Back to Journals » International Medical Case Reports Journal » Volume 17
Rhino-Orbital Cerebral Mucormycosis in a Healthy Female Child: Case Report
Authors Yusuf AA, Ibrahim IG , Hirsi IM, Adali A , Hassan YY, Yasar MZ , Abdullahi IM, Hassan MS
Received 13 December 2023
Accepted for publication 18 March 2024
Published 26 March 2024 Volume 2024:17 Pages 241—246
DOI https://doi.org/10.2147/IMCRJ.S454697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Abdisalam Abdullahi Yusuf,1 Ismail Gedi Ibrahim,2,3 Ibrahim Mohamed Hirsi,1 Ali Adali,1 Yonis Yusuf Hassan,1 Mehmet Zeki Yasar,1 Ismail Mohamoud Abdullahi,4 Mohamed Sheikh Hassan3,5
1Department of Pediatric, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia; 2Department of Radiology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia; 3Faculty of Medicine and Surgery, Mogadishu University, Mogadishu, Somalia; 4Department of Pathology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia; 5Department of Neurology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia
Correspondence: Abdisalam Abdullahi Yusuf, Email [email protected]
Abstract: Mucormycosis is a potentially fatal condition with a high mortality rate, particularly when there is extra nasal involvement, and it is rare for patients with fungal brain disease to survive. It mostly affects patients who are metabolically or immunologically compromised, which constitutes one of the three classical stages of the progression of Rhino-Orbito-Cerebral Mucormycosis (ROCM). Stage I: infection of the nasal mucosa and paranasal sinuses; Stage II: orbital involvement; Stage III: cerebral involvement.Here, we report a case of rhino-orbital cerebral mucormycosis in a 14-year-old girl with no known risk factor who presented with periorbital edema, right eye proptosis, fever, and extreme facial pain, which progressively worsened to confusion and left leg weakness in 3 days after admission. The final diagnosis was rhino-orbital-cerebral mucormycosis. The infection was successfully treated using liposomal amphotericin and surgical debridement to remove infected orbital tissue. Mucormycosis is a potentially fatal disease that necessitates prompt diagnosis and treatment. Children are rarely infected with mucormycosis. The majority of studies show that people are typically between 40 and 50 years old. ROCM is typically diagnosed using clinical symptoms and histopathologic evaluation; however, imaging is critical in determining the presence of intracranial lesions. The standard treatment for ROCM is amphotericin B at a recommended dose of 1.0– 1.5 mg/kg/day for weeks or months, depending on the clinical response and severity of adverse drug reactions, particularly nephrotoxicity.Rhino-orbital cerebral mucormycosis in a healthy female child is uncommon; early diagnosis and prompt treatment with Amphotericin B should be necessary. Devastating consequences will result from a delayed diagnosis.
Keywords: mucormycosis, orbital cellulitis, liposomal amphotericin B, cerebral involvement
Introduction
Mucormycosis is a subacute, acute, and generally rapidly progressing infection caused by angiotropic fungi of the order Mucorales, which are known to be highly fatal, especially in immunosuppressed patients.1 According to the 2017 report by Leading International Fungal Education (LIFE), the annual prevalence of mucormycosis was approximately 910,000 cases worldwide, with nearly 98.9% of these cases occurring in India. The estimated average mortality rate for this condition was about 38.2% per year.2 Mucormycosis most commonly affects the nasal mucosa, but it can also invade the sinuses, orbits, and brain.3 ROCM typically progresses in three stages: Stage I involves an infection of the nasal mucosa and paranasal sinuses; Stage II involves involvement of the orbits; and Stage III involves involvement of the brain, which primarily affects people with metabolic or immunological compromise.4 Patients with rhino-orbito-cerebral mucormycosis present with pain and paresthesia in their faces, headaches, swollen orbits and noses, inflammation, drooping eyelids, proptosis, external and internal ophthalmoplegia, vision loss, and blackish necrosis of the palate and nasal mucosa.5 However, extra-nasal involvement increases mortality rates, and survival in cases of fungal brain disease is rare6. In this study, we present a case of ROC mucormycosis in a healthy female child with a classical presentation consisting of right eye proptosis, sudden vision loss, and fever for two weeks and gradually altered consciousness and hemiplegia on the side opposite the lesion who was hospitalised in our paediatric inpatient ward.
Case Presentation
A 14-year-old female patient with a fever, right eye proptosis, periorbital edema, redness, extreme facial pain, and loss of vision for two weeks was hospitalized in our pediatric inpatient ward. At the time of the examination, she had a blood pressure of 130/70 mmHg, a pulse rate of 108, and 99% saturation of oxygen (SpO2) in room air. During the initial examination, the patient appeared confused and febrile, and the right eye was excessively edematous, hyperemic, and exophthalmic (Figure 1). Because of the patient’s confusion, her neurological examination was limited, but it revealed weakness in the left leg. No seizures had been observed. The left eye had ptosis, the right pupil was dilated and fixed, and extraocular movements were restricted during the cranial nerve examination. Her vision in the left eye was diminished.
 |
Figure 1 Orbital photograph showing proptosis, exophthalmic and edema with hyperemia. |
Blood tests at admission revealed a hemoglobin level of 10 g/dL, a total leukocyte count of 16,810/mm3, a differential count of 80% neutrophils and 20% lymphocytes, and a CRP of 78 mg/L. The results of the liver and renal function tests, HIV, COVID-19, and blood sugar were normal. The patient was not malnourished. There were no additional risk factors, and the patient was immunocompetent. There was no recent history of facial injuries. It was assumed that the patient had primary orbital cellulitis due to a bacterial infection when she was admitted. Three days of taking vancomycin and ceftriaxone led to her condition deteriorating; she also began to lose consciousness, and her ocular findings progressed. Brain and orbit MRI revealed a diffusely heterogeneous lobulated lesion (T1-isointense and T2-hyperintense) with a central signal void in the right intraorbital region, resulting in exophthalmos as well as cerebral infarcts (Figures 2 and 3).
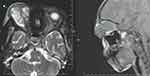 |
Figure 2 Orbital MRI showed space-occupying that’s T1-isointense and T2-hyperintense heterogeneous lobulated lesion with central signal void in the right intraorbital region resulting exophthalmos. |
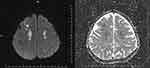 |
Figure 3 DWI and ADC MRI: Lesions showing acute diffusion restriction were detected in both parietal, frontal and right occipital lobes (acute/subacute ischemic infarct). |
A direct microscopy of the orbital tissue swab sample taken from the right eye was sent to a microbiology lab by using a KOH mount and calcofluor white. The orbital tissue sample was stained with hematoxylin-eosin under direct microscopy, which revealed fungal hyphae with broad-based non-septate hyphae (Figure 4). After discontinuing antibiotics, antifungal therapy was started with liposomal amphotericin B at a dose of 2 mg per kg every 24 hours. There was prominent improvement at day six after treatment (see Figure 5). Following three weeks of liposomal amphotericin B infusion and surgical debridement of infected orbital tissue with appropriate dressing, the right eye returned to its original size, and the edema was resolved. Enoxaparin, a low-molecular-weight heparin, was administered alongside her antifungal treatment due to the suspicion of thrombosis associated with Rhino-Orbito-Cerebral Mucormycosis (ROCM). Gradually, the patient began to see with his left eye but could not move it. After five weeks of the liposomal amphotericin B treatment, the patient’s general situation has improved (Figure 6).
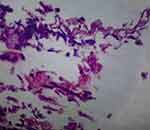 |
Figure 4 The smear shows fungal hyphae with broad based non-septate hyphae stained with H&E stain. |
 |
Figure 5 Day 6 photo taken following the initiation of antifungal therapy. |
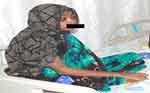 |
Figure 6 The final day of the hospital discharge. |
Discussion
According to epidemiological data, mucormycosis infection in children is uncommon, and it is also difficult to determine the true burden in the pediatric setting due to limited awareness of the healthcare profession and the rarity of the disease. Based on most research, the typical age of presentation is between 40 and 50 years old.7 Mucormycosis is a potentially fatal illness that requires timely diagnosis and treatment. In numerous scenarios, an invasive fungal infection frequently affects the central nervous system (CNS), making it a potentially lethal illness.8 This disease has different clinical manifestations, such as in the lungs, gastrointestinal tract, soft tissues, paranasal (sino-orbital), and brain tissues, with the potential to progress to widespread illness.9
Many research studies have shown that the main risk factors predisposing to mucormycosis infection include iron overload, long-term corticosteroid use, intravenous drug use, hematological malignancy, severe neutropenia, significant trauma, poorly controlled diabetes, prematurity, and malnutrition.10 The mucormycosis infection also affects the skin and subcutaneous tissues, persists for years, occurs in immunocompetent individuals without any obvious risk factors, and eventually results in severe deformity.11 In contrast to our study, we presented a case of ROCM in a 14-year-old female with no known history of trauma or risk factors for immunocompromising effects or any chronic disease. Detection of fungal hyphae in tissue samples by direct microscopy, histopathology, and isolation of the pathogen from culture is essential for a definitive diagnosis of ROCM. Rapid detection of suspected mucormycosis infection by direct microscopy (KOH fixation and staining with Calcofluor White) allows early and successful treatment. Additional details about vascular invasion, infarcts, and perineural invasion can be obtained from the histopathological analysis of tissues. It also aids in the diagnosis of culture-negative cases and helps differentiate between a true fungal infection and a culture contaminant, which is crucial for saprophytic fungi. Generally, R. arrhizus is the most frequently isolated species worldwide.12
In our case, the diagnosis relies on clinical symptoms; histopathologic analysis and imaging play a key role in determining the extent of involvement and the presence of intracranial lesions. The following symptoms and signs should be taken into consideration as “red flags”: cranial nerve palsy, diplopia, sinus pain, proptosis, periorbital edema, orbital apex syndrome, and palate ulcers.13
Fungal hyphae enter the artery lumen, causing vascular obstruction, thrombosis, and infarction, which results in tissue necrosis and hypoxia.14 Ophthalmic venous thrombosis and bony destruction are characteristic findings mentioned in different reports.15 In comparison, our case had stage 4 ROCM with cerebral involvement and a frontal lobe infarction but did not have bony destruction.
It has been shown that effective treatment can be given without orbital exenteration because of early clinical regression with medication and a lower fungal burden.16 Liposomal amphB (LamphB) could improve drug distribution to infected locations and minimize toxicity while increasing the therapeutic index of the drug.17 Amphotericin B at the recommended dose of 1.0–1.5 mg/kg/day for weeks or months, depending on clinical response and severity of adverse drug reactions, especially nephrotoxicity, is the most common therapy for RCM.18 Our patient was treated with an antifungal approach (liposomal amphB (LamphB)), as it was shown to have a favorable outcome, with early debridement taken after intravenous amphotericin B. Within the first week, it appeared to be very successful in controlling rhino-orbito-cerebral mucormycosis. In addition, low-dose heparin can also be used in our case. With appropriate medical treatment along with regular debridement, the patient was successfully improved clinical follow-up.
Conclusion
Rhino-orbital cerebral mucormycosis in a healthy female child is uncommon; early diagnosis and prompt treatment with Amphotericin B should be necessary. Devastating consequences will result from a delayed diagnosis.
Ethical Approval
We obtained written informed consent from the child’s parents for publication of this case report and the accompanying images.
Author Contributions
Each author contributed significantly to the work reported, whether it was through conception, study design, execution, data acquisition, analysis, and interpretation, or in all of these areas; they all contributed to draft, revise, or critically review the article; they approved the final version that was published; they all agreed on the journal to which the article was submitted; and they all agreed to take responsibility for the work in its entirety.
Funding
No funding for this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clinics. 2016;30(1):143–163. doi:10.1016/j.idc.2015.10.011
2. Dong N, Jordan AE, Shen X, et al. Rhino-orbital cerebral mucormycosis in a patient with diabetic ketoacidosis: a case report and literature review. Front Neurol. 2022;13:815902. doi:10.3389/fneur.2022.815902
3. Yadav SP, Goel AK. Rhino-orbital mucormycosis—a case report. Int J Pediat Otorhinolaryngol Extra. 2010;5(1):9–12. doi:10.1016/j.pedex.2008.12.008
4. DiBartolo MA, Kelley PS. Rhino-orbital-cerebral mucormycosis (ROCM): a comprehensive case review. Aviat Space Environ Med. 2011;82:913–916. doi:10.3357/asem.3036.2011
5. Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007;50(4):290–296. doi:10.1111/j.1439-0507.2007.01364.x
6. Weprin BE, Hall WA, Goodman J, Adams GL. Long-term survival in rhinocerebral mucormycosis. J Neurosurg. 1998;88:570–575. doi:10.3171/jns.1998.88.3.0570
7. Alabaz D, Yilmaz G, Uguz A, et al. Mucormycosis in a pediatric population: a review of 20 cases from southern Turkey, Turk. J Pediatr. 2021;63(1):11–22.
8. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi:10.1016/S2666-5247(20)30115-4
9. Muggeo P, Calore E, Decembrino N, et al. Invasive mucormycosis in children with cancer: a retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses. 2019;6:165–170. doi:10.1111/myc.12862
10. Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clinl Infect Dis. 2012;54(Issue suppl_1):S23–S34. doi:10.1093/cid/cir866
11. Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93–101. PMID: 29538730; PMCID: PMC6251532. doi:10.1093/mmy/myx101
12. Vallabhaneni S, Mody RK. Gastrointestinal mucormycosis in neonates: a review. Curr Fungal Infection Rep. 2015;9(4):269–274. doi:10.1007/s12281-015-0239-9
13. Corzo-Leon DE, Chora-Hernandez LD, Rodriguez-Zulueta P, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2017. doi:10.1093/mmy/myx017
14. Honavar SG. CodeMucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361–1365. doi:10.4103/ijo.IJO_1165_21
15. Press GA, Weindling SM, Hesselink JR, et al. Rhinocerebral mucormycosis: MR manifestations. J Comput Assist Tomogr. 1988;12:7449. doi:10.1097/00004728-198809010-00005
16. Pelton RW, Peterson E, Patel BC, Davis K. Successful treatment of rhino-orbital mucormycosis without exenteration: the use of multiple treatment modalities. Ophthalmic Plastic Reconstructive Surg. 2001;17(1):62–66. doi:10.1097/00002341-200101000-00012
17. Yohai RA, Bullock JD, Aziz AA, et al. Survival factors in rhino-orbital-cerebral mucormycosis. SurvOphthalmol. 1994;39:3–22.
18. Ferguson BJ. Mucormycosis of the nose and paranasal sinuses, Otolaryngol. Clin North Am. 2000;33:349–365.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
