Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
Risk Factors for Hepatitis B Virus Infection in North Ethiopia: A Case–Control Study
Authors Weldebrhan D , Berhe H, Tesfay Y
Received 11 March 2023
Accepted for publication 4 July 2023
Published 19 July 2023 Volume 2023:15 Pages 79—91
DOI https://doi.org/10.2147/HMER.S407069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Video abstract presented by Desalegn Bekru.
Views: 94
Desalegn Weldebrhan,1 Hailemariam Berhe,2 Yohannes Tesfay2
1Department of Adult Health Nursing, Faculty of Nursing, Mekelle University, Tigray, Ethiopia; 2Department of Reproductive Health and Maternity Nursing, Faculty of Nursing, Mekelle University, Tigray, Ethiopia
Correspondence: Desalegn Weldebrhan, Email [email protected]
Background: Hepatitis B virus infection (HBV) is an important clinical and public health problem that contributes to liver-related public health morbidity and mortality. Although childhood vaccination was introduced in 1980, hospital admissions, morbidity and mortality rates from HBV infection increased in Ethiopia. Risk factors for HBV infection and associated complications generally vary from case to case. No epidemiological studies have identified the risk factors for HBV infection in northern Ethiopia. Therefore, this study aimed to identify risk factors for HBV infection in specialist and teaching hospitals in Ayder.
Methods: From March 2019 to May 2019, an unmatched hospital-based case–control study has been carried out on a total of 213 patients [71 cases and 142 controls] in northern Ethiopia. Cases were selected sequentially and two consecutive controls were selected for each case by a simple random method. The data were collected using pretested questionnaires structured by the interviewer as part of a face-to-face interview. Data were entered in Epi Data version 3.1, exported and analyzed with SPSS version 22. Binary and multivariable logistic regression analyses were used. Statistical significance was given as P < 0.05.
Results: Multivariate logistic regression analysis revealed that patients with familial exposure to hepatitis (AOR 3.7, 95% CI: 1.5– 9.01), prior traditional medical procedure (AOR 1.2, 95% CI: 1.08– 3.4), any history of dental procedures (AOR 3.8, 95% CI: 1.8– 9.01) were associated risk factors to hepatitis B virus infection, and awareness of sexually transmitted hepatitis B virus infection (AOR 0.084, 95% CI: 0.01-0.6) is less likely to be infected with hepatitis B virus infection.
Conclusion: This study (findings) demonstrated that contact with a case of hepatitis in the family, history of dentist visits, prior traditional medical procedure, and lack of awareness of its transmission through sexual contact have been identified as independent risk factors for the development of hepatitis B virus infection.
Keywords: hepatitis B, infection, risk factors
Introduction
Hepatitis B is a potentially life-threatening liver infection induced by the hepatitis B virus (HBV), which is an enveloped deoxygenated ribonucleic acid (DNA) virus. It is an important global health problem that causes chronic infection and increases the risk of dying from cirrhosis and liver cancer. It can range from asymptomatic infection or mild disease to severe or rarely fulminant hepatitis.1–3
The hepatitis B virus is a highly contagious disease, which is 50–100 times more contagious than the human immunodeficiency virus (HIV), and 10 times more contagious than the hepatitis C virus. Many carriers of the hepatitis B virus are unaware of their infection and are therefore referred to as “silent killers”.4 The virus is highly contagious that can be transmitted from mother to child and through contact with contaminated body fluids such as unprotected sex, contaminated medical equipment and blood donations.5
Hepatitis B virus (HBV) infection is one of the major diseases affecting humanity, which has been shown to cause serious public health problems.6 It is estimated that at the end of 2015, around 2 billion people were infected with HBV worldwide and around 325 million people suffered from chronic hepatitis.7 The World Health Organization (WHO) estimates that 257 million people worldwide, or 3.5% of the population suffer from chronic HBV infection. The Africa and Western Pacific region esteemed for 68% of those infected. The prevalence was highest in Africa (6.1%) and the Western Pacific (6.2%). A total of around 257 million people were living with an HBV.8
There are large geographical variations, with the highest prevalence (8%) found in Southeast Asia, China, the Pacific Islands and sub-Saharan Africa.9 Chronic hepatitis B (CHB) has a variable natural history, ranging from an inactive carrier state with an excellent long-term prognosis to progressive liver fibrosis that can lead to the development of cirrhosis and hepatocellular carcinoma (HCC).4 Approximately 20–30% of adults with CHB develop these complications, and an estimated 686,000 people die prematurely due to HBV every year.4
Despite the introduction of global hepatitis B vaccination and effective antiviral therapy, hepatitis B virus infection is indigenous in sub-Saharan Africa, and the estimated overall Seroprevalence of hepatitis B surface antigen remains high at 6.1% within the range of 4.6%–8.5%.10 Ethiopia, as part of sub-Saharan Africa, is recognized a high HBV endemic area. In recent studies, the pooled HBV seropositivity prevalence of hepatitis B virus (HBV) was 7.4%. However, this varies from 5% to 11% between different subgroups ranging from 5.2% in human immunodeficiency virus (HIV) infected individuals, 8.0% in community-based studies, 8.4% in blood donors, 11.0% in Immigrants and 6.9% in other groups.11
According to the latest estimates from the Global Burden of Disease study and the WHO, viral hepatitis is responsible for around 1.34 million deaths annually. This corresponds to the annual number of deaths from HIV/AIDS (1.3 million) and tuberculosis (1.3 million).12 Left untreated, HBV and HCV infection can lead to liver cirrhosis (720,000 deaths) and hepatocellular carcinoma (470,000) deaths. These long-term complications are life-threatening and account for 96% of deaths from viral hepatitis. Mortality from viral hepatitis has increased by 22% since 2000. If people with HBV and HCV infection go undiagnosed and untreated, the number of deaths from viral hepatitis will continue to increase.8
The viral hepatitis pandemic is placing a high burden on lives, communities, and healthcare systems. In general, it is responsible for an estimated 1.4 million deaths per year from acute infection and hepatitis-related complications. About 47% of those complications-related deaths are due to hepatitis B virus.13 In many countries, viral hepatitis is the most common of liver transplantation. The end-stage treatments are expensive, easily costing hundreds of thousands of dollars per person.14
As pointed out in various scientific literatures, HBV infection is caused by demographic, healthcare service-related and behavioural factors.15–17 Studies conducted in developed countries showed that contact history of hepatitis cases in households, blood transfusion, jaundice, body piercing, male gender, low level of education, lack of knowledge, hemodialysis, and past hospitalization was found to be important independent risk factors.16–20
Studies on health care costs and access to treatment for viral hepatitis in Ethiopia showed that the majority of those infected are unaware of their disease status, most likely due to the general lack of awareness of HBV infection among the Ethiopian population. The results of the expert survey suggest that the low public awareness of viral hepatitis means that patients generally suspect a positive infection only after diagnosis. Lack of awareness and the silent nature of hepatitis brings to late presentation, and severe complications.21
Federal Ministry of Health (FMOH) has prepared and implemented various strategic plans to reduce the HBV burden in Ethiopia, including the provision of selective vaccination programs for high-risk groups such as health workers and people in close contact with the population, and routine screening of patients with suspected viral hepatitis.22 Nevertheless, HBV vaccination is a vital prevention mechanism.23 Due to the high cost of the vaccine ($29.99), access is less likely to be available to individuals.24
The vast majority of studies into risk factors for hepatitis B virus infection take place in developed countries, while only a negligible amount of prevalence surveys are conducted in developing countries, including Ethiopia. Some variables that have been shown to be predictors of HBV infection in one study may not necessarily be a risk factor for HBV infection in another study, supporting the argument that potential determinants vary across geographical locations. Therefore, it may be difficult to generalize the result to the developed regions outside the study area.
Looking at studies in Ethiopia, it is almost all about the magnitude, but studies on risk factors were limited and could not be extrapolated to other settings. Above all, there are few published previous studies in this area that could identify the risk factors of the problem. Therefore, the aim of this study was to fill this information gap by identifying the risk factors of hepatitis B virus infection in the Ayder comprehensive speciality hospital and updating the previous knowledge on this problem.
Methods and Materials
Study Area and Period
The study was conducted from March 2019 to May 2019 at Ayder Comprehensive Specialized Hospital and Teaching Hospital in Northern Ethiopia. Ayder Comprehensive Specialized Hospital (ACSH) one of the largest referral and teaching hospitals in Ethiopia provides specialist care-up service to patients with different specialists, including those with gastrointestinal and hepatology problems. Since 2010, ACSH has started offering its referral and non-referral services to 10 million in its service area of Tigray [6.6 million] with 53 districts and 15 governmental general hospitals,25 Afar, some Eritreans [refugees], and Southeastern parts of the Amhara regional States.26 This is a government owned, not for profit and offers service at a minimum cost. The total number of outpatients of the ACSH in 2011 was 180,000 and the monthly outpatient flow in the Gastroenterology and Hepatology department averaged 490.
Ayder comprehensive specialized hospital has two senior gastroenterologists and hepatologists, resident fellows working at a time during the specified study period. The service includes outpatient care, follow-up care and Endoscopy. The experts working in the Department of Hepatology are expected to be involved in the treatment of patients with various forms of liver diseases including malignancies.
Study Design and Population
A hospital-based, unmatched, case–control study was overlooked with cases [patients with hepatitis B virus infection (HBsAg+)] and controls [patients without liver disease] admitted to the gastroenterology and hepatology department of the comprehensive speciality hospital Ayder in Northern Ethiopia. All patients who were admitted to gastroenterology and hepatology units were our study population.
Inclusion Criteria
Cases: All patients with various forms of liver disease who tested positive for hepatitis B surface antigen (HBsAg+) by rapid strip test and who were in follow-up treatment for hepatitis B virus. Additionally, the unit was maintained during the study period with an asymptomatic (inactive) chronic carrier status, even including positive HBsAg tests during blood donation screening or routine health checkups as cases.
Hepatitis B virus infection can be detected by diagnosing the presence of hepatitis B surface antigen (HBsAg), which represents active acute and chronic infections; whereas, the hepatitis B pre-core Antigen (HBeAg) indicates high viral reproduction, while hepatitis B surface antibodies (HBsAb) and hepatitis B pre-core antibodies (HBeAb) are indicative of HBV resolution.27
Controls: Patients without liver diseases who were HBsAg-negative were referred for upper gastrointestinal endoscopy. Cases and controls were ascertained (confirmed) by a physician through history, clinical manifestation (objective findings) and laboratory testing during visits to gastrointestinal and hepatology units, respectively.
Exclusion Criteria
Case: HBsAb-positive patients who were critically ill.
Control: Patients without liver disease who were seriously ill.
Study Variables
The Sociodemographic factors highlighted in the literature that predispose patients to HBV infection were age, gender, marital status, religion, exposure with hepatitis cases in the household, place of residence, and education level of participants.16,20,28 The healthcare service-related factors included a history of blood transfusion, a history of hospitalization, a history of surgery, a history of needle stick injury, a history of dentist visits and a history of blood testing in a laboratory,20,29 and behavioural factors included a history of treatment for sexually transmitted diseases, history of ear and nose piercing (female), history of barber shaving (male), history of tattoo ever, previous procedures in traditional medicine, and awareness of hepatitis B virus infection including heard about hepatitis B virus transmission through blood, transmission through sexual contact, and knowledge about prevention/vaccination.17,20,29
Sample Size Determination and Sampling Procedure
Sample size was determined using Epi Info 7.0 Stat Calc. This was calculated by taking the power at 80%, the confidence level at 95%, the percentage of control exposed at 6.7, the odds ratio of 3.9 from contact with a household hepatitis case against HBV infection,16 and the Ratio of a case to accepted control was 1:2. History of contacts with household hepatitis cases was selected because this exposure variable was the largest sample size for cases and controls among the other variables. Allowing for a non-response rate of 10%, the total sample size was 213 with 71 cases and 142 controls. The cases were selected sequentially from among the patients admitted to the hepatology department. The next-door two corresponding controls were selected by a simple random method on the same day in the gastroenterology department part of the gastroenterology and hepatology departments.
Data Collection Tool and Process
The data collection tool was initially developed in English and translated into the local language (Tigrigna) and back into English. The review was carried out by Tigrigna, English language experts and health professional experts in terms of the consistency of the language translation. A questionnaire was created by reviewing various literature on the subject of hepatitis B virus infection. Most of the questions were taken from questionnaires from other studies to investigate risk factors of hepatitis B virus infection.20,29,30 Data were collected through face-to-face interviews with patients by trained experienced health professionals using pre-tested and interviewer-structured questionnaires. They were interviewed by trained health workers at Ayder comprehensive specialized hospital about their socio-demography, healthcare service-related factors, behavioural factors and awareness of hepatitis B virus infection. The data were collected during a shift by four trained assistant lecturers in nursing and supervised by the investigators. Continuous follow-up and monitoring were carried out by the principal investigator throughout the data collection period.
Operational Definition
Traditional medicine procedure: Practices that involve uvulectomy, dental extraction at home, herbal medicine, and other traditional mutilation-related procedures.
History of blood test in the laboratory: Participants who had a previous history of blood tests in the laboratory but it does not consider during the study period.
Severely (Critically) ill: Study participants who were unresponsive (unaware) to time place, and person.
Data Quality Assurance and Management
The two-day training was conducted for data collectors and supervisors on the objective of the study, data collection tool, data collection procedures, and ethical considerations in data collection. Before the actual data collection, a pretest was carried out with 5% of the sample and the necessary adjustments were made to the tool. A pre-test was conducted at Axum comprehensive speciality hospital outside the study area. Close monitoring was carried out by the researchers during the time of data collection. Each respondent’s data was reviewed by the manager for completeness, clarity, consistency, and accuracy.
Data Analysis Procedure
Data were entered using Epidata version 4.2 and exported to SPSS version 22 for cleansing and analysis. The basic socio-demographic characteristics, health service-related factors, behavioural factors, and awareness of hepatitis B virus infection of cases and controls were compared to identify any differences. The normality of the continuous variables was assessed using the Shapiro–Wilk test. Continuous variables were pooled using means and standard deviations. In contrast, frequencies and percentages were used for categorical variables. Bivariate associations between independent variables and HBV infection were tested using the chi-square test and the association was analyzed by calculating the crude odds ratio (OR) at a 95% confidence interval using binary logistic regression. Multivariate logistic regression was examined for the relationship between independent variables and hepatitis B virus infection to remove the confounding effect. To control for possible residual confounders due to factors that were previously thought to be confounders, we also included those variables for which bivariate analysis gave a p-value of 0.25 or less. We concluded that a cut-off value of 0.25 would allow us to test the effect of all factors not previously known to have a confounding effect on the relationship between HBV infection and independent variables, without including the factors that were reasonably least likely. Multicollinearity between independent variables was assessed using the variance inflation factor (VIF) and was all less than 2.1. The Hosmer and Lemeshow test was habituated to test the goodness-of-fit of regression models. The test static was 0.69 (p > 0.05) showing that the model fitted the data reasonably. In the final multivariable-adjusted model, we included all statistically significant covariates.
Ethical Consideration
Ethical approval was obtained from the Institutional Review Board (IRB) of the College of Health Science at Mekelle University. The study complies with the declaration of Helsinki. Official letters of support were obtained from the School of Nursing. Then officials at different levels of the hospital were declared through formal letters. The responsible authorities in the gastroenterology and hepatology departments were informed of the purpose of the study and written informed consent was obtained from the participants to confirm their willingness. They have been informed that they have the right to refuse or withdraw from the interview at any time. The confidentiality of the information was ensured and maintained throughout the study process.
Result
Socio-Demographic Characteristics of the Participants
A total of 213 (71 cases and 142 controls) took part in the study. The mean age (SD) of the cases and controls was 40.1±13.5 and 42±15.5 years, respectively. The majority of cases (23.9%) and controls (27.5%) were in the 31 and 40 years age group, while 9.9% of cases and 6.3% of controls were under 20 years old. About two-thirds (67.6%) of the cases were from urban residential areas, while among controls (55.6%) were from urban residences. Almost two-thirds (64.8%) of the cases were married and (28.2%) single, while in the control group (67.5%) were married and (25.4%) single (Table 1).
 |
Table 1 Socio-Demographic Characteristics of HBV Patients and Controls, North Ethiopia, 2019 [n = 213] |
Healthcare Services-Related Factors of Participants
Of the total, 16,9% of cases had a history of blood transfusions, while 16.2% of controls reported a history of blood transfusions. While (53.5%) of the cases reported a history of hospitalization, 62% had a similar experience. The proportion of cases and controls reported in the laboratory was 62% and 57%, respectively (Table 2).
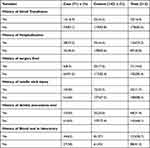 |
Table 2 Health Care Services Related Factors of HBV Patients and Controls, North Ethiopia, 2019 [n = 213] |
Behavioural Factors of Participants
Sixty-nine (69%) of cases had a history of STI treatment, while 98% of the controls reported a history of STI treatment. More than a fifth (21.1%) of cases and 18.3% of controls reported a history of ear and nose piercing (females). Of all participants, 47.9% of the cases had prior traditional medical procedures, while 46.5% of the controls reported prior traditional medical procedures. Of these, 8.5% are due to uvulectomy (Table 3).
 |
Table 3 Behavioral Factors of HBV Patients and Controls, North Ethiopia, 2019 [n = 213] |
Awareness About Hepatitis B Virus Infection for Cases and Controls
Of all cases, more than two-thirds (69%) had heard of hepatitis B virus infection, while 39.4% of controls reported having heard of hepatitis B virus infection. In the case of transmission through sexual contact, more than half (52.1%) of the cases were aware, while (8.5%) of the controls reported being aware of sexually transmitted hepatitis (Table 4).
 |
Table 4 Awareness of Hepatitis B Virus Infection Among HBV Patients and Controls, North Ethiopia, 2019 [n = 213] |
Risk Factors of Hepatitis B Virus Infection Participants
Variables such as place of residence, history of needle stick injuries, history of shaving at barber ever (male), heard of hepatitis B virus, transmission by blood and knowledge of prevention showed a statistical association with hepatitis B virus infection in the bivariate analysis but they were not significantly associated after adjustment in multiple logistic regression.
Both crude and adjusted analyses showed that exposure to hepatitis cases in the household, history of dental procedures, prior traditional medical procedure, and lack of awareness of HBV infection transmission through sexual contact were ascertained to be after controlling for possible confounders independent predictors for the occurrence of hepatitis B virus infection.
Patients who had contact with hepatitis cases in their household were almost four times more likely to develop hepatitis B virus infection than those patients who did not have hepatitis B virus infection (AOR 3.7, 95% CI: 1.5–9.01), prior traditional medicine procedure (AOR 1.2, 95% CI: 1.08–3.4), and history of dental procedures have a higher risk of HBV infection compared to those who have never had dental procedures (AOR 3.8, 95% CI: 1.8–9.01). Those who were aware of hepatitis B virus infection transmission through sexual contact were less likely to have had HBV infection than those who were unaware of transmission through sexual contact (AOR 0.084, 95% CI: 0.01-0.6) (Table 5).
 |
Table 5 Multivariable Analysis of Risk Factors Among Study Participants HBV Patients and Controls, at Ayder Comprehensive Specialized Hospital, North Ethiopia, 2019 [Cases = 71 Controls = 142] |
Discussion
A total of 213 patients participated in this case–control study conducted at the Ayder comprehensive speciality hospital, in Northern Ethiopia, including 71 laboratory-confirmed (HBsAg+) HBV-infected patients and 142 (HBsAg-) patients entering and receiving advancing training were referred to the upper gastrointestinal endoscopy. There is a rising burden of HBV infection in many countries, which may be due to its socio-demographic, healthcare service-related and behavioural factors. Adequate information about epidemiological factors is critical in formulating national policies and refocusing health resources to control the transmission of HBV infection. Because no single factor is entirely conducive to the occurrence of HBV infection, and there is a lack of information about factors contributing to occurrences of HBV infection, this study attempted to examine the different variables. Ethiopia as part of sub-Saharan Africa is considered a severely endemic and widespread area for HBV infections.11 The results of this study showed that a history of contact with hepatitis cases in the household, history of dental procedures, previous traditional medical procedures, and lack of awareness of transmission through sexual contact were positively related to hepatitis B virus infection.
Our study has clearly demonstrated that exposure to hepatitis cases in households is an independent risk factor for the spread of hepatitis B virus infection. This finding is endured by the study conducted in Malatya City (Turkey), Karaj Hepatitis Center (Iran), and China.17,18,31 In addition, a study conducted in Kashmir (India) has shown that HBV has a high familial frequency.4 This association could be because physical contacts between family members are common and the accredited presence of HBV DNA in the saliva, blood, sweat and other fluids of peoples infected with HBV shows the event of virus transmission through adulterated instruments, cutaneous and non-cutaneous contacts.32
This finding is inconsistent with the study conducted in a resource-constrained setting of a primary care clinic in eastern Nigeria,29 which indicated that a family history of hepatitis B virus infection was found to be insignificant. This could likely be explained by the reason that case selection was confirmed by laboratory and clinical features in our setup and in the country’s found to be significant; however, in Eastern Nigeria cases, it was confirmed only by sign and symptom (clinical features) beyond the sample size was relatively small (140) and the study setting lacked the resource available to confirm the disease.
According to this study, exposure to previous traditional medical procedures was significantly associated with hepatitis B virus infection. This finding is comparable to studies conducted in Nigeria,29 Uganda,33 and the city of Axum Northern Ethiopia,34 which found that approximately 87.8% of mothers practiced at least one traditional practice with their children, uvula cutting was performed in 86.9% of the children followed by the extraction of deciduous teeth in 12.5%. This vulnerability could be because in Ethiopia traditional medicine is generally practiced by lay people, and there are no rules or guidelines to regulate and monitor this practice.
It has been repeatedly suggested that a history of dental procedures/treatment is an important risk factor for HBV infection in developing countries. In some studies conducted in Bahrain, Iraq, Palestine, Iran, and Kashmir (India) one of the reported risk factors for HBV was a history of dental procedures.4,17,30,35,36 Our study found that a history of dental procedures ever was an independent risk factor for HBV acquisition. This could likely be explained by the fact that hepatitis B virus infection spreads through contact with blood, saliva, and other bodily fluids or through indirect contact with contaminated objects including instruments, devices, and surfaces. Through these pathways, the disease can be transmitted from dental staff to patient, or vice Versa, and also from one patient to another.37
However, this contradicts the study conducted in China.31 This could be due to a shortage of knowledge, limited resources and poor practice in clinical infection control in developing countries. In addition, infection control measures may be inadequate and present a major opportunity for transmission of blood-borne pathogens such as the hepatitis B virus through dental care. This can be accepted by the study conducted in Palestine in 2009,38 which lay out that only 54.6% of dentists wear gloves when treating patients, 53.6% use 70% alcohol solutions as a disinfectant and 83.2% of them use dry heat sterilization technique that has proven to be inefficient.
The finding of this study also stated that those who were aware of the transmission of HBV infection transmission through sexual contact were less likely to be infected with hepatitis B virus infection than those who were unaware of the transmission through sexual contact. This finding is similar to the unmatched case–control study conducted in rural areas of Patina, India, which showed that those who were unaware of transmission through sexual contact were more likely to contract hepatitis B virus infection as compared to participants having awareness of transmission through sexual contact.20 This could be because infection with the hepatitis B virus can also be transmitted through semen and blood. Therefore, awareness of this can be the prerequisite for preventing the contraction of the disease.
Several studies have shown that male gender was the risk factor for HBV accession.17,30,36 However, this was inconsistent with our study, and the study conducted in Southern India.20
Our study found that healthcare-related risk factors including history of blood transfusion, history of hospitalization, history of surgery, and needle stick injury were found to be insignificant risk factors for HBV infection. This was confirmed by a study conducted in India.39 However, this is in contradiction with the study done in Palestine and Patina (India).20,30 This could likely be explained by the fact that a lower percentage of our participants had a history of surgery and blood transfusion. In general, we concluded that a history of hospitalization and healthcare injections were more important risk factors in the previous literature.
Although we found no significant association between a history of tattoos ever, a history of shaving at a barber ever (male), history of ear and nose piercing (female) with hepatitis B virus infection in our study, other studies conducted in some countries however, provided to be significant.4,20,31 The possible explanations could be that most of the participants came from the urban residential area when traditional practice changed to a modern practice and they used both disinfectants and sterilization.
Limitations of the Study
In this study, several measures were taken to alleviate potential limitations. In order to, minimize recall bias, incident cases from HBV patients were included in our study and all data collectors were trained in a standard method of data collection and the same standardized questionnaire was applied to ask for cases and controls. Since most episodes of the hepatitis B virus are asymptomatic, these were likely control cases reminiscent of exposure potentially associated with HBV infection.
Conclusion
This study found that exposure to household hepatitis cases, history of dental procedures, prior traditional medical procedures, and lack of awareness of transmission through sexual contact were independent risk factors for the episode of hepatitis B virus infection. According to the demography, there was no important difference between cases and controls related to age, sex, marital status, and religion. Therefore, raising people’s awareness of the transmission and prevention of hepatitis B virus infection has strategic importance in order to avoid contracting the disease.
Abbreviations
ACSH, Ayder Comprehensive Specialized Hospital; AIDS, Acquired Immune Deficiency Syndrome; AOR, Adjusted Odds Ratio; CHB, Chronic Hepatitis B; CI, confidence interval COR, Crude Odds Ratio; DNA, Deoxyribonucleic Acid; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HCC, Hepatocellular Carcinoma; HDV, Hepatitis D Virus; MOH, Ministry of Health; MU, Mekelle University; OR, Odds Ratio; STI, Sexually Transmitted Infection; SPSS, Statistical Software for the Social Sciences; WHO, World Health Organization; RH, Reproductive Health; ie, such that.
Declarations
Ethical clearance and approval were acquired from the institutional review board of Mekelle University, CHS. The letter of approval for the mission was obtained from the Chief Clinical Director of Ayder Comprehensive Specialized Hospitals. In addition, the purpose and aim of the study were described to the study participants before the study was conducted and oral informed consent was obtained since most of the participants were illiterate. Study participants were instructed that they had full right to withdraw from the study during the interview. Confidentiality of participants and any special data security requirements were observed and assured by not exposing patients’ names and information. In addition, the questionnaires and all other information were cached on a personal computer that is protected with a password. According to the university ethical clearance rule and regulation by the Reference Number of MU/1299/2019.
Data Sharing Statement
Data sets used during the current study are available upon reasonable request from the relevant author.
Consent to Publication
Not applicable as there are no issues of image or other confidentiality.
Acknowledgments
First of all, our special thanks and deepest gratitude goes to Mekelle University for the financial support in carrying out this research. Our heartfelt thanks also go to the study participants, data collectors and supervisors who participated in the study. Our thanks also go to the administrator of the hospital, the ward manager, and the head nurse of the gastroenterology and hepatology department of the respective hospital.
Funding
Mekelle University was the funding source (reference no. 1299/2019). The university supported us financially with the transport.
Disclosure
The authors declare that there are no competing interests in this work.
References
1. Guidance D. Chronic hepatitis B virus infection: developing drugs for treatment guidance for industry chronic hepatitis B virus infection: developing drugs for treatment guidance for industry. FDA; 2018:1–24. Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
2. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
3. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–686. doi:10.1016/j.virol.2015.02.031
4. Naqshbandi I, Yasir S, Qadri A, Yasmeen N, Bashir N. Seroprevalence and risk factors of hepatitis b virus infection among general population of srinagar kashmir. Int J Contemp Med Res. 2016;3(4):1050–1054.
5. World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2017;92(27):369–392.
6. World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection; 2015:166. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/.
7. World Health Organization. World hepatitis day — access to treatment for hepatitis B virus infection — worldwide, 2016; 2018:2017–2018.
8. World Health Organization. Global hepatitis report; 2017. Available from: http://www.who.int/hepatitis.
9. Croagh CMN, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. 2014;20(30):10395–10404. doi:10.3748/wjg.v20.i30.10395
10. Spearman CW, Afihene M, Ally R, et al. Series Viral hepatitis in sub-Saharan Africa 1 Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017;2(12):900–909. doi:10.1016/S2468-1253(17)30295-9
11. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(761):1–14. doi:10.1186/s12879-016-2090-1
12. Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–1210.
13. Health G, Strategy S, Ending T, Hepatitis V. Viral hepatitis 2016–2021. 2016:4–56. Available from: http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E.
14. Razavi HA. Economic burden of hepatitis C-Associated diseases: Europe, Asia Pacific, and the Americas. J Med Econ. 2014;31(2):ISSN 1478–3223.
15. Mehmet D, Meliksah E, Serif Y, Gunay S, Tuncer Ö, Zeynep S. Prevalence of hepatitis B infection in the southeastern region of turkey: comparison of risk factors for HBV Infection in Rural and Urban Areas. Jpn J Infect. 2005;58(7):15–19.
16. Talaat M, Radwan E, El Sayed N, Ismael T, Hajjeh R, Mahoney,FJ. Case-control study to evaluate risk factors for acute hepatitis B virus infection in Egypt. East Mediterr Heal J. 2010;16(1):4–9. doi:10.26719/2010.16.1.4
17. Sali S, Bashtar R. Risk factors in chronic hepatitis B infection in Iran: hepat mon; 2005:109–115.
18. Ozer A, Yakupogullari Y, Beytur A, Beytur L, Koroglu M, Aydogan F. Risk factors of hepatitis B virus infection in Turkey: a population-based, case-control study. Hepat Mon. 2011;11(4):263–268.
19. Rajamoorthy Y, Mohd N, Mudatsir M, Abdul K, Radam A. Risk behaviours related to hepatitis B virus infection among adults in Malaysia: a cross-sectional household survey. Clin Epidemiol Glob Heal. 2020;8(1):76–82. doi:10.1016/j.cegh.2019.04.011
20. Lohani P, Kumar A, Singh R, Sinha RK, Mukherjee M. A study of risk factors of hepatitis B infection among rural adult population of Patna, Bihar. Int J Community Med Public Heal. 2017;4(12):4654–4660. doi:10.18203/2394-6040.ijcmph20175346
21. Bane A, Patil A, Khatib M. Healthcare cost and access to care for viral hepatitis in Ethiopia. Inter J Innovat Appl Stud. 2014;9(4):1718–1723.
22. Yazie TD. An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infect Dis. 2019;1(1):1–13.
23. Prüss-üstün A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48(6):482–490. doi:10.1002/ajim.20230
24. Akibu M, Nurgi S, Tadese M, Dibekulum W. Attitude and vaccination status of healthcare workers against hepatitis B I.: discovery Service for de la salle health sciences institute. Scientifica. 2018;2018(1):1–8. doi:10.1155/2018/6705305
25. Sciences MUCOH. Ayder comprehensive specialized hospital; 2019.
26. Sciences MUCOH. Ayder comprehensive specialized hospital; 2020. Available from: https://issuu.com/fragiofre/docs/investigating_the_observers_and_users_experience_/s/21305286.
27. Krajden M, Art GM, Petric M. The laboratory diagnosis of hepatitis B virus. Can J Infect Dis Med Microbiol. 2005;16(2):65–72. doi:10.1155/2005/450574
28. Halatoko WA, Patassi A, Yanogo P, et al. Risk factors of hepatitis B virus surface antigen carriage and serological profile of HBsAg carriers in Lomé Togo, 2016. BMC Public Health. 2019;19(32):1–7. doi:10.1186/s12889-018-6320-x
29. Nwokediuko S. Risk factors for hepatitis B virus transmission in Nigerians: a case-control study. J Gastroenterol. 2010;10(1):1–5.
30. Nazzal Z, Sobuh I. Risk factors of hepatitis B transmission in northern Palestine: a case–control study. BMC Res Notes. 2014;7(1):1–4. doi:10.1186/1756-0500-7-190
31. Zhang HW, Yin JH, Li YT, et al. Risk factors for acute hepatitis B and its progression to chronic hepatitis in Shanghai, China. Hepatology. 2008;57(1):1713–1720.
32. Hatami H, Salehi M, Sanei E, Khosravi S, Ala- SM. Intra-familial transmission of hepatitis B virus Infection in Zahedan. Iran Red Crescent Med J. 2013;15(1):4–8. doi:10.5812/ircmj.2282
33. Nsibirwa S, Anguzu G, Kamukama S, Ocama P, Nankya-mutyoba J. Herbal medicine use among patients with viral and non-viral Hepatitis in Uganda: prevalence, patterns and related factors. BMC Complement Med Ther. 2020;8(1):1–11.
34. Gebrekirstos K, Abebe M, Fantahun A. A cross-sectional study on factors associated with harmful traditional practices among children less than 5 years in Axum town, north Ethiopia, 2013. BMC Reprod Heal. 2014;11(46):1–7.
35. Hussein NR, Daniel S. A study of hepatitis B virus associated risk factors in patients attending hepatitis unit in Duhok City, Iraq. Arch Clin Infect Dis. 2017;2017:1–5.
36. Janahi EM. Prevalence and Risk Factors of Hepatitis B Virus Infection in Bahrain, 2000 through 2010. PLoS One. 2014;9(2):1–5. doi:10.1371/journal.pone.0087599
37. Woods R. The prevention of hepatitis Larry I. Lutwick, MD Abstract B transmission in dental practice Dental Care Transmission of HBV. Int Dent J. 1984;34(2):122–126.
38. Monou M, Monou M, Mosleh SA, Al-Subu MM, Kassinos D. Dental solid and hazardous waste management and safety practices in developing countries: Nablus district, Palestine Dental solid and hazardous waste management and safety practices in developing countries: Nablus district, Palestine. Waste Manag Res. 2009;28(1):436–444. doi:10.1177/0734242X09337657
39. Biswas DK, Bhunia R, Das P. An Outbreak of Hepatitis B in a Rural Area of West Bengal, India. Sri Lankan J Infect Dis. 2015;5(2):51–57. doi:10.4038/sljid.v5i2.8077
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
