Back to Journals » OncoTargets and Therapy » Volume 17
Response to Savolitinib in a Patient with Advanced Poorly Differentiated Lung Carcinoma Positive for a Novel EML4-MET Gene Fusion
Authors Ouyang G , Shu P, Xue Y, Luo F, Li Y
Received 30 September 2023
Accepted for publication 17 January 2024
Published 30 January 2024 Volume 2024:17 Pages 79—84
DOI https://doi.org/10.2147/OTT.S442685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Ganlu Ouyang,1,2,* Pei Shu,3,4,* Yinyin Xue,1,2,* Feng Luo,1,2 Yan Li1,2
1Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3Devision of Thoracic Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 4Clinical Trial Center, National Medical Products Administration Key Laboratory for Clinical Research and Evaluation of Innovative Drugs, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Li
Department of Medical Oncology, Cancer Center / Lung Cancer Center, West China Hospital, Sichuan University, No. 37, Guo Xue Xiang, Chengdu, Sichuan Province, 610041, People’s Republic of China
, Tel +86 18980606806
, Email [email protected]
Background: Cellular-mesenchymal to epithelial transition factor (c-MET) alterations have significant therapeutic implications in non-small cell lung cancer (NSCLC). Although MET fusion is a rare genomic event, advances in detection technologies have enabled the identification of various MET fusion partner genes. However, standard therapeutic options for MET fusion in NSCLC cases remain undefined. This report presents a novel fusion variant, EML4-MET, encompassing exons 1 to 13 of EML4 and exons 15 to 21 of MET, including the entire MET kinase domain, and discusses the response of this case to savolitinib treatment.
Case Presentation: A 65-year-old woman was diagnosed with advanced poorly differentiated lung carcinoma. Molecular profiling of circulating tumor DNA (ctDNA), carried out by next-generation sequencing (NGS), identified a novel EML4-MET fusion. The patient was administered the MET receptor tyrosine kinase inhibitor savolitinib at 400 mg daily. One month later, computed tomography (CT) revealed some lesions with volume reduction. However, COVID-19 diminished the efficacy of savolitinib. Regrettably, the patient succumbed to respiratory and circulatory failure due to disease progression in March 2023.
Conclusion: This case uncovers a new type of MET fusion and expands the range of potential MET fusion targets in NSCLC. The patient responded to savolitinib, suggesting a reference basis for the treatment of similar cases with EML4-MET fusion in the future. Additional research is warranted to assess the biological significance of the EML4-MET fusion in NSCLC.
Keywords: non-small cell lung cancer, EML4-MET, savolitinib
Introduction
The cellular-mesenchymal to epithelial transition factor (c-MET) gene, which encodes a receptor tyrosine kinase, is important in cell cycle regulation. Thanks to recent advances in detection technologies, various MET alterations, including MET exon 14 skipping mutations, MET amplifications, and MET fusions, are increasingly identified in non-small cell lung cancer (NSCLC).1 MET fusion, an uncommon structural rearrangement, has been reported in approximately 0.26% of NSCLC cases.1–3 Currently, no standardized therapeutic options for NSCLC patients with MET fusion are available. Several isolated case reports have suggested the potential efficacy of MET tyrosine kinase inhibitors (TKIs) in NSCLC patients with MET fusion.2,4,5 However, multiple fusion partners for MET have been reported, and the therapeutic efficacy of MET TKIs on these newly identified rare MET fusion variants remains undefined, which requires further research. Here, we report a novel fusion variant, EML4-MET, found in NSCLC and discuss the response of the case to savolitinib treatment. We present the following case in accordance with the CARE reporting checklist.
Case Presentation
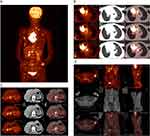
In August 2022, a 65-year-old Chinese woman, a never-smoker, was admitted to our hospital. She had a three-month history of progressive dyspnea, cough, and bloody sputum. The patient’s overall health was poor due to a five-year history of hypertension and a 30-year history of rheumatoid arthritis. Her family history regarding cancer was unclear. She had an Eastern Cooperative Oncology Group (ECOG) performance scale score between 3 and 4. Positron emission tomography-computed tomography (PET-CT) revealed a space-occupying lesion in the right upper lobe of the lung, along with multiple masses in the pericardium, various skeletal sites (including bilateral ribs, thoracic and lumbar vertebrae, iliac bone, and bilateral femoral bones), right latissimus dorsi muscle, and left adrenal gland (Figure 1). Immunohistochemistry (IHC) showed positivity for pan cytokeratin (PCK), cytokeratin 7 (CK7), cytokeratin 5/6 (CK5/6) and Ki67 (60%), and negative for Napsin A, thyroid transcription factor 1 (TTF1), P63, chromogranin A (CgA), and synaptophysin (Syn). Morphologically, the appearance was consistent with poorly differentiated carcinoma, with adenocarcinoma features (Figure 2). A diagnosis of stage IVB (cT4N3M1c) poorly differentiated carcinoma was confirmed by PET-CT and pathological examination of a coarse needle biopsy from the right axillary lymph node. Further, a blood sample was collected for next-generation sequencing (NGS)-based molecular profiling of circulating tumor DNA (ctDNA) (conducted by OrigiMed, Shanghai, China), which identified a novel EML4-MET fusion, comprising exons 1 to 13 of EML4 and exons 15 to 21 of MET (including the complete MET kinase domain) (Figure 3). The allele frequency of EML4-MET was 0.4%. NGS also identified a TP53 gene mutation (exon 10, abundance: 6.8%) and an NTRK3 mutation (exon 10, abundance: 5.3%).
 |
Figure 2 H&E, typical image of pathology. Immunohistochemical staining images of pan cytokeratin (PCK) (+), cytokeratin 7 (CK7) (+), cytokeratin 5/6 (CK5/6) (+) and Ki67 (60%). |
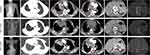
Following diagnosis, the patient opted out of chemotherapy and was prescribed anlotinib for a two-week course. However, due to hemoptysis, an adverse event, anlotinib was discontinued. Following a multi-disciplinary treatment (MDT) discussion in October 2022, oral savolitinib treatment (400 mg once daily) was started. After one month, CT showed that the patient had achieved stable disease (SD), with no significant size changes for some lesions (right upper lung lobe, pericardium, skeleton, right latissimus dorsi muscle, and left adrenal gland). There were also reductions in pericardial and right pleural effusions, revealing savolitinib as a viable therapeutic option for this patient. We therefore continued the treatment with savolitinib. However, the patient was diagnosed with coronavirus disease 2019 (COVID-19) in December 2022. Next, the patient had progressive exertional dyspnea after a 3-month treatment of savolitinib, due to treatment delay and COVID-19 complications. A subsequent CT scan revealed significantly increased right pleural and pericardial effusions, as well as a small left pleural effusion, indicating progressive disease (PD) (Figure 4). Following progression on savolitinib, the patient underwent thoracic and pericardial catheter drainage. She was then administered chemotherapy with pemetrexed disodium (0.6 g) for two weeks. Unfortunately, in March 2023, the patient succumbed to respiratory failure due to disease progression. The patient provided informed consent for the publication of this case.
Discussion
This study reports the identification of a novel EML4-MET fusion variant in NSCLC. However, no previous studies have examined the therapeutic implications of this fusion. The case had a response to off-label treatment with savolitinib, a MET-TKI with activity against MET exon 14 skipping mutation. To the best of our knowledge, this study provides the first clinical evidence for the efficacy of savolitinib in NSCLC with EML4-MET fusion.
Savolitinib, a highly selective small-molecule MET-TKI, received conditional approval by the China National Medical Products Administration (NMPA) in 2021 for the clinical treatment of NSCLC with MET exon 14 skipping mutations.6–8 Previous reports have also indicated that savolitinib is effective in NSCLC patients with MET amplification after previous treatment with EGFR-TKIs.9 However, the efficacy of MET-TKIs, especially savolitinib, is not fully assessed in MET fusion-positive NSCLC. Previous case studies reported responses to another MET-TKI, crizotinib, in MET fusion-positive NSCLC cases.2,5,10 Zhuo et al found that the most frequent breakpoint within the MET fusion gene is in intron 14, causing the retention of exons 15–21, which span the entire kinase region. This study also reported that MET gene fusions with upstream genes often result from inversions,1 akin to the EML4-MET fusion gene in the current case. Therefore, the mechanism of EML4-MET fusion mutations might parallel that of MET exon 14 skipping mutation, which is characterized by loss of negative regulation in the juxtamembrane region.4,11 Moreover, EML4-MET fusion mutations including the 3’ MET kinase domain via fusion at exon 15 may become activated due to the constitutive dimerization of MET.4,12 This led our MDT experts to postulate that the present case with EML4-MET fusion might respond to savolitinib. However, future studies are required to explore the specific mechanism of action of this fusion gene, given the heterogeneous responses of various MET fusion partners to MET-TKIs.
In February 2023, Sun et al reported a lung squamous cell carcinoma case harboring an EML4-MET fusion who, upon crizotinib treatment, achieved a partial response (PR).13 This study suggested that lung squamous cell carcinoma patients with MET fusions might benefit from crizotinib. However, the reported therapeutic efficacy only lasted one month. In the current case, a SD response was obtained after one month of savolitinib treatment but progressed to PD after three months owing to COVID-19. Therefore, the long-term effectiveness of crizotinib in this MET fusion case remains uncertain. Given that Sun et al only reported a short follow-up period, the long-term efficacy of crizotinib requires further investigation. Moreover, while the case reported by Sun et al was squamous carcinoma, the current patient had poorly-differentiated adenocarcinoma. Even though they share the same mutated gene, pathological differences might affect the efficacy of MET-TKIs. Furthermore, the current patient with the EML4-MET fusion mutation did not respond to chemotherapy, corroborating Sun et al, suggesting further investigation into these rare genomic alterations is required.
In addition, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, causing COVID-19, has a major impact on cancer.14 Cancer treatment was frequently delayed during the COVID-19 pandemic.15 In addition, lung cancer patients are at higher risk of a worse outcome of COVID-19.16 In this study, the patient was diagnosed with COVID-19 after a 1-month treatment with savolitinib, and had progressive disease after a 3-month treatment with savolitinib. The MDT experts speculated that PD in this patient was caused by treatment delay and COVID-19 complications. However, whether the tumor was too malignant or COVID-19 caused disease progression is unknown. Further real-world studies are required to confirm the impact of COVID-19 on targeted therapies in NSCLC.
This case report had a limitation. The blood sample was subjected to NGS analysis due to a limited specimen remaining after pathological examination. Therefore, it could not be definitively ascertained whether the fusion forms identified in the blood sample and tumor tissue were different.
Conclusions
In conclusion, a novel EML4-MET fusion was reported in a patient with NSCLC, and the efficacy of savolitinib in this case was described. For future clinical applications, the specific mechanisms of action of this fusion gene warrant investigation, due to the heterogeneous response to treatment.
Data Sharing Statement
The data will be shared on reasonable request to the corresponding author.
Ethics Approval and Consent to Participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Ethics committee on Biomedical Research, West China Hospital of Sichuan University (2023-2236). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Consent for Publication
Written Informed Consent for publication has been obtained from patient.
Acknowledgments
The authors thank the academic support from OrigiMed Diagnostics.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Zhuo M, Liang Z, Yi Y, et al. Analysis of MET kinase domain rearrangement in NSCLC. Lung Cancer. 2020;145:140–143. doi:10.1016/j.lungcan.2020.04.040
2. Liu J, Shen L, Qian Y, Liu Y, Su M, Yi L. Durable response to crizotinib in an advanced lung adenocarcinoma patient harboring rare CD47-MET fusion: a case report. Transl Cancer Res. 2022;11(8):2931–2935. doi:10.21037/tcr-22-141
3. Ai X, Yu Y, Zhao J, et al. Comprehensive analysis of MET mutations in NSCLC patients in a real-world setting. Therapeut Adv Med Oncol. 2022;14:17588359221112474. doi:10.1177/17588359221112474
4. Blanc-Durand F, Alameddine R, Iafrate AJ, et al. Tepotinib efficacy in a patient with non-small cell lung cancer with brain metastasis harboring an HLA-DRB1-MET gene fusion. Oncologist. 2020;25(11):916–920. doi:10.1634/theoncologist.2020-0502
5. Ma Q, Kong L, Zhong D. Case report: dramatic response to crizotinib in a patient with non-small cell lung cancer positive for a novel ARL1-MET fusion. Front Oncol. 2022;12:804330. doi:10.3389/fonc.2022.804330
6. Markham A. Savolitinib: first Approval. Drugs. 2021;81(14):1665–1670. doi:10.1007/s40265-021-01584-0
7. Zhu X, Lu Y, Lu S. Landscape of savolitinib development for the treatment of non-small cell lung cancer with MET alteration-A narrative review. Cancers. 2022;14(24). doi:10.3390/cancers14246122
8. Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, Phase 2 study. Lancet Respir Med. 2021;9(10):1154–1164. doi:10.1016/S2213-2600(21)00084-9
9. Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21(3):373–386. doi:10.1016/S1470-2045(19)30785-5
10. Zhu YC, Wang WX, Xu CW, et al. Identification of a novel crizotinib-sensitive MET-ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol. 2018;29(12):2392–2393. doi:10.1093/annonc/mdy455
11. Zhu YC, Wang WX, Song ZB, et al. MET-UBE2H fusion as a novel mechanism of acquired EGFR resistance in lung adenocarcinoma. J Thorac Oncol. 2018;13(10):e202–e204. doi:10.1016/j.jtho.2018.05.009
12. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5(1):4846. doi:10.1038/ncomms5846
13. Sun D, Wu W, Wang L, et al. Identification of MET fusions as novel therapeutic targets sensitive to MET inhibitors in lung cancer. J Transl Med. 2023;21(1):150. doi:10.1186/s12967-023-03999-7
14. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discovery. 2020;10(6):783–791. doi:10.1158/2159-8290.CD-20-0422
15. Aapro M, Lyman GH, Bokemeyer C, et al. Supportive care in patients with cancer during the COVID-19 pandemic. ESMO open. 2021;6(1):100038. doi:10.1016/j.esmoop.2020.100038
16. de Joode K, Dumoulin DW, Tol J, et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi:10.1016/j.ejca.2020.09.027
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.