Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Retrospective Analysis of the Clinical Characteristics of Patients with Breast Cancer Treated with Telomerase Peptide Immunotherapy Combined with Cytotoxic Chemotherapy
Authors Kim JY , Yang DW , Kim S , Choi JG
Received 28 August 2023
Accepted for publication 8 December 2023
Published 21 December 2023 Volume 2023:15 Pages 955—966
DOI https://doi.org/10.2147/BCTT.S431333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert Clarke
Jong Yeup Kim,1 Dong Won Yang,1 Sangjae Kim,2 Jong Gwon Choi3
1Department of Biomedical Informatics, College of Medicine, Konyang University, Daejeon, Republic of Korea; 2Department of Research and Development, Teloid Inc., Los Angeles, CA, 90010, USA; 3Department of Oncology-Hematology, Konyang University Hospital, Daejeon, Republic of Korea
Correspondence: Jong Gwon Choi, Oncology-Hematology, Konyang University Hospital, 158 Gwanjeodong-Ro, Seo-Gu, Daejeon, Republic of Korea, Email [email protected]
Purpose: Telomerase activation, a critical step in cancer progression, occurs in approximately 95% of breast cancer cases. Telomerase is an attractive therapeutic target for breast cancer owing to its unique expression pattern. GV1001, a telomerase-derived peptide, is loaded onto human leukocyte antigen (HLA) class II antigen-presenting cells and binds to CD4+ T cell activating immune responses. This study aimed to evaluate the effectiveness and safety of co-administration of GV1001 and cytotoxic chemotherapy in patients with heavily-treated metastatic breast cancer.
Patients and methods: We analyzed 63 patients with breast cancer who received both GV1001 and cytotoxic chemotherapy. The GV 1001 administration methods involves 0.56 mg intradermal injection three times during the first week, one time at weeks 2, 3, 4, and 6, and then once every 28 days. The primary endpoint of this study was quality of life according to EORTC QLO-C30 and EQ-5D, while the secondary endpoint was the antitumor response according to RECIST 1.1, progression-free survival, overall survival, and toxicity profile.
Results: In 34 patients with HR+ breast cancer evaluable for tumor response, the disease control rate (DCR) and overall response rate (ORR) were 58.8% and 26.4%, respectively. The DCR and ORR were 66.6% and 28.5% in 21 patients with HER-2+ and 50% and 25% in patients with triple-negative breast cancer (TNBC), respectively. The median progression free survival was 10.4, 8.7, and 5.6 months in HR+, HER-2+, TNBC, respectively. The overall survival was 19.7, 13.2, and 9.4 months for patients with HR+, HER-2+, and TNBC, respectively. Most patients had an improved quality of life with statistically significant differences in some variables. The patients in this study experienced no additional toxicities other than the cytotoxic chemotherapy-associated side effects.
Conclusion: GV1001 is a relatively safe anticancer vaccine for patients with heavily-treated breast cancer and can to improve the quality of life.
Keywords: telomerase, GV1001, quality of life, safety, breast cancer
Introduction
Telomerase, an enzyme that exists in the human body, is not well expressed in normal cells; however, it is overexpressed in most cancer cells. Tumor cells protect the telomere that exists at the end of the DNA so that they do not undergo apoptosis due to aging and divide beyond the normal cell division frequency, extending the lifespan of the cell.1 Tertomotide is a telomerase-based peptide that kills cancer cells via the autoimmune system and has been developed to treat patients with cancer. It is an immune treatment approach that allows immune cells (T cells) to be recognized as antigens by screening 16 peptides that make up the telomerase, and then translocate to the cancerous region where the telomerase is active to kill cancer cells.2 Tertomotide (code name GV1001) has the advantage of being a cancer vaccine that can be applied to the treatment strategies of various cancers with telomerase overexpression.3 GV1001 is a telomerase-targeting peptide vaccine composed of 16 amino acids derived from the activated part of the human telomerase reverse transcriptase catalytic subunit (hTERT, 611–626, EARPALLTSRLRFIPK).4 When antigen-presenting cells (APCs) recognize a peptide, it is taken up and processed. The APC presents the processed peptide to the T cell with HLA combined with the peptide, and in turn activates the T cell. Combined with MHC class II, the peptide induces both CD4+ and CD8+ responses, enhances the immune response, and reduces the risk of immune avoidance.5 An activated T cell kills encountered cancer cells presenting the same telomerase peptide/HLA complex. Since hTERT is expressed in most cancer types, it is often selected as the target antigen of cancer vaccination; hence, hTERT-targeted vaccines could be used in general. Recently, it was reported that inoculation with the telomerase-derived peptide GV 1001 induced telomerase-specific CD4+ and CD8+ T cell responses in hTERT-transfected dendritic cells,6 leading to therapeutic effects in patients with metastatic pancreatic cancer and non-small cell lung cancer.7,8 Other studies have reported that GV1001 has a direct antitumor effect because it is a cell-penetrating peptide.9 To date, GV1001 is undergoing clinical trials for solid cancers, such as pancreatic cancer, hepatocellular carcinoma, prostate cancer, non-small cell lung cancer, and malignant melanoma, which reported well-tolerated or mild adverse events only.10–14
However, the treatment effect of solid cancers is limited when using a cancer vaccine alone;15 therefore, it is necessary to combine it with other treatments to improve disease prognosis. Recent studies have focused on combining hTERT targeting approaches with other treatments. Several studies have demonstrated the synergistic effects of telomerase inhibitors along with NK cell-based therapy, paclitaxel, and arsenic trioxide.16–18
In this study, we aimed to determine whether the antitumor response and quality of life were improved by administering GV1001 to patients with breast cancer who were undergoing treatment or could not proceed with aggressive treatment.
Methods
Patients and Study Design
Between May 2018 and December 2020, 63 patients with breast cancer who underwent multiple chemotherapies were enrolled in this study. The types of clinicopathology varied, including hormone-positive, human epidermal growth factor receptor (HER)-2, and triple-negative breast cancer (TNBC). The inclusion criteria were: (1) histologically confirmed breast cancer; (2) non-resectable metastatic disease (The American Joint Committee On Cancer staging 8th edition, stage IV); (3) measurable tumor lesions; (4) age between 18 and 80 years; (5) Eastern Cooperative Oncology Group (ECOG) performance status 0–2; and (6) adequate hematological, renal, and hepatic function. The exclusion criteria were: (1) clinically confirmed brain metastases; (2) severe cardiac disease; (3) severe active infections; and (4) need for administering immunosuppressive medication. No screening for brain metastases was performed. Prior to participation in the study, patients had to undergo investigations regarding past medical history, physical examination, and blood tests, including WBC with differential, hemoglobin, platelet counts, electrolytes, liver function tests, standard urine analysis, serum tumor marker (CA 15–3), and chest computed tomography (CT) or PET-CT scan. All patients provided written consent before enrollment in the study. This study was approved by the Institutional Review Board of Konyang University Hospital and complied with the ethical guidelines for good clinical practice and provisions of the Helsinki Declaration and local law.
Vaccination Schedule and Treatment
The GV1001 vaccine was supplied as a freeze-dried peptide in sterile vials. GV1001 was manufactured by Samsung Pharm Co., Ltd., South Korea and supplied by Gemvax & Kael Co., Ltd. GV1001 was administered as 0.56 mg intradermal injection three times a week for the first week and then once a week for weeks 2, 3, 4, and 6. Afterwards, the injection was administered once every 28 days for six months. A total of 12 doses were administered in one cycle. Each patient underwent chemotherapy and did not discontinue the treatment during GV1001 administration.
Clinical Evaluation
Tumor response was evaluated using CT at 8-week intervals and classified according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1).
In RECIST 1.1, complete response (CR) is defined as the disappearance of all target lesions, partial response (PR) is defined as more than 30% reduction from the baseline sum of diameters, progressive disease (PD) is defined as more than 20% increase than the smallest sum of diameters in the study, and stable disease (SD) is defined as the state of neither having sufficient shrinkage to qualify for PR nor a sufficient increase to qualify for PD. Physical examination, blood screening, and ADR assessment of adverse drug reactions were performed during each visit. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and were considered treatment-related if they were possibly drug-related or suspected.
Quality of Life (QOL) Measurement
The QOL after GV1001 administration was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and EuroQol-5D-3L questionnaire (EQ-5D-3L). EORTC QLQ-C30 is a system for evaluating the QoL of patients with cancer and consists of 30 questions. The tool comprises three subcategories: a Global Health Status/QoL scale, functional scales, and symptom scales. The functional scales include 15 items on physical, role, cognitive, emotional, and social functioning. The symptom scale includes 13 items, namely fatigue, nausea/vomiting, pain, dyspnea, appetite loss, constipation, diarrhea, and financial difficulties. Each item was evaluated on a four-point Likert scale, with the exception of the Global Health Status/QoL scale, which was evaluated on a seven-point scale. A higher Global Health Status/QoL scale or functional scale score indicates a better quality of life. In contrast, the higher symptom scales indicate more severe symptoms.
The EQ-5D descriptive system is a preference-based Health-Related Quality of Life (HRQL) measurement with one question for each of the five dimensions: mobility, self-management, routine activities, pain/inconvenience, and anxiety/depression. Each patient chooses the statement in each dimension that best describes their health condition on the day of examination. Their responses are coded as numbers (1, 2, or 3) corresponding to the respective level of severity: 1 indicates no problems, 2 indicates some problems, and 3 indicates extreme problems. Patients who completed the EORTC QLQ-C30 and EQ-5D-3L questionnaires two times before and after 12 doses of GV 1001 injection were included in this study.
Statistical Analysis
Kaplan–Meier analysis was used to analyze progression-free survival (PFS) and overall survival (OS). A P-value less than 0.05 was considered statistically significant. The general characteristics of the participants were expressed as frequency and percentage. Student’s t-test for paired samples was used to compare between the values at baseline and those at different monitoring times during treatment. Two sample Student’s t-test for independent samples was used to compare the mean scores before and after treatment. SPSS software program (Version 27.0; SPSS, Chicago, IL, USA) was used for statistical analysis. Stuart Maxwell test was performed to verify the relationship between the participants’ mobility, self care, usual activity, pain/discomfort, and anxiety/depression by R software program (version 4.3.1).19
Ethical Approval
GV1001 treatment was approved by the KFDA and Konyang University Hospital Review Committee. Informed consent was obtained from all the enrolled patients. The study was conducted in compliance with the World Medical Association Declaration of Helsinki.
Results
Patient Characteristics
Between May 2018 and December 2020, 63 patients with advanced-stage IV breast cancer were assigned GV 1001. Baseline patient demographics and disease characteristics are shown in Table 1. The median age was 56 years (range, 34–81 years), and 60% of the patients had an ECOG performance status of 0–1. In terms of receptor subtypes, 34 patients (54%) were estrogen receptor (ER)-positive, 21 (33%) were human epidermal growth factor receptor-2 (HER-2)-positive, and 8 (12%) had triple-negative breast cancer (TNBC). Visceral, bone, and brain metastases were observed in 54 (85%), 9 (14%), and 5 patients (8%), respectively. Patients received a median of five (range, 1–9) prior lines of chemotherapy for metastatic breast cancer. A total of 35 patients (55%) were treated with three or more lines of cytotoxic chemotherapy, excluding adjuvant chemotherapy. All patients had previously received anthracycline or taxane treatment and 38 (60%) were treated with eribulin. A total of 39 patients (62%) were treated with only one cycle of GV 1001 and 24 patients (38%) were treated more than two cycles of GV 1001.
 |
Table 1 Baseline Characteristics of the Patients |
Tumor Response
The treatment responses according to the RECIST 1.1 criteria are shown in Table 2, where CR was observed in one (1.5%) patient in the HER-2 positive group. In the subgroup analysis, PR was observed in nine (26.4%), five (23.8%), and two (25%) patients with hormone receptor (HR)-positive, HER-2 positive, and TNBC, respectively. The objective response rates (ORR) was 26.4%, 28.5%, and 25% in patients with HR positive, HER-2 positive, and TNBC, respectively. The disease control response (DCR) was 58.8%, 66.6%, and 50% in patients with HR positive, HER-2 positive, and TNBC, respectively. Based on investigator assessment, the median PFS was 10.4, 8.7, and 5.6 months in the patients with HR positive, HER-2 positive, and TNBC, respectively (Figure 1). The OS was 19.7, 13.2, and 9.4 months in the patients with HR positive, HER-2 positive, and TNBC, respectively (Figure 2).
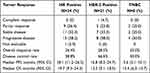 |
Table 2 Treatment Efficacy (N=63, %) |
Toxicity
Combination therapy with cytotoxic chemotherapy and GV1001 was well tolerated. Twenty seven patients developed transient severe neutropenia (CTCAE grade ≥ 3) and 23 patients had severe thrombocytopenia (CTCAE grade ≥ 3), which was considered to be related to cytotoxic chemotherapy. No other grade 3/4 toxicities were associated with treatment. In most patients, grade 1–2 side effects were recorded, which were most commonly nausea, vomiting, muscle pain, dizziness, or local skin reactions at the GV1001 injection site. Urticaria (grade ≤2) was observed in 6 patients. However, there was no evidence of long-term toxicity in the patients with extended survival despite administering repeated GV1001 booster vaccinations (Table 3).
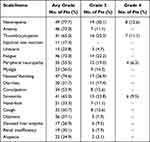 |
Table 3 Frequency of Adverse Events |
Quality of Life (QOL)
EORTC QLQ-C30
Global health status: The overall health condition and quality of life experienced by the patients after receiving the GV 1001 treatment showed a significant increase compared to those in the pre-treatment condition (p < 0.05).
- Functional scale: The difference in QLQ-C30 evaluation before and after GV 1001 treatment was verified. After treatment, the physical (P <0.05) and emotional (P <0.05) functions were evaluated as higher than their values before treatment in the functional area, while the role and social functions did not show any significance.
- Symptom scale/Items: In three and six single symptoms, fatigue (P <0.05) was evaluated to be significantly lower after GV1001 injection than that before treatment. Other symptoms tended to show improvements; however, this was not statistically significant (Table 4, Figure 3).
 |
Table 4 Quality of Life Score Derived from EORTC QLQ-C30 Questionnaire After GV1001 Treatment |
EQ-5D-3L
Mobility: Mobility showed an overall improvement after treatment, especially in the no-problem question. However, the difference was not statistically significant.
- Self-care: After GV1001 treatment, the number of patients who reported no problems increased. The patients with self-care enabled showed a statistically significant decrease (P <0.05).
- Usual activity: The number of patients who checked for “no problem” showed a significant increase, while the number of patients who checked the “enable” showed a significant decrease (P <0.05).
Pain/discomfort: Patients who complained of severe pain showed a decreased pain. However, the number of patients who checked “some problems” increased which could be attributed to a decrease in the number of patients complaining of severe pain.
Anxiety/Depression: Patients complaining of anxiety generally showed a decrease in number, and patients who reported “no problem” showed an increase in number which could be attributed to improvement in the patient’s general condition (Table 5).
 |
Table 5 Quality of Life Score Derived from EQ-5D Questionnaire After GV1001 Treatment |
Discussion
This study presents the findings of a retrospective study on a novel peptide-based cancer vaccine for advanced breast cancer. Our data showed that vaccination with GV1001 was well tolerated and increased physical performance was correlated with an improved quality of life. GV1001 vaccine improved the symptoms of the disease, such as dyspnea, insomnia, loss of appetite, constipation, and diarrhea. The cytotoxic chemotherapy-related side effects were similar to those reported in other studies, and there was no overlapping toxicity due to GV1001 treatment. Tissue-resident dendritic cells at the vaccination site present GV1001 peptides to naïve T cells in the lymph nodes. The dendritic cells expressing GV1001 cross-bind to CD4+T helper 1 (Th1) cells and CD8+ T cells, allowing CD4+ T cells to directly stimulate CD8+T cells through IL-2 secretion with the co-stimulation of APCs.20 The GV1001 specific T cells leave the lymph nodes, enter the systemic circulation, and translocate to the tumor microenvironment.21 Tumor cells presenting hTERT to MHC Class II can be killed either directly through cytokine secretion by CD8+ cells or indirectly through macrophage activation.22,23 Dissolved tumor cells release hTERT or neoantigens, which are phagocytized by APCs and presented again to T cells, leading to the extension of the immune response through epitope dissolution and expansion. Eventually, hTERT, which is expressed during tumor development, can be a target antigen that can activate the CD4+Th1 response, regardless of the rapidly evolving genetic composition of the tumor.24,25 Activated CD4+Th1 cells enhance the immune response through the release of inflammatory cytokines such as interleukin (IL)-2 or IL-10. Important characteristics of CD4 + T cells in cancer cell death include effective antigen presentation by APC, activation and proliferation of CD 8+ T cells, homing of T cells into the tumor environment, and formation of memory T cells.26–28
Choi et al revealed that GV1001 downregulates the expression of enolase 1-induced pro-inflammatory cytokines. When enolase 1 was stimulated, p38 mitogen-activated protein kinase (MAPK) and NF-κB were activated, but they were successfully suppressed when treated with GV1001. Accordingly, GV1001 may be an effective anti-inflammatory peptide that decreases the production of pro-inflammatory cytokines through the suppression of p38 MAPK and NF-κB activation. In addition, administration of GV1001 reduced the expression of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β, and IL-6, in peripheral blood mononuclear cells.29 Moreover, Ko et al reported that the downregulation of p38 MAPK and ERK-signaling by GV1001 reduced the production of lipopolysaccharide (LPS)-induced inflammatory mediators IL-6 and TNF-α on dental pulp cells without significant cytotoxicity. Furthermore, high-dose GV1001 treatment markedly inhibited the phosphorylation of the MAPKs, ERK and p38, in LPS-stimulated dental pulp cells. These findings provide mechanistic insights into the anti-inflammatory actions of the GV1001 peptide in LPS-stimulated pulpitis without significantly affecting cell viability.30 Park et al showed that H2O2 significantly reduced the viability of neural stem cells and that GV1001 has antioxidant effects against H2O2. Finally, they confirmed the antioxidant effects of GV1001 on intracellular signaling proteins in neural stem cells injured by oxidative stress.31 These anti-inflammatory and anti-oxidant effects can help improve the QOL of patients by suppressing the inflammatory reactions caused by tumors and reducing the secretion of cytotoxic cytokines.
Telomeres are expressed in more than 90% of breast cancer cells, but are not expressed in most normal cells; therefore, they can be diagnostic and treatment targets. High telomerase activity in breast cancer is associated with genetic abnormalities at the 3q (gain), 8q (gain), and 17p (deletion) sites. Genetic abnormalities that are commonly observed in breast cancer, such as hTR (3q), c-myc (8q), and p53 (17p), are associated with the regulation of telomerase.32 Yashima et al revealed that the average telomerase level gradually increased according to the severity of histopathological changes, with 14% in benign breast disease, 92% in cancer-site lesions, and 94% in invasive breast cancer.33 Hoos et al showed a close connection between telomerase activity and breast cancer stage.34 A significant association has also been observed between telomerase-positive infiltrating breast cancer and lymphatic invasion, a basic stage of breast cancer metastasis and predictor of survival, rendering telomerase as a useful prognostic indicator.35 Clark et al reported in a study examining patients with lymph node-positive breast cancer that the increased telomerase activity is associated with decreased disease-free survival.36 While most progress in understanding the role of telomerase in breast cancer has been achieved in diagnosis, there is an increasing number of studies examining its role in the treatment of breast cancer using telomerase inhibitors. Breast cancer cells with short telomeres are the most affected cells by telomere inhibitors, whereas normal stem cells with long telomeres are relatively resistant.37 The effectiveness of telomerase inhibitors depends on the initial telomere length and rate of cell division; thus, observing changes in tumor size can occur within several weeks to months.38 Telomerase is broken down by proteasomes to form hTERT-derived peptides that are presented as antigens on the surface of cancer cells.39 This can induce antitumor reactions through CD4+ or CD8+ cytotoxic T lymphocytes activated by GV1001.4 Therefore, combining telomere inhibitors with current therapies to reduce the tumor burden may provide a better treatment outcome in terms of preventing breast cancer and its recurrence. This study has limitations with respect to statistical significance because there was no control group and the number of patients was small. However, to the best of our knowledge, this is the first study to analyze the clinical significance of patients who received both cytotoxic chemotherapy and GV1001 immunotherapy for breast cancer.
Conclusion
Although the number of patients included in this study was small, these data showed that tumor-specific immune responses to hTERT peptides can be induced in patients with breast cancer. The immunologic response rate was considerable compared to that in previous GV1001 trials without concomitant chemotherapy, although low toxicity was retained. The study findings warrant further studies on GV1001 combined with chemotherapy. Moreover, it supports the general concept that combining cancer vaccination with a telomerase inhibitor (GV1001) and current chemotherapies to reduce tumor burden may thus provide a better regimen to target breast cancer and prevent recurrence. Therefore, a future prospective research study needs to compare between a group of patients who only received standard chemotherapy and other patients who received both standard chemotherapy and GV1001 immunotherapy for breast cancer. Based on the findings of this study and that of other studies on immune responses and clinical efficacy, measures should be taken to increase the magnitude and duration of the immune response to GV1001.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
GV1001 was provided by Gemvax & Kael Co. Ltd. (Seoul, Korea).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol Life Sci. 2016;73:1659–1670. doi:10.1007/s00018-016-2146-9
2. Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10(6):673–679. doi:10.1016/S1074-7613(00)80066-7
3. Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov. 2006;5:577–584. doi:10.1038/nrd2081
4. Jafri MA, Ansari SA, Alqahtani MH, et al. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69. doi:10.1186/s13073-016-0324-x
5. Liu JP, Chen W, Schwarer AP, et al. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805:35–42. doi:10.1016/j.bbabio.2010.04.123
6. Inderberg-Suso EM, Trachsel S, Lislerud K, Rasmussen AM, Gaudernack G. Widespread CD4+ T-cell reactivity to novel hTERT epitopes following vaccination of cancer patients with a single hTERT peptide GV1001. Oncoimmunol. 2012;1:670–686. doi:10.4161/onci.20426
7. Staff C, Mozaffari F, Frodin JE, et al. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int J Oncol. 2014;45:1293–1303. doi:10.3892/ijo.2014.2496
8. Brunsvig PF, Aamdal S, Gjertsen MK, et al. Telomerase peptide vaccination: a Phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi:10.1007/s00262-006-0145-7
9. Mizukoshi E, Kanek S. Telomerase-targeted cancer immunotherapy. Int J Mol Sci. 2019;20:1823. doi:10.3390/ijms20081823
10. Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomized, Phase 3 trial. Lancet Oncol. 2014;15:829–840. doi:10.1016/S1470-2045(14)70236-0
11. Greten TF, Forner A, Korangy F, et al. A Phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. doi:10.1186/1471-2407-10-209
12. Kyte JA, Gaudernack G, Dueland S, et al. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011;17(13):4568–4580. doi:10.1158/1078-0432.CCR-11-0184
13. Su Z, Dannull J, Yang BK, et al. Telomerase mRNA transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi:10.4049/jimmunol.174.6.3798
14. Hunger RE, Lang K, Markowski CJ, et al. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother. 2011;60:1553–1564. doi:10.1007/s00262-011-1061-z
15. Matthew JL, Judit SA, Gabrielle SL, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911–926. doi:10.1038/s43018-022-00418-6
16. Mazloumi Z, Rafat A, Dizaji Asl K, et al. A combination of telomerase inhibition and NK cell therapy increased breast cancer cell line apoptosis. Biochem Biophys Res Commun. 2023;640:50–55. doi:10.1016/j.bbrc.2022.11.090
17. Shi Y, Sun L, Chen G, et al. A combination of the telomerase inhibitor, BIBR1532, and paclitaxel synergistically inhibit cell proliferation in breast cancer cell lines. Target Oncol. 2015;10(4):565–573. doi:10.1007/s11523-015-0364-y
18. Nasrollahzadeh A, Bashash D, Kabuli M, et al. Arsenic trioxide and BIBR1532 synergistically inhibit breast cancer cell proliferation through attenuation of NF-κB signaling pathway. Life Sci. 2020;257:118060. doi:10.1016/j.lfs.2020.118060
19. Stuart A. A test for homogeneity of the marginal distributions in a two-way classification. Biometrika. 1955;42:412–416. doi:10.1093/biomet/42.3-4.412
20. Zanetti M. A second chance for telomerase reverse transcriptase in anticancer immunotherapy. Nat Rev Clin Oncol. 2017;14:115–128. doi:10.1038/nrclinonc.2016.67
21. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
22. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2020;28:5–17. doi:10.1038/s41417-020-0183-x
23. Haabeth OA, Tveita AA, Fauskanger M, et al. How do CD4(+) T cells detect and eliminate tumor cells that either lack or express MHC class II molecules. Front Immunol. 2014;5:174. doi:10.3389/fimmu.2014.00174
24. Dosset M, Castro A, Carter H, Zanetti M. Telomerase and CD4 T cell immunity in cancer. Cancers. 2020;12:1687. doi:10.3390/cancers12061687
25. Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180(5):3122–3131. doi:10.4049/jimmunol.180.5.3122
26. Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi:10.1126/science.1251102
27. Janssen EM, Droin NM, Lemmens EE, et al. CD4 T-cell help controls CD8 T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi:10.1038/nature03337
28. Oh DY, Kwek SS, Raju SS, et al. Intratumoral CD4(+) T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell. 2020;181:1612–1625. doi:10.1016/j.cell.2020.05.017
29. Choi J, Kim H, Kim Y, et al. The anti-inflammatory effect of GV1001 is mediated by the downregulation of ENO1-induced pro-inflammatory cytokine production. Immune Netw. 2015;15:291–303. doi:10.4110/in.2015.15.6.291
30. Ko YJ, Kwon KY, Kum KY, et al. The anti-inflammatory effect of human telomerase-derived peptide on P. gingivalis lipopolysaccharide-induced inflammatory cytokine production and its mechanism in human dental pulp cells. Mediators Inflamm. 2015;2015:385127. doi:10.1155/2015/385127
31. Park HH, Yu HJ, Kim S, et al. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology. 2016;55:131–141. doi:10.1016/j.neuro.2016.05.022
32. Loveday RL, Greenman J, Drew PJ, et al. Genetic changes associated with telomerase activity in breast cancer. Int J Cancer. 1999;84(5):516–520. doi:10.1002/(SICI)1097-0215(19991022)84:5<516::AID-IJC12>3.0.CO;2-Y
33. Yashima K, Milchgrub S, Gollahon LS, et al. Telomerase enzyme activity and RNA expression during the multistage pathogenesis of breast carcinoma. Clin Cancer Res. 1998;4:229–234.
34. Hoos A, Hepp HH, Kaul S, et al. Telomerase activity correlates with tumor aggressiveness and reflects therapy effect in breast cancer. Int, J, Cancer. 1998;79(1):8–12. doi:10.1002/(SICI)1097-0215(19980220)79:1<8::AID-IJC2>3.0.CO;2-5
35. Mokbel KM, Parris CN, Ghilchik M, et al. Telomerase activity and lymphovascular invasion in breast cancer. Eur J Surg Oncol. 2000;26(1):30–33. doi:10.1053/ejso.1999.0736
36. Clark GM, Osborne CK, Levitt D, et al. Telomerase activity and survival of patients with node-positive breast cancer. J Natl Cancer Inst. 1997;89(24):1874–1881. doi:10.1093/jnci/89.24.1874
37. Lu L, Zhang C, Zhu G, et al. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13(3):R56. doi:10.1186/bcr2893
38. Bolzán AD. Effect of chemotherapeutic drugs on telomere length and telomerase activity. Telomere Telomerase. 2016;3:e1488.
39. Xu Y, Goldkorn A. Telomere and telomerase therapeutics in cancer. Genes. 2016;7(6):22. doi:10.3390/genes7060022
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



