whole-genome DNA methylation in skin lesions from patients with psoriasis vulgaris. J Autoimmun. 2013;41:17–24. doi:10.1016/j.jaut.2013.01.001
34. Tsoi LC, Spain SL, Knight J, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44(12):1341–1348. doi:10.1038/ng.2467
35. Tang L, Liang Y, Xie H, et al. Long non-coding RNAs in cutaneous biology and proliferative skin diseases: advances and perspectives. Cell Prolif. 2020;53(1):e12698. doi:10.1111/cpr.12698
36. Zhu K, Li S, Liang L. MicroRNA-146a polymorphisms are associated with psoriasis vulgaris. MedRxiv. 2022;1:2.
37. Shen H, Wang D, Zhan M, et al. MicroRNA‐146a and microRNA‐146b deficiency correlates with exacerbated disease activity, and their longitude increment relates to etanercept response in psoriasis patients. J Clin Lab Anal. 2022;36(2):e24198. doi:10.1002/jcla.24198
38. Mostafa SA, Mohammad MH, Negm WA, et al. Circulating microRNA203 and its target genes’ role in psoriasis pathogenesis. Front Med. 2022;9:988962. doi:10.3389/fmed.2022.988962
39. Jia HY, Zhang K, Lu WJ, et al. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Mol Cell Biol. 2019;20(1):
40. Yan S, Xu Z, Lou F, et al. NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat Commun. 2015;6(1):7652. doi:10.1038/ncomms8652
41. Moldovan LI, Tsoi LC, Ranjitha U, et al. Characterization of circular RNA transcriptomes in psoriasis and atopic dermatitis reveals disease-specific expression profiles. Exp Dermatol. 2021;30(8):1187–1196. doi:10.1111/exd.14227
42. Rakhshan A, Zarrinpour N, Moradi A, et al. A single nucleotide polymorphism within HOX Transcript Antisense RNA (HOTAIR) is associated with risk of psoriasis. Int J Immunogenet. 2020;47(5):430–434. doi:10.1111/iji.12482
43. Nour ZA, Elwan Y, Nassar Y, et al. Possible role of LncRNA MEG3-microRNA-21 and endoplasmic reticulum (ER) stress proteins in the pathogenesis of psoriasis vulgaris. Rep Biochem Mol Biol. 2022;11(3):367. doi:10.52547/rbmb.11.3.367
44. Shefler A, Patrick MT, Wasikowski R, et al. Skin-expressing lncRNAs in inflammatory responses. Front Genet. 2022;13:835740. doi:10.3389/fgene.2022.835740
45. Botchkareva NV. The molecular revolution in cutaneous biology: noncoding RNAs: new molecular players in dermatology and cutaneous biology. J Invest Dermatol. 2017;137(5):e105–e111. doi:10.1016/j.jid.2017.02.001
46. Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin Exp Dermatol. 2008;33(3):312–315. doi:10.1111/j.1365-2230.2008.02804.x
47. Qiao M, Li R, Zhao X, et al. Up-regulated lncRNA-MSX2P1 promotes the growth of IL-22-stimulated keratinocytes by inhibiting miR-6731-5p and activating S100A7. Exp Cell Res. 2018;363(2):243–254. doi:10.1016/j.yexcr.2018.01.014
48. Rebane A, Akdis CA. MicroRNAs: essential players in the regulation of inflammation. J Allergy Clin Immunol. 2013;132(1):15–26. doi:10.1016/j.jaci.2013.04.011
49. Lee WR, Chou WL, Lin ZC, et al. Laser-assisted nanocarrier delivery to achieve cutaneous siRNA targeting for attenuating psoriasiform dermatitis. J Control Release. 2022;347:590–606. doi:10.1016/j.jconrel.2022.05.032
50. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi:10.1038/nrg1379
51. Hanna E, Rémuzat C, Auquier P, et al. Gene therapies development: slow progress and promising prospect. J Mark Access Health Policy. 2017;5(1):1265293. doi:10.1080/20016689.2017.1265293
52. Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9(1):40–46. doi:10.1038/nm804
53. Li J, Li X, Zhang Y, et al. Gene therapy for psoriasis in the K14-VEGF transgenic mouse model by topical transdermal delivery of interleukin-4 using ultradeformable cationic liposome. J Gene Med. 2010;12(6):481–490. doi:10.1002/jgm.1459
54. Zhang Y, Li J, Liu CY, et al. A novel transdermal plasmid-dimethylsulfoxide delivery technique for treatment of psoriasis. Dermatology. 2010;221(1):84–92. doi:10.1159/000314154
55. Chang T, Sun L, Wang Y, et al. Inhibition of keratin 17 expression with antisense and RNAi strategies: exploring novel therapy for psoriasis. Exp Dermatol. 2011;20(7):555–560. doi:10.1111/j.1600-0625.2010.01235.x
56. Zibert JR, Wallbrecht K, Schön M, et al. Halting angiogenesis by non-viral somatic gene therapy alleviates psoriasis and murine psoriasiform skin lesions. J Clin Invest. 2011;121(1):410–421. doi:10.1172/JCI41295
57. Guinea-Viniegra J, Jiménez M, Schonthaler HB, et al. Targeting miR-21 to treat psoriasis. Sci Transl Med. 2014;6(225):225re1. doi:10.1126/scitranslmed.3008089
58. Xu N, Meisgen F, Butler LM, et al. MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J Immunol. 2013;190(2):678–688. doi:10.4049/jimmunol.1202695
59. García-Rodríguez S, Arias-Santiago S, Blasco-Morente G, et al. Increased expression of microRNA-155 in peripheral blood mononuclear cells from psoriasis patients is related to disease activity. J Eur Acad Dermatol Venereol. 2017;31(2):312–322. doi:10.1111/jdv.13861
60. Liu Q, Wu DH, Han L, et al. Roles of micro RNAs in psoriasis: immunological functions and potential biomarkers. Exp Dermatol. 2017;26(4):359–367. doi:10.1111/exd.13249
61. Bumcrot D, Manoharan M, Koteliansky V, et al. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi:10.1038/nchembio839
62. Desmet E, Van Gele M, Grine L, et al. Towards the development of a RNAi-based topical treatment for psoriasis: proof-of-concept in a 3D psoriasis skin model. Exp Dermatol. 2018;27(5):463–469. doi:10.1111/exd.13414
63. Mandal A, Kumbhojkar N, Reilly C, et al. Treatment of psoriasis with NFKBIZ siRNA using topical ionic liquid formulations. Sci Adv. 2020;6(30):eabb6049. doi:10.1126/sciadv.abb6049
64. Budakoti M, Panwar AS, Molpa D, et al. Micro-RNA: the darkhorse of cancer. Cell Signal. 2021;83:109995. doi:10.1016/j.cellsig.2021.109995
65. Hawkes JE, Nguyen GH, Fujita M, et al. MicroRNAs in Psoriasis. J Invest Dermatol. 2016;136(2):365–371. doi:10.1038/JID.2015.409
66. Fu D, Yu W, Li M, et al. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol Lett. 2015;166(1):55–62. doi:10.1016/j.imlet.2015.05.014
67. Meisgen F, Xu N, Wei T, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol. 2012;21(4):312–314. doi:10.1111/j.1600-0625.2012.01462.x
68. Wu R, Zeng J, Yuan J, et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 2018;128(6):2551–2568. doi:10.1172/JCI97426
69. Rapalli VK, Singhvi G, Dubey SK, et al. Emerging landscape in psoriasis management: from topical application to targeting biomolecules. Biomed Pharmacother. 2018;106:707–713. doi:10.1016/j.biopha.2018.06.136
70. Yang Z, Zeng B, Tang X, et al. MicroRNA-146a and miR-99a are potential biomarkers for disease activity and clinical efficacy assessment in psoriasis patients treated with traditional Chinese medicine. J Ethnopharmacol. 2016;194:727–732. doi:10.1016/j.jep.2016.08.028
71. Zhang W, Yi X, Guo S, et al. A single-nucleotide polymorphism of miR-146a and psoriasis: an association and functional study. J Cell Mol Med. 2014;18(11):2225–2234. doi:10.1111/jcmm.12359
72. Chimenti MS, Perricone C, D’Antonio A, et al. Genetics, epigenetics, and gender impact in axial-spondyloarthritis susceptibility: an update on genetic polymorphisms and their sex related associations. Front Genet. 2021;12:671976. doi:10.3389/fgene.2021.671976
73. van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi:10.4049/jimmunol.0802999
74. Wu R, Li X, Li S, et al. Decreased microRNA-126 expression in psoriatic CD4 + T cells promotes T-helper 17 cell differentiation and the formation of dermatitis in imiquimod-induced psoriasis-like mice. J Dermatol. 2022;49(4):432–440. doi:10.1111/1346-8138.16272
75. Yan JJ, Qiao M, Li RH, et al. Downregulation of miR-145-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. Br J Dermatol. 2019;180(2):365–372. doi:10.1111/bjd.17256
76. Srivastava A, Nikamo P, Lohcharoenkal W, et al. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol. 2017;139(2):550–561. doi:10.1016/j.jaci.2016.07.025
77. Hermann H, Runnel T, Aab A, et al. MiR-146b probably assists miRNA-146a in the suppression of keratinocyte proliferation and inflammatory responses in psoriasis. J Invest Dermatol. 2017;137(9):1945–1954. doi:10.1016/j.jid.2017.05.012
78. Meng Y, Li J, Ye Z, et al. MicroRNA-148a facilitates inflammatory dendritic cell differentiation and autoimmunity by targeting MAFB. JCI Insight. 2020;5(8):e133721. doi:10.1172/jci.insight.133721
79. Srivastava A, Luo L, Lohcharoenkal W, et al. Cross-talk between IFN-γ and TWEAK through miR-149 amplifies skin inflammation in psoriasis. J Allergy Clin Immunol. 2021;147(6):
80. Guo W, Xu F, Zhuang Z, et al. Ebosin ameliorates psoriasis-like inflammation of mice via miR-155 targeting tnfaip3 on IL-17 pathway. Front Immunol. 2021;12:662362. doi:10.3389/fimmu.2021.662362
81. Huang C, Zhong W, Ren X, et al. MiR-193b-3p-ERBB4 axis regulates psoriasis pathogenesis via modulating cellular proliferation and inflammatory-mediator production of keratinocytes. Cell Death Dis. 2021;12(11):963. doi:10.1038/s41419-021-04230-5
82. Xue Y, Liu Y, Bian X, et al. MiR-205-5p inhibits psoriasis-associated proliferation and angiogenesis: wnt/β-catenin and mitogen-activated protein kinase signaling pathway are involved. J Dermatol. 2020;47(8):882–892. doi:10.1111/1346-8138.15370
83. Feng H, Wu R, Zhang S, et al. Topical administration of nanocarrier miRNA-210 antisense ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Dermatol. 2020;47(2):147–154. doi:10.1111/1346-8138.15149
84. Abdallah F, Henriet E, Suet A, et al. MiR-21-3p/IL-22 axes are major drivers of psoriasis pathogenesis by modulating keratinocytes proliferation-survival balance and inflammatory response. Cells. 2021;10(10):2547. doi:10.3390/cells10102547
85. Liu A, Zhang B, Zhao W, et al. MicroRNA-215-5p inhibits the proliferation of keratinocytes and alleviates psoriasis-like inflammation by negatively regulating DYRK1A and its downstream signalling pathways. Exp Dermatol. 2021;30(7):
86. Bian J, Liu R, Fan T, et al. miR-340 alleviates psoriasis in mice through direct targeting of IL-17A. J Immunol. 2018;201(5):1412–1420. doi:10.4049/jimmunol.1800189
87. Wu Y, Liu L, Bian C, et al. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Commun Signal. 2018;16(1):58. doi:10.1186/s12964-018-0271-9
88. Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48(4):398–406. doi:10.1038/ng.3525
89. Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44(9):1006–1014. doi:10.1038/ng.2359
90. Han G, Williams CA, Salter K, et al. A role for TGFbeta signaling in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130(2):371–377. doi:10.1038/jid.2009.252
91. Li AG, Wang D, Feng XH, et al. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23(8):1770–1781. doi:10.1038/sj.emboj.7600183
92. Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9(6):650–657. doi:10.1038/ni.1613
93. Singh TP, Schön MP, Wallbrecht K, et al. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS One. 2013;8(1):e51752.
94. Mao CM, Yang X, Cheng X, et al. Establishment of keratinocyte-specific Cre recombinase transgenic mice. Yi Chuan Xue Bao. 2003;30(5):407–413.
95. Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi:10.1016/S1074-7613(02)00391-6
96. Meng Z, Lu M. RNA Interference-induced innate immunity, off-target effect, or immune adjuvant? Front Immunol. 2017;8:331. doi:10.3389/fimmu.2017.00331
97. Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437(7057):369–375. doi:10.1038/nature03963
98. Traber GM, Yu AM. RNAi-based therapeutics and novel RNA bioengineering technologies. J Pharmacol Exp Ther. 2023;384(1):133–154. doi:10.1124/jpet.122.001234
99. Li Z, Rana TM. Molecular mechanisms of RNA-triggered gene silencing machineries. Acc Chem Res. 2012;45(7):1122–1131. doi:10.1021/ar200253u
100. Caplen NJ, Parrish S, Imani F, et al. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci U S A. 2001;98(17):9742–9747. doi:10.1073/pnas.171251798
101. Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):
102. McCaffrey AP, Meuse L, Pham TT, et al. RNA interference in adult mice. Nature. 2002;418(6893):
103. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):
104. Raoul C, Abbas-Terki T, Bensadoun JC, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11(4):423–428. doi:10.1038/nm1207
105. Fan T, Wang S, Yu L, et al. Treating psoriasis by targeting its susceptibility gene Rel. Clin Immunol. 2016;165:47–54. doi:10.1016/j.clim.2016.03.009
106. Hsieh WL, Huang YH, Wang TM, et al. IFI27, a novel epidermal growth factor-stabilized protein, is functionally involved in proliferation and cell cycling of human epidermal keratinocytes. Cell Prolif. 2015;48(2):187–197. doi:10.1111/cpr.12168
107. Xiao CY, Zhu ZL, Zhang C, et al. Small interfering RNA targeting of keratin 17 reduces inflammation in imiquimod-induced psoriasis-like dermatitis. Chin Med J. 2020;133(24):2910–2918. doi:10.1097/CM9.0000000000001197
108. Luan C, Chen X, Zhu Y, et al. Potentiation of psoriasis-like inflammation by PCSK9. J Invest Dermatol. 2019;139(4):
109. Gao Y, Lu J, Bao X, et al. Inhibition of phospholipases suppresses progression of psoriasis through modulation of inflammation. Exp Biol Med. 2021;246(11):1253–1262. doi:10.1177/1535370221993424
110. Chen Y, Yan H, Song Z, et al. Downregulation of TNIP1 expression leads to increased proliferation of human keratinocytes and severer psoriasis-like conditions in an imiquimod-induced mouse model of dermatitis. PLoS One. 2015;10(6):e0127957.
111. Yang L, Zhang T, Zhang C, et al. Upregulated E3 ligase tripartite motif-containing protein 21 in psoriatic epidermis ubiquitylates nuclear factor-κB p65 subunit and promotes inflammation in keratinocytes. Br J Dermatol. 2021;184(1):111–122. doi:10.1111/bjd.19057
112. Jakobsen M, Stenderup K, Rosada C, et al. Amelioration of psoriasis by anti-TNF-alpha RNAi in the xenograft transplantation model. Mol Ther. 2009;17(10):1743–1753. doi:10.1038/mt.2009.141
113. Bracke S, Carretero M, Guerrero-Aspizua S, et al. Targeted silencing of DEFB 4 in a bioengineered skin-humanized mouse model for psoriasis: development of si RNA SEC osome-based novel therapies. Exp Dermatol. 2014;23(3):199–201. doi:10.1111/exd.12321
114. Johansen C, Funding AT, Otkjaer K, et al. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176(3):1431–1438. doi:10.4049/jimmunol.176.3.1431
115. Dam TN, Kang S, Nickoloff BJ, et al. 1α,25-dihydroxycholecalciferol and cyclosporine suppress induction and promote resolution of psoriasis in human skin grafts transplanted on to SCID mice. J Invest Dermatol. 1999;113(6):1082–1089. doi:10.1046/j.1523-1747.1999.00811.x
116. Wang X, Sun J, Hu J, Slominski AT. IMQ Induced K14-VEGF mouse: a stable and long-term mouse model of psoriasis-like inflammation. PLoS One. 2015;10(12):e0145498. doi:10.1371/journal.pone.0145498
117. Mellett M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138(9):2010–2023. doi:10.1016/j.jid.2018.03.1525
118. Wan T, Pan Q, Ping Y. Microneedle-assisted genome editing: a transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci Adv. 2021;7(11):eabe2888. doi:10.1126/sciadv.abe2888
119. Wolfram JA, Diaconu D, Hatala DA, et al. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174(4):1443–1458. doi:10.2353/ajpath.2009.080858
120. Jin J, Xue N, Liu Y, et al. A novel S1P1 modulator IMMH002 ameliorates psoriasis in multiple animal models. Acta Pharm Sin B. 2020;10(2):276–288. doi:10.1016/j.apsb.2019.11.006
121. Li Y, Golden JB, Camhi MI, et al. Protection from psoriasis-related thrombosis after inhibition of IL-23 or IL-17A. J Invest Dermatol. 2018;138:2.
122. Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi:10.1016/S0140-6736(15)00149-X
123. Capon F. The Genetic basis of psoriasis. Int J Mol Sci. 2017;18(12):2526. doi:10.3390/ijms18122526
124. Bhat P, Garibyan L. The potential of CRISPR-guided therapies in the dermatology clinic. JID Innov. 2022;2(2):100103. doi:10.1016/j.xjidi.2022.100103
125. D’Adamio S, Silvaggio D, Lombardo P, et al. The safety of anti-interleukins monoclonal antibodies for the treatment of psoriasis. Expert Opin Drug Saf. 2019;18(11):1031–1041. doi:10.1080/14740338.2019.1663168
126. Parab S, Doshi G. The experimental animal models in psoriasis research: a comprehensive review. Int Immunopharmacol. 2023;117:109897. doi:10.1016/j.intimp.2023.109897
127. Swindell WR, Johnston A, Carbajal S, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS One. 2011;6(4):e18266. doi:10.1371/journal.pone.0018266
128. Garber K. Psoriasis: from bed to bench and back. Nat Biotechnol. 2011;29(7):563–566. doi:10.1038/nbt.1906
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.