Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Risk of Subsequent Breast Cancer in Women with Early Stage HER2-Positive Breast Cancer in a Large Community Health Plan
Authors Haque R , Chen LH , Oestreicher N, Lalla D, Chlebowski RT
Received 20 May 2023
Accepted for publication 9 August 2023
Published 16 August 2023 Volume 2023:15 Pages 637—645
DOI https://doi.org/10.2147/BCTT.S420061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Reina Haque,1,2 Lie Hong Chen,1 Nina Oestreicher,3 Deepa Lalla,3 Rowan T Chlebowski4
1Kaiser Permanente Southern California, Department of Research & Evaluation, Pasadena, CA, USA; 2Kaiser Permanente School of Medicine, Department of Health Systems Science, Pasadena, CA, USA; 3PUMA Biotechnology, Los Angeles, CA, USA; 4Lundquist Research Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, USA
Correspondence: Reina Haque, Kaiser Permanente, Research & Evaluation, Kaiser Permanente Bernard J. Tyson School of Medicine, Health Systems Science, 100 South Los Robles, 2nd Floor, Pasadena, CA, 91101, USA, Tel +1 626 564-5707, Email [email protected]
Purpose: Clinical outcomes have improved for women with early stage, HER2-positive breast cancer following the FDA approval of adjuvant trastuzumab use in 2006. However, only limited information exists on such patients’ outcomes in real-world settings outside of clinical trials. We examined the risk of subsequent breast cancer in women with HER-2 positive disease, and the impact of trastuzumab use, in a large California community-based health plan.
Patients and Methods: A cohort of 3550 women with HER2-positive breast cancer (stages I–III) from 2009– 2017 were followed through December 2018. We calculated subsequent breast cancer (SBC) rates overall and by trastuzumab use. Multivariable Cox proportional hazards modeling was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for SBC by trastuzumab use.
Results: Within the cohort diagnosed with HER2-positive disease, 81% received adjuvant trastuzumab. After 4.1 mean years follow-up (maximum 10 years), the risk of SBC was 22% lower with adjuvant trastuzumab use (hazard ratio [HR] = 0.78, 95% confidence interval [CI]: 0.66– 0.92) compared with non-use. The cumulative incidence of SBC precipitously rose two years after diagnosis and by the 10th year, the cumulative incidence was 31% among those who had trastuzumab therapy versus 34% without this therapy.
Conclusion: In community practice settings, the cumulative incidence of SBC in patients with early stage HER2-positive BC was 31% at 10 years in a cohort treated with adjuvant trastuzumab. Trastuzumab use was associated with a 22% reduced risk of developing SBC. This residual disease burden suggests breast cancer outcomes may be improved with further treatment given the advent of next-generation HER2-targeted therapies.
Keywords: breast cancer, survival, mortality, HER2 positive, recurrence, relapse, cohort study, real world
Introduction
About 15–20% of breast cancers have amplification of the human epidermal growth factor receptor 2 (HER2) gene or HER2 protein overexpression (“HER2-positive”), which is associated with poor clinical outcomes.1 Following the demonstration that trastuzumab, a humanized monoclonal antibody that binds close to the trans-membrane domain of the HER2 protein, resulted in improved survival in HER2-positive metastatic breast cancer in combination with chemotherapy,2 clinical trials of adjuvant trastuzumab were initiated.3 In 2005, early results were reported from three Phase III adjuvant trials in patients with HER2-positive, early breast cancer comparing chemotherapy alone with chemotherapy plus trastuzumab. Findings from a joint analysis including 4046 patients from National Surgical Adjuvant Breast and Bowel Project trial (NSABP B-31) and North Central Cancer Treatment Group trial (NCCTG N9831)4 and 5102 patients from the Herceptin Adjuvant (HERA) trial5 were published. Both showed substantial reductions in recurrence risk with trastuzumab in addition to chemotherapy of a magnitude not previously seen. Comparable findings were subsequently reported for trastuzumab in addition to non-anthracycline chemotherapy.2 After 8.4 years median follow-up of the NSABP and NCCTG trials, a 37% increase in disease-free survival (DFS) and a 40% increase in overall survival (OS)6 were shown. Similarly, in the HERA trial after 11 years median follow-up, a 24% increase in DFS and a 24% increase in OS was reported.7 Taken together, these findings represent major treatment advances for patients with HER2-positive breast cancer.
During the years of this present study, the US FDA indications for adjuvant trastuzumab use were based on recurrence risk. Trastuzumab is indicated for patients with HER2-positive early breast cancers who were: (1) node positive; (2) node negative and also ER-negative (estrogen receptor) and PR-negative (progesterone receptor); and (3) node negative, ER-positive and PR-positive with at least one high-risk feature (defined as having tumor size >2 cm, age <35 years, or tumor grade 2 or 3). While newer HER2-directed therapy regimens may now be available, such as pertuzumab, a humanized monoclonal antibody which binds to the dimerization domain of the HER2 protein resulting in cell-mediated cytotoxicity, this drug was initially for metastatic breast cancer8–10 and was only recently FDA approved in December 201711 for adjuvant therapy for patients with early stage breast cancer based on the APHINITY trial findings.12,13 Hence, population-based estimates of recurrence risk in patients with early stage HER2-positive tumors treated with and without adjuvant trastuzumab are not established.
Despite the treatment advances described above, approximately one quarter of women with HER2-positive, early breast cancer who received trastuzumab-based adjuvant therapy in clinical trial settings, developed recurrence within 8–10 years.6,7,14,15 In combined analysis of NCCTG N9831 and NSABP B-31, after 8 years median follow-up, information on recurrence-free survival (RFS), which included deaths from breast cancer was determined. In women receiving adjuvant trastuzumab, 10-year RFS was 81.4% and 77.8% in hormone-receptor positive and negative cases, respectively.16
In the literature, data on outcomes in women with HER2-positive, early breast cancer in clinical practice settings outside of clinical trial reports are rare.17 Specifically, the recurrent course of HER2-positive breast cancer in real-world settings is not established. Thus, our goal was to examine the risk of subsequent breast cancer (SBC) events in a large diverse group of women with early HER2-positive breast cancer in a managed health-care plan.
Methods
Study Design, Subjects and Setting
This cohort study was conducted at Kaiser Permanente Southern California (KPSC), a vertically integrated health-care system that includes 15 hospitals with over 200 medical clinics that serves nearly 4.7 million members. A cohort of 23,876 women with breast cancer (AJCC TNM stage I–IV), diagnosed from 2009–2017 was identified from the KPSC health plan’s NCI-SEER affiliated tumor registry. Women were followed through December 2018. Of these, 3777 women had HER2-positive stage I–IV disease. Data elements were extracted from the tumor registry and electronic health records including manual chart reviews. The final study group included patients with stage I–III disease, N = 3550. The KPSC Institutional Review Board (IRB) reviewed and approved this study; as the study was based on electronic health records (EHR) collected during routine health-care, written or verbal patient informed consent was waived for the collection, analysis and publication of the retrospectively obtained data for this non-interventional study. The KPSC IRB operates in accordance with the Declaration of Helsinki. After the EHR data linkages, we de-identified the final analytic dataset to maintain patient confidentiality. Additionally, access to the final dataset was limited to the statistician (LHC), and it was stored on the KPSC mainframe which requires knowledge of programming language and multiple passwords, thereby enhancing confidentiality.
Study Outcome
We identified subsequent breast cancer (SBC) events as those that occurred ≥6 months after initial surgery date through the end of subjects’ follow-up. Six months was used as the cut-off as the health plan’s SEER-affiliated tumor registry completes data extraction of primary tumor characteristics and their treatments at this point. The earliest of the following events that occurred before the study’s end (December 2018) was determined to be the SBC event: (1) developed recurrence at least six months post initial diagnosis; (2) developed a second primary breast cancer in the contralateral breast; or (3) died due to breast cancer. Recurrence was defined as invasive breast cancer occurring in the ipsilateral breast, or regional or distant metastasis. For 2/3 of the cohort (N = 2405), recurrences were identified through manual review of available pathology reports. For the remaining 1/3 of the cohort without pathology reports (n = 1145), we reviewed all available radiology reports post 6 months after the surgery date (n = 505) or extracted electronic health data to identify health-care utilization patterns and diagnoses indicative of recurrence or metastases (n = 640). This hybrid approach of manually reviewing pathology text supplemented with an automated data algorithm that examined encounter, procedure and clinical codes indicative of SBC was validated and yielded robust test characteristics (sensitivity 96.7%; specificity 92.1%).18 Second primary breast cancers in the contralateral (opposite) breast were identified through the tumor registry. Breast cancer deaths (ICD-10-CM C50.x) and their dates were ascertained from the health plan’s inpatient, national and state death database. SBC was examined as a composite outcome to ensure adequate sample size for robust statistical analyses.
Trastuzumab Use and Covariates
Trastuzumab use and dates were captured from the infusion databases. Use of pertuzumab, although less common, was also captured. Covariates included race and ethnicity; geocoded median household income; hospital; body mass index at diagnosis; Charlson Comorbidity Index one year before breast cancer diagnosis; age and stage at diagnosis; diagnosis year; histology; primary cancer treatments (surgery and adjuvant chemotherapy, radiation, and hormonal treatments [tamoxifen, aromatase inhibitors]); tumor size; lymph node status; and ER, PR, and HER2 status assessed by immunohistochemical or fluorescence in situ hybridization (FISH) techniques. Geocoded median household income (based on census tract and block group numbers of patient addresses), was determined via linkage with the 2010 US Census database to obtain socioeconomic information.19 Dates and causes of death were extracted from inpatient records and state and national death databases.
Statistical Analysis
Differences in demographics, tumor characteristics, primary cancer treatments, and covariates were examined by trastuzumab use by comparing frequency distributions and χ2 or Fisher exact tests (P-values were two-sided). Additionally, we computed person-year rates for SBC by exposure to adjuvant trastuzumab. We followed women from the index date up to the date of SBC, health plan disenrollment, date of death or end of study (December 2018), whichever occurred first. Multivariable Cox proportional hazards regression was used to estimate the adjusted hazard ratios (HR) and corresponding 95% confidence intervals for the association between SBC and adjuvant trastuzumab accounting for covariates. All adjuvant treatments, including trastuzumab use, were treated as time-dependent variables. HRs were adjusted for age; year of diagnosis; stage; hospital; race and ethnicity; body mass index; Charlson comorbidity status; geocoded median household income; ER status; PR status; lymph nodes (positive/negative); tumor size; grade; histology; surgery type; and primary adjuvant therapy (receipt of radiation, chemotherapy, hormonal therapy); and annualized numbers of office visits. The proportional hazard assumption was evaluated by assessing interactions between covariates with time and with Schoenfeld residuals; no significant violations were found. We graphed the cumulative incidence of SBC adjusted for the aforementioned covariates by trastuzumab use status.
We had a low percentage of missing values of the covariates, therefore, missing values (<3% for most variables) were handled as an additional category in all models. This was determined based on a complete-case assessment, restricting the analysis to individuals with no missing covariate data. Given there were no material differences in these two approaches, we presented the results based on the full cohort. We used SAS 9.4 (SAS Institute, Cary NC) for data collection and analyses.
Results
In the cohort of 3550 women with HER2-positive breast cancer (15% of the base cohort of N = 23,876), the majority were diagnosed with stage I or II disease (stage I: 41%; stage II: 43%; stage III: 16%). Table 1 displays the demographic characteristics of the cohort by trastuzumab use. Roughly 80% (N = 2875) of the cohort underwent trastuzumab therapy. The cohort was diverse and included: 16% Asian/Pacific Islanders (PI); 13% Black women; 24% Hispanic women; 46% White women; and 1% of other/mixed backgrounds. Baseline demographics characteristics were generally similar in those who received trastuzumab versus those who did not. However, trastuzumab use was greater among those who were younger (mean age: 56 vs 64 years); had no comorbid conditions (65% vs 58%); and had obesity (37% vs 29%), than those who did not receive this therapy, respectively (P<0.05 for all variables).
 |
Table 1 Baseline Demographic Characteristics of Women with HER2-Positive Breast Cancer (AJCC TNM Stages I–III) Diagnosed 2009–2017 by Adjuvant Trastuzumab |
Table 2 displays tumor characteristics and primary cancer treatment by trastuzumab use. Trastuzumab use was greater in those who underwent mastectomy (59% vs 52%); higher grade (59% vs 46%); and had positive lymph nodes (37% vs 20%) compared with trastuzumab non-users, respectively (P<0.001 for all variables).
 |
Table 2 Tumor Characteristics Among Women with HER2-Positive Breast Cancer by Adjuvant Trastuzumab Use |
About 20% of the cohort did not undergo trastuzumab therapy; as expected, they were more likely to be older, have more comorbidity, diagnosed with Stage I disease, and have smaller sized tumors (Table 1 and Table 2). Less than 20 women (0.5%) used neoadjuvant trastuzumab (data not shown).
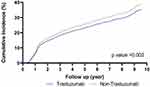
Overall, we identified 1101 women who developed SBC during the ensuing 10 years of follow-up (mean 4.1 years, interquartile range [IQR]: 1.5–6.3). The mean time to SBC was 2.0 years (IQR: 0.9–2.5 years). The SBC rate was lower in women who underwent adjuvant trastuzumab therapy (73.5/1000 PY) than those who did not (90.8/1000 PY), corresponding to 22% lower risk (adjusted HR = 0.78, 95% CI: 0.66–0.91) (Table 3). Compared with women diagnosed at stage I, those with stage II–III disease were 1.19 times more likely to develop SBC (adjusted HR = 1.19, 95% CI: 1.01–1.40). Tumor size ≥2 cm was associated with a 23% greater SBC risk (HR = 1.23, 95% CI: 1.00–1.51) versus tumors <2 cm. The fully adjusted multivariable model included these covariables (Model 2, Table 3): age at diagnosis; diagnosis year; stage; race/ethnicity; geocoded income; ER/PR; surgery type; adjuvant therapy (hormonal, radiation, other chemotherapy); lymph node status; histology; BMI; comorbidity; and neoadjuvant trastuzumab. Correspondingly, Figure 1 shows that adjusted cumulative incidence of subsequent breast cancer continued to rise steeply over 10 years, and more sharply in the non-trastuzumab group (P = 0.002). Around 10 years, the cumulative SBC incidence was nearly 31% in those who had trastuzumab, while it reached nearly 34% for those not treated with trastuzumab.
 |
Table 3 Rates and Hazard Ratios of Subsequent Breast Cancer (SBC) in Women with HER2-Positive Breast Cancer |
Discussion
In this large cohort of women with HER2-positive breast cancer in a large community health plan, trastuzumab use was significantly associated with a 22% lower risk of SBC events compared with women who did not undergo this therapy. Despite the favorable findings associated with adjuvant trastuzumab use, SBC events persisted with an adjusted cumulative risk of approximately 31% over the 10-year follow-up. Our findings on SBC risk are consistent with clinical trials results that examined local/regional recurrences.6,7 In the combined NSABP and NCCTG report, DFS events were 37% lower in the trastuzumab group.6 In the HERA trial, DFS events were 24% lower in the trastuzumab group.7
To our knowledge, this is the largest population-based study to examine breast cancer outcomes in patients with HER2-positive breast cancer by trastuzumab use, providing information on outcomes of women with HER2-positive disease whose therapy did and did not include trastuzumab. A prior registry-based report from Finland of 6977 women with breast cancer diagnosed during 2005–2018 included 832 (14.2%) HER2-positive cases where 76.5% received HER2-targeted therapy.17 While outcomes by adjuvant trastuzumab use were not provided in that Finnish study, the 5-year “real world” DFS (rwDFS) of the HER2-positive women was 76.2%. There were similar findings in a cohort of 2046 Italian women with early breast cancer treated with trastuzumab in clinical practice in 2006–2009 where 75% of women were disease-free after 4 years of follow-up.20 In both reports, the recurrence risks were higher in the observational studies compared with the clinical trials.6,7
Up until the last year of our study data collection (2018), the FDA had not approved any HER2-targeted therapy for use following trastuzumab-based adjuvant therapy. In 2017, neratinib, an oral pan-HER2 tyrosine kinase inhibitor, was FDA approved as extended adjuvant therapy. In the ExteNET trial of 2840 women, neratinib, when given after trastuzumab-based adjuvant or neoadjuvant therapy, significantly improved invasive DFS and fewer central nervous system recurrences were observed.21 Diarrhea was the most common grade III toxicity, which can be reduced by preemptive prophylaxis or neratinib dose escalation.15,22 In 2019, ado-trastuzumab emtansine (T-DM1), an antibody-drug conjugate, was approved as adjuvant therapy for patients with residual disease post-neoadjuvant therapy. In the KATHERINE trial, among 1480 women with HER2-positive early breast cancer with residual invasive disease following trastuzumab-based neoadjuvant therapy, the risk of invasive DFS, which included death as a component measure, was significantly reduced by 50% with adjuvant T-DM1 compared with the standard of continuing trastuzumab after surgery.23 Also, the FDA granted regular approval to pertuzumab for adjuvant treatment in 2017 for early stage breast cancer.24 Despite these newer HER2-directed therapies, long enough follow-up time has not yet accrued to comprehensively understand the benefit and risk profiles; thus, future studies are needed to evaluate the impact of these newer HER2-directed therapies.
This study has several strengths. First, our results from unselected patients in a large community health plan enhances the generalizability of results from clinical trials. For example, 54% of the women in our cohort were of diverse racial/ethnic backgrounds. Second, the identification of SBC events, based in part on use of comprehensive EHRs, has been previously validated.18 Third, we were able to examine outcomes without the confounding effect of variable healthcare access. Additionally, we were able to account for a comprehensive set of covariates – both clinical and demographic factors – such as comorbidity status, geocoded income, tumor characteristics, and adjuvant cancer therapies.
This study has certain limitations. Therapy associations with breast cancer outcomes in a population-based cohort may differ from randomized clinical trial evidence because in community practices, physician and patient choices and underlying comorbidities may influence therapy selection. To try to address this, we accounted for comorbidity in the analysis. Also, the low use of neoadjuvant therapy in this cohort for early-stage breast cancer limited our evaluation. However, given that the FDA only recently approved pertuzumab in 2017 and ado-trastuzumab emtansine (T-DM1) in May 2019 for use in women with early stage as additional options for HER-2 directed adjuvant therapies, future studies are warranted to examine their long-term effects in population-based studies.25 Another area of investigation for the HER2-targeted therapies approved after trastuzumab will be to examine their effectiveness, and more broadly their risk-benefit profiles in typical clinical practice settings.
Conclusion
In summary, in community practice settings, trastuzumab use was associated with a statistically significant 22% reduction in SBC. Despite trastuzumab therapy, cumulative risk of SBC events persisted even at 10 years. These findings extend those from clinical trials in women with HER2-positive breast cancer. Current study findings support the need for clinicians in community practices to consider use of some of the newer HER2-targeted therapy options.
Data Sharing Statement
This dataset includes identifiers; thus investigators cannot share the dataset at this time. However, de-identified dataset may be made available to requestors following data use agreements and KPSC IRB approval. Please contact corresponding author.
Ethics Approval and Informed Consent
The KPSC Institutional Review Board reviewed and approved this study; as the study was based on electronic health records (EHR), written or verbal patient consent was waived.
Acknowledgments
The authors thank the patients of Kaiser Permanente for helping to improve care with information collected through the electronic health record systems.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Kaiser Permanente Southern California received research support from PUMA Biotechnology.
Disclosure
Dr Reina Haque reports Kaiser Permanente Southern California received research support from AstraZeneca for a study outside submitted work. Dr Nina Oestreicher is a former employee of Puma Biotechnology. Dr Deepa Lalla is affiliated with PUMA Biotechnology. Dr Rowan T Chlebowski reports personal fees from Up-to-Date, Novartis, and AstraZeneca, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/ neu oncogene. Science. 1987;235:177–182. doi:10.1126/science.3798106
2. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi:10.1056/NEJM200103153441101
3. Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi:10.1056/NEJMe058196
4. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi:10.1056/NEJMoa052122
5. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi:10.1056/NEJMoa052306
6. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi:10.1200/JCO.2014.55.5730
7. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi:10.1016/S0140-6736(16)32616-2
8. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi:10.1056/NEJMoa1413513
9. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13:25–32. doi:10.1016/S1470-2045(11)70336-9
10. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized Phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–2284. doi:10.1093/annonc/mdt182
11. Howie LJ, Scher NS, Amiri-Kordestani L, et al. FDA approval summary: pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res. 2019;25:2949–2955. doi:10.1158/1078-0432.CCR-18-3003
12. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi:10.1056/NEJMoa1703643
13. Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39:1448–1457. doi:10.1200/JCO.20.01204
14. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–1028. doi:10.1016/S0140-6736(13)61094-6
15. Chan A, Moy B, Mansi J, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21:80–91.e7. doi:10.1016/j.clbc.2020.09.014
16. Chumsri S, Li Z, Serie DJ, et al. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: combined analysis of NCCTG N9831 (alliance) and NRG oncology/NSABP B-31. J Clin Oncol. 2019;37:3425–3435. doi:10.1200/JCO.19.00443
17. Teerenhovi H, Tuominen S, Nurmi-Rantala S, et al. Real-world clinical outcomes in biological subgroups of breast cancer in the hospital district of Southwest Finland. Oncologist. 2021;26:e1372–e1380. doi:10.1002/onco.13813
18. Haque R, Shi J, Schottinger JE, et al. A hybrid approach to identify subsequent breast cancer using pathology and automated health information data. Med Care. 2015;53:380–385. doi:10.1097/MLR.0000000000000327
19. Chen FM, Breiman RF, Farley M, Plikaytis B, Deaver K, Cetron MS. Geocoding and linking data from population-based surveillance and the US Census to evaluate the impact of median household income on the epidemiology of invasive Streptococcus pneumoniae infections. Am J Epidemiol. 1998;148(12):1212–1218. doi:10.1093/oxfordjournals.aje.a009611
20. Bonifazi M, Franchi M, Rossi M, et al. Long term survival of HER2-positive early breast cancer treated with trastuzumab-based adjuvant regimen: a large cohort study from clinical practice. Breast. 2014;23:573–578. doi:10.1016/j.breast.2014.05.022
21. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi:10.1016/S1470-2045(17)30717-9
22. Barcenas CH, Hurvitz SA, Di Palma JA, et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol. 2020;31:1223–1230. doi:10.1016/j.annonc.2020.05.012
23. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi:10.1056/NEJMoa1814017
24. US Food & Drug Administration. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pertuzumab-adjuvant-treatment-her2-positive-breast-cancer.
25. US Food & Drug Administration. FDA grants regular approval to pertuzumab for adjuvant treatment of HER2-positive breast cancer. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pertuzumab-adjuvant-treatment-her2-positive-breast-cancer.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.