Back to Journals » Infection and Drug Resistance » Volume 17
Reliability of Droplet Digital PCR Alone and in Combination with Interleukin-6 and Procalcitonin for Prognosis of Bloodstream Infection
Authors Yin S, Lin Y, Wang B , Peng Y, Wang Z, Zhu X, Liang H, Li X, Wang M
Received 18 September 2023
Accepted for publication 23 February 2024
Published 15 March 2024 Volume 2024:17 Pages 1051—1071
DOI https://doi.org/10.2147/IDR.S439683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Sheng Yin,1 YingRui Lin,1 Bingqi Wang,1 Yizhi Peng,2 Zeyou Wang,1 Xiaolin Zhu,1 Hao Liang,1 Xianping Li,1 Min Wang1
1Department of Laboratory Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, People’s Republic of China; 2Department of Laboratory Medicine, Hunan Cancer Hospital, Central South University, Changsha, Hunan, 410031, People’s Republic of China
Correspondence: Min Wang, Department of Laboratory Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, People’s Republic of China, Tel +86 13298697558, Email [email protected]
Purpose: Bloodstream infection(BSI) is linked with high mortality, underscoring the significance of prompt etiological diagnosis for timely and precise treatment. This study aims to investigate the diagnostic value of droplet digital polymerase chain reaction(ddPCR) in combination with conventional inflammatory markers [interleukin-6(IL-6) and procalcitonin(PCT)] concerning disease progression and treatment prognosis in BSI patients. Furthermore, the study aims to explore a more efficient clinical application strategy.
Patients and Methods: This prospective case seried study centers on 176 patients suspected of or confirmed with BSI. Blood samples were collected to extract nucleic acids for identifying pathogens (bacteria, fungi, and viruses) and determining copy loads via ddPCR.
Results: The sensitivity of ddPCR was markedly higher compared to the culture method (74.71% vs 31.03%). A positive correlation existed between bacterial load and levels of inflammatory markers [IL-6 (P= 0.0182), PCT (P= 0.0029), and CRP (P= 0.0005)]. In suspected BSI cases, the combination of ddPCR and inflammatory markers could predict sepsis risk [ROC: Area under the curve(AUC)=0.6071, P= 0.0383]. Within confirmed BSI patients, the ddPCR bacterial load of those with SOFA< 7 was lower than that of the SOFA≥ 7 (P= 0.0334). ddPCR (OR: 1.789, P= 0.035) monitoring combined with PCT (OR: 1.787, P= 0.035) holded predictive value for SOFA progression (AUC=0.7913, P= 0.0003). Similarly, BSI survivors displayed a lower burden than non-survivors (P= 0.0170). Additionally, ddPCR combinated with IL-6 provided a more accurate and expedited insight into clinical outcomes prediction for BSI confirmed patients (AUC=0.7352, P= 0.0030). Serial monitoring of bacterial load by ddPCR effectively mirrored the clinical course of BSI in patients. Notably, patients with positive ddPCR virus infection exhibited significantly reduced lymphocyte counts (P= 0.0003).
Conclusion: In a clinical context, qualitative ddPCR results and quantitative continuous monitoring can more precisely assess sepsis progression and treatment prognosis in BSI patients. Furthermore, ddPCR results offer quicker and more accurate reference points for clinical antibacterial and antiviral interventions.
Keywords: bloodstream infection, droplet digital polymerase chain reaction, interleukin-6, procalcitonin, prognosis
Introduction
Bloodstream infection(BSI) in the intensive care unit has garnered significant clinical attention due to its high mortality rate. This is particularly relevant during the COVID-19 epidemic, as patients with severe pneumonia are susceptible to developing severe BSI, which may culminate in sepsis.1,2 Globally, an estimated 48.9 million patients succumb annually to BSI. However, current empiric treatment options for bacterial infections have limited efficacy, and the indiscriminate use of broad-spectrum antibiotics can lead to severe economic and health burdens on both patients and society.3 The diagnosis of BSI is currently mainly based on blood culture, which is considered the gold standard. However, the accuracy of blood culture results can be affected by several factors, including the number of blood pathogens, the timing and site of sampling, as well as various culture-related conditions such as culture time and environmental factors.4 In addition, the process of bacterial enrichment and plate inoculation required for blood culture, coupled with the need for mass spectrometry for accurate identification, contributes to a turnaround time(TAT) of more than five days for the official final report. Unfortunately, this long waiting time forces clinicians to rely on broad-spectrum drug resistance measures based on the patient’s condition and prior experience, leaving a significant proportion of patients with ineffective treatment. This situation is particularly dire for patients with sepsis in the intensive care unit, who may succumb to uncontrolled BSI that persists for nearly a week.5
At present, procalcitonin, interleukin-6 (IL-6), and C-reactive protein (CRP) are used to detect early infection in clinical practice. Procalcitonin (PCT) is the peptide precursor of the calcitonin hormone. The level of procalcitonin in healthy individuals was below the clinical detection limit (0.01μg/L). Procalcitonin levels rise within 24h in response to proinflammatory stimuli, particularly those of bacterial origin.6 Therefore, it is usually classified as an acute phase reactant of infection. With the inflammatory cascade and systemic response of severe infection, blood procalcitonin levels may rise by many orders of magnitude, with higher values associated with more severe disease. IL-6 is secreted by macrophages in response to bacterial infection. IL-6 plays a role in both innate and adaptive immune responses that can protect the host from various infections.7 When there is an inflammatory stimulus, CRP levels can increase from less than 10 μg/L to 50,000-fold and peak at 48h.8 The changes from various time-phase reactive proteins and infection are in the same direction. Droplet digital PCR (ddPCR) as an indicator to detect the species and load of pathogenic microorganisms in bloodstream infection, is also closely related to the severity of infection. To address the clinical demand for swift diagnosis of BSI, several institutions are engaged in developing new and advanced diagnostic methods.9 ddPCR represents a newer approach to PCR analysis that has demonstrated enhanced precision and sensitivity compared to traditional quantitative PCR methods.10 With its ultra-high sensitivity and absolute quantitative characteristics, digital PCR technology offers a promising solution for rapid detection of pathogenic fungi, bacteria, and viral nucleic acids in peripheral blood, allowing for the timely identification of BSI. Unlike conventional cultures, digital PCR technology can achieve reporting times of two to three hours. Moreover, it also demonstrates potential in quantifying pathogenic bacteria, detecting Drug-resistance genes, and dynamically monitoring disease progression. The adoption of clinical detection schemes based on digital PCR detection technology is expected to improve the clinical efficacy for patients suffering from BSI.
A compendium of studies conducted across distinct medical centers collectively underscores the multifaceted potential of ddPCR in clinical applications (Table 1). ddPCR assumed a dual role, facilitating both dynamic disease progression monitoring and offering drug guidance to inform antibiotic administration. BSI patients’ white blood cell count, procalcitonin, and C-reactive protein revealed a discernible correlation with ddPCR load.11 A study was undertaken on ddPCR’s sensitivity and rapidity in differentiating Acinetobacter baumannii and Klebsiella pneumoniae.12 Notably, ddPCR’s pragmatic applicability emerged in expediting pathogen and antimicrobial resistance(AMR) gene detection for critically patients, juxtaposed with metagenomic next-generation sequencing, a preferred choice in diagnosing BSI where conventional methodologies fail to identify causative agents.13,14 Furthermore, the clinical significance of ddPCR extended to monitoring pathogen load and species within BSI patients, intricately intertwined with patient clinical outcomes.15 However, these studies have not extensively explored methods to harness ddPCR results for optimal clinical efficacy.16 ddPCR’s quintessential feature lies in its capacity to quantify pathogen concentrations within blood samples.17 Nevertheless, the crux of mortality in BSI patients rests on unbridled bacterial proliferation leading to sepsis. Ergo, robust clinical interpretation of ddPCR outcomes necessitates integration with pertinent inflammatory indicators to derive precise diagnostic inferences. In this vein, the present study endeavors to provide constructive insights to enhance the clinical deployment of ddPCR.
 |
Table 1 The Related Research of ddPCR in Bloodstream Infection |
The present investigation directed its focus towards evaluative scrutiny encompassing the quantitative and qualitative manifestations of ddPCR. This scrutiny, in conjunction with pertinent clinical inflammatory markers including interleukin-6, PCT, and CRP, was steered towards a comprehensive monitoring of disease progression and the attendant clinical outcomes associated with BSI. Furthermore, the inquiry extended to the meticulous evaluation of ddPCR’s diagnostic precision in discerning both bacterial and viral agents. Concurrently, an appraisal of ddPCR’s clinical surveillance efficacy unfolded, particularly concerning its deployment within patients exhibiting signs suggestive of BSI (Figure 1).
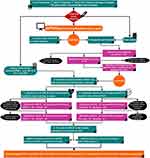 |
Figure 1 Flowchart of enrollment. The experimental design of this study and the inclusion and exclusion categories of patients are introduced in detail. |
Materials and Methods
Case and Patient Information Collection
This was a single-center prospective cohort study conducted at a teaching hospital in Changsha, China. The study protocol was reviewed and approved by the Hospital Ethics Committee (Ethical Review Committee of the Second Xiangya Hospital of Central South University, Changsha, China, No. 2022xyeyy051). All studies of individuals, samples, or data in this paper were conducted in accordance with the principles laid down in the Declaration of Helsinki. The study enrolled hospitalized patients over 18 years old with suspected BSI from November 2022 to February 2023. Inclusion criteria required at least two of the following clinical manifestations or laboratory test results: (i) peak body temperature over 38°C, (ii) increased white blood cell count without an alternative explanation, (iii) elevated levels of CRP, erythrocyte sedimentation rate, or IL-6 as determined by the clinician, (iv) increased PCT levels without an alternative explanation, (v) presence of BSI risk factors, and (vi) suspected or confirmed sepsis. Simultaneous blood samples, defined as samples collected within 2 hours, were sent for ddPCR testing, blood culture, and other laboratory tests, including CRP, PCT, IL-6, biochemical tests, and routine blood tests, as needed. Patients without key clinical parameters were excluded. The primary objective of the study was to evaluate the diagnostic accuracy of ddPCR for bacteria, fungi, and viruses, as well as the clinical value of ddPCR in patients with suspected BSI. Follow-up samples were collected until the patient recovered and was discharged or until the disease worsened leading to death. All participants signed an informed consent form (consent to be included in the study, to collect information from their electronic medical records and to collect blood samples for ddPCR analysis) by themselves or their guardians. In this prospective cohort study, a total of 176 hospitalized patients with suspected BSI were enrolled, most of whom were from the intensive care unit (ICU) (110, 62.5%). Each patient provided 2 blood samples, which was subjected to both ddPCR detection and blood culture.
Detection of Inflammatory Factors
IL-6 and PCT were determined by the double antibody sandwich method. Experiments were performed according to the kit instructions (Elecsy Brahms IL-6-09015612500V4/PCT-09318747500V1, Roche Diagnostics, Switzerland). Antigen-antibody complexes were formed by incubating 30μL of serum samples with a biotinylated monoclonal antibody. Ruthenium-labeled monoclonal antibody was incubated with magnetic beads coated with streptomycin. The complex is bound to the magnetic beads by the action of biotin and streptomycin. The reaction solution was sucked into the measuring cell, and the magnetic beads were adsorbed on the electrode surface by electromagnetic action. A certain voltage was applied to the electrode to make the complex chemiluminescence, and the luminescence intensity was measured by a photomultiplier. Elecsys software automatically calculated the test results through the calibration curve. The whole process was carried out on the automatic inspection instrument (Cobas e801, Roche Diagnostics, Switzerland). C-reactive protein in serum was determined by immunoturbidimetry. C-reactive protein was measured according to the manufacturer’s instructions (447,280, Beckman Coulter, USA). Goat polyclonal C-reactive protein antibody was mixed with 50uL of serum. Suspension particles were formed due to antigen-antibody reactions. The ratio of the scattered light enhancement of the solution determined by the instrument (Immage 800, Beckman Coulter, USA) was automatically calculated. Reference ranges: IL-6 (0–7.0 pg/mL), PCT (0–0.05 ng/mL), and CRP(0–10.0 mg/L).
Nucleic Acid Extraction from Peripheral Blood
In this study, patients suspected of BSI underwent blood collection for both blood culture and digital polymerase chain reaction (PCR) testing. Plasma cell-free DNA was extracted from 10 mL of blood using the Easy-CF2 plasma cell-free DNA extraction kit manufactured by Pilotene (China). A mixture of 2 mL of plasma, with 10 μL of internal control added to each sample, and 200 μL of proteinase K was thoroughly mixed. Subsequently, 50–60 μL of eluent PTBC was added to the seventh well from the left of the reagent strip, followed by the addition of the mixed sample to the first well from the left of the reagent strip. The reagent strip was then subjected to fully automatic nucleic acid extraction using a program customized according to Table 2. After the completion of the extraction process, the purified DNA was obtained from the 7th well from the left side of each reagent strip and transferred to a centrifuge tube for storage at −20°C.
 |
Table 2 Pathogen and Fluorescent Correspondence |
Droplet Digital PCR
In this study, the presence of bacteria, fungi, and drug-resistant gene loads in peripheral blood was assessed using the BSI pathogenic microorganism genus and drug-resistant gene nucleic acid combined detection kit (digital PCR method) (NY7, Pilotene, China). Similarly, the human herpesvirus 1–5 combined detection kit (digital PCR method) (8,101,014, Pilotene, China) was employed to detect viral load in peripheral blood. Table 3 provides details of the infectious strains and antibiotic resistance genes identified by digital PCR nucleic acid. For each tube of reaction solution (dNTP, buffer, enzyme, primer, and probe), 15 μL of the nucleic acid extraction products of the sample to be tested, negative quality control, and positive quality control were added, followed by vortexing and brief centrifugation. Subsequently, 14 μL of the reaction solution containing the sample was added to the sampling cup of each channel of the digital PCR microdroplet chip. Droplet generation was performed using the droplet generator (DG32, Pilotene, China). After droplet generation, the chip was inserted into a PCR amplification instrument (TC1, Pilotene, China) and subjected to amplification, consisting of 95°C for 5 minutes; [95°C for 5 seconds, 60°C for 15 seconds]*40 cycles; 25°C for 1 minute. The chip was then placed in the biochip reader (CS71, Pilotene, China), and fluorescence channels FAM, VIC, ROX, CY5, CY5.5, and A425 were selected(Table 3). The positioning channel was set to VIC, and the chip hole position was adjusted before microarray scanning and analysis. Micro-droplets exhibiting fluorescent signals were considered positive, while micro-droplets lacking fluorescent signals were deemed negative. The copy number of the target molecule was calculated by dividing the number of positive droplets by the total number of droplets. For conventional bacterial fluorescence channels, a detection value of ≥0.5 copies/μL was considered positive. However, in the case of candida, streptococcus, and coagulase-negative staphylococcus, a detection value of ≥1.0 copies/μL was required to confirm positivity. For the virus fluorescence channel, a detection value of ≥0.35 copies/μL was considered positive.18,19
 |
Table 3 Nucleic Acid Extractor Editing Program Steps |
Statistical Analysis
To analyze the impact of digital PCR results on antibacterial and antiviral treatment, a retrospective analysis of clinical patient test results and disease course records was conducted. A detailed analysis of clinical diagnosis results and antibacterial regimens before and after ddPCR testing was also conducted. The general characteristics of patients and laboratory results were reported as means ± standard deviation. Binomial variables were assessed using the chi-square test as independent variables, and statistical significance was defined as a P-value of <0.05. Differences between ddPCR subgroups were compared using the Mann–Whitney test. Pearson correlation analysis was used for linear analysis of associations between ddPCR and inflammatory markers. The cut-off value of ddPCR/PCT/IL-6/CRP was determined using ROC curve analysis. Statistical analysis was performed using SPSS 25.0 and GraphPad Prism 8.4.0.
Results
Diagnostic Performance of ddPCR
The baseline characteristics of the study population are presented in Table 4. Derived from the outcomes procured within our clinical laboratory, an assessment of 176 subjects suspected of BSI unveiled that blood culture yielded positive results in 15.34% (27/176) of cases, whereas ddPCR testing identified positivity in 36.93% (65/176) of instances. In particular, 10 cases (5.68%) displayed positive ddPCR results for fungi, as depicted in Figure 2A. Notably, within this subset, ddPCR analyses delineated that 10.80% (19/176) exhibited mixed bacterial infections, while 2.84% (5/176) displayed a concurrent presence of both bacterial and fungal elements. Among patients with positive ddPCR findings, 57.66% (64/111) received active clinical interventions guided by appropriately prescribed medications. Additionally, 38.74% (43/111) of patients underwent significant prescription adjustments based on the diagnostic revelations of ddPCR(Figure 2B). The gold standard employed for the adjudication of BSI was anchored in the comprehensive amalgamation of laboratory outcomes, clinical assessments, and the ultimate patient prognosis. Within the cohort, a definitive total of 87 patients (49.43%) exhibited genuine bloodstream infections. However, only 9 individuals evidenced concordance between their blood culture outcomes and ddPCR findings. Notably, the sensitivity of ddPCR was calculated at 74.7% (65/87, [95% CI]65.4–84.0%), whereas blood culture’s sensitivity was reported at 31.0% (27/87, [95% CI]21.1–41.0%). Impressively, the detection rate of ddPCR outperformed that of conventional culture significantly (74.71% vs 31.03%, Pearson chi-square=33.305, P<0.0001). Furthermore, the proportion of negative blood cultures but positive ddPCR results (surplus detection rate) stood at 87.1% (51/60, [95% CI]79.1–95.2%). Remarkably, the Klebsiella pneumoniae and Acinetobacter baumannii emerged as the predominant pathogens discerned through ddPCR. The convergence between ddPCR and blood culture findings was most robust in the context of Klebsiella detection, whereas the sensitivity to Staphylococci detection exhibited marked variability (Figure 2C). Given ddPCR’s exceptional sensitivity in detecting bacterial components within BSI cases, it standed poised as an indispensable tool for the identification of intricate infectious pathogens in sepsis-afflicted patients. Delving deeper into the temporal aspected of pathogen diagnosis, it was notable that the time required for ddPCR testing (7.91 hours) was substantially abridged (P<0.0001) in comparison to the duration demanded by blood culture (138.03 hours) (Figure 2D).
 |
Table 4 General Characteristics* |
Correlation Analysis Between Ddpcr and Laboratory Results of Inflammation Indicators
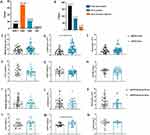
The present investigation undertook a comprehensive correlation analysis to ascertain the interrelationship between ddPCR outcomes and established inflammation indicators. Remarkably, the study’s findings elucidated the existence of statistically significant positive correlations between IL-6 (R=0.3040, [95% CI]0.05431–0.5180, P=0.0182, Figure 3A), PCT (R=0.3775, [95% CI]0.1367–0.5762, P=0.0029, Figure 3B), and CRP (R=0.4368, [95% CI]0.2057–0.6218, P=0.0005, Figure 3C) concentrations and the pathogen load detected through ddPCR. However, discerned from the analysis were the non-significant associations existing between white blood cell count, neutrophil count, lymphocyte count, and ddPCR load (Figure 3D–F). Subsequently, within the cohort of patients exhibiting affirmative bacterial detection through ddPCR, intriguing patterns emerged in relation to routine inflammatory markers [IL-6 (≥100 ng/mL, P=0.0986, Figure 3G), PCT (≥0.50 ng/mL, P=0.0452, Figure 3H), and CRP (≥70 mg/L, P=0.004, Figure 3I)]. Significantly elevated bacterial loads were observed among patients displaying augmented levels of the aforementioned markers in contrast to those with lower expression levels. This substantiates the proposition that the quantitative ddPCR assessment of bacterial load in BSI potentially carried augmented clinical diagnostic import in comparison to the qualitative ddPCR findings. Hence, the application of ddPCR as a modality for quantifying bloodstream bacterial load might harbor substantial implications for refined clinical diagnosis.
Significance of ddPCR Bacterial Qualitative Results for Early Warning of Sepsis
In this study, we examine the potential of ddPCR bacterial qualitative and quantitative results as a means of early detection for sepsis. The investigation involved the assessment of 126 suspected BSI individuals, who underwent comprehensive evaluations of Sequential Organ Failure Assessment (SOFA) score,1 ddPCR, leukocyte, neutrophil, and lymphocyte counts, IL-6, PCT and CRP levels. SOFA scores were used to track patient status during ICU stay to determine the degree of organ function or failure rate of the patient. The score is based on the failure of the respiratory system, cardiovascular system, liver system, coagulation system, renal system and nervous system. Mortality was increased in patients with a SOFA score of 7 or greater.20 Therefore, we take 7 as the grouping node. Among suspected cohort, a subgroup of 66 patients exhibited a SOFA score of less than 7, while 56 patients presented a SOFA score equal to or greater than 7. Significant disparities in IL-6 (P=0.0002, Figure 4D), PCT (P<0.0001, Figure 4E), and CRP levels (P=0.0035, Figure 4F) were discerned between the subset of SOFA<7 and ≥7 group. Conversely, the analysis found no statistical significance in white blood cell (P=0.1963, Figure 4A), lymphocyte (P=0.9676, Figure 4B), and neutrophil (P=0.1499, Figure 4C) count. Employing logistic regression analysis, we assessed the utility of ddPCR qualitative results as a diagnostic tool for predicting the progression of sepsis (OR=1.876, [95% CI]0.909–3.871, P=0.089). The resultant regression equation for constructing a predictive model was. The receiver operating characteristic (ROC) curve analysis, aimed at predicting sepsis progression outcomes, revealed that the amalgamation of ddPCR and conventional validation factors yielded a new predictor with an area under the curve (AUC) of 0.6071 ([95% CI] 0.5088–0.7054, P=0.0383), offering a sensitivity of 60.00% and specificity of 60.61% (Figure 4H). By contrast, relying solely on conventional inflammatory markers yielded an AUC of 0.5539 ([95% CI]0.4532–0.6546, P=0.2970), with a sensitivity of 75.00% and specificity of 37.88% (Figure 4G). The integration of ddPCR bacterial qualitative results with IL-6, PCT, and CRP demonstrated a notable enhancement in diagnostic specificity for SOFA progression in suspected BSI. However, for confirmed BSI patients, more sensitive clinical indicators are needed for the risk of sepsis, SOFA progression and monitoring. ddPCR has the ability to quantify the number of bacteria in blood. And as mentioned before, the bacterial load results of ddPCR are correlated with clinical inflammation indicators. Therefore, we further verified the diagnostic significance of ddPCR quantitative results in the confirmed BSI. In the context of refining diagnostic accuracy for sepsis SOFA, an exploration into the synergistic potential of ddPCR in conjunction with PCT has been undertaken. A cohort of 60 individuals exhibiting positive ddPCR bacterial outcomes has been meticulously selected from the pool of BSI patients. Among this cohort, 25 patients manifested SOFA<7, while 35 patients’ SOFA≥7. The analysis of these data delineated conspicuous distinctions in the ddPCR bacterial quantitative results between groups (P=0.0334, Figure 4I). Correspondingly, notable variations were observed in IL-6 (P=0.0446, Figure 4N) and PCT (P=0.0051, Figure 4O) levels between these two groups, while parameters such as CRP (P=0.2393, Figure 4M), white blood cell count (P=0.1191, Figure 4J), lymphocyte count (P=0.1030, Figure 4K), and neutrophil count (P=0.1753, Figure 4L) exhibited no statistically significant differences. Employing logistic regression analysis, the potential of ddPCR bacterial load in prognosticating sepsis progression was rigorously evaluated. The outcomes underscored that the fusion of ddPCR (OR=1.789, [95% CI]1.043–3.069, P=0.035) with PCT (OR=1.787, [95% CI]1.059–3.016, P=0.035) embodied prognostic significance and diagnostic utility. The formulation of a predictive model was accomplished through the derivation of the regression equation. The diagnostic efficacy of this amalgamated approach was gauged by generating and contrasting ROC utilizing paired samples concurrently detected by ddPCR and PCT. The exclusive utility of ddPCR (AUC=0.7087, [95% CI]0.5683–0.8491, P=0.0098) for prognosticating SOFA progression was vividly portrayed in Figure 4Q. In tandem, when ddPCR was harnessed in concert with PCT (AUC = 0.7913, [95% CI]0.6708–0.9118, P=0.0003), sensitivity and specificity of 56.67% and 91.3% respectively were observed in prognosis (Figure 4R). Akin to this, the isolated utilization of PCT (AUC=0.7674, [95% CI]0.6317–0.9031, P=0.0009) evinced a sensitivity of 63.33% and specificity of 86.96% in predicting sepsis progression (Figure 4P). The cumulative utilization of ddPCR quantitative findings, paired with PCT, in sepsis SOFA diagnosis culminated in a substantial augmentation of diagnostic specificity.
Diagnostic Significance of Bacterial ddPCR for Prognostic Assessment of BSI
We investigated the diagnostic utility of ddPCR bacterial qualitative results in predicting the prognosis of patients diagnosed with BSI. The research entailed a comprehensive analysis of clinical parameters, including white blood cell counts, neutrophil counts, lymphocyte counts, IL-6, PCT, and CRP levels, among 131 patients. Out of these, 65 patients achieved clinical recovery, while 66 patients succumbed to the infection. The study revealed noteworthy distinctions between ddPCR bacterial qualitative results in surviving and deceased patients. Statistically significant variations were particularly evident in IL-6 (P<0.0001, Figure 5E) and PCT (P<0.0001, Figure 5F) levels between the survivor and deceased groups. Conversely, no statistically significant differences were observed in CRP (P=0.1216, Figure 5D), white blood cell count (P=0.3133, Figure 5A), lymphocyte count (P=0.0693, Figure 5B), and neutrophil count (P=0.1041, Figure 5C) between groups. Logistic regression analysis was employed to evaluate the potential diagnostic efficacy of ddPCR qualitative results in forecasting clinical outcomes in BSI. The findings demonstrated that when combined with the prevailing clinical diagnostic markers [IL-6 (OR=1.715, [95% CI]1.238–2.375, P=0.001), PCT (OR=1.517, [95% CI]1.126–2.042, P=0.006) and ddPCR (OR=1.316, [95% CI]0.596–2.907, P=0.497)] did not independently exhibit clear prognostic significance. The regression model for the combined IL-6+PCT index to formulate patient outcome predictions was expressed as. Integration of novel predictor ddPCR typing results with inflammatory markers yielded AUC of 0.7746 ([95% CI]0.6959–0.8533, P<0.0001), boasting a sensitivity of 60.00% and specificity of 60.61% (Figure 5H). In contrast, employing conventional inflammatory indicators exclusively (AUC = 0.7691, [95% CI]0.6996–0.8486, P<0.0001) demonstrated a sensitivity of 75.00% and specificity of 37.88% (Figure 5G). The ROC analysis did not emphasized the substantial enhancement in diagnostic specificity for clinical outcome prognosis when utilizing ddPCR qualitative results in conjunction with PCT. However, for patients who have been diagnosed with BSI, effective indicators to predict the patient’s clinical prognosis are also necessary. Therefore, we further verified the diagnostic significance of ddPCR quantitative results for prognosis in a group of diagnosed BSI patients. The potential of enhancing diagnostic precision for predicting the prognosis of patients with BSI has been investigated through the strategic amalgamation of ddPCR with IL-6. A discerning selection process was conducted among a pool of suspected BSI patients, yielding a cohort of 60 individuals with confirmed positive ddPCR bacterial results (23 patients exhibited favorable prognoses, 37 patients met a less fortunate outcome). The dataset gleaned from these patients encompassed comprehensive clinical outcomes. Within this cohort, the obtained results illuminated statistically significant disparities in the ddPCR bacterial quantitative findings between survivors and those who experienced unfortunate demises (P=0.0170, Figure 5I). Moreover, pronounced distinctions were observed specifically in the IL-6 levels (P=0.0268, Figure 5O) between survivors and none. In contrast, parameters including PCT (P=0.0839, Figure 5M), CRP (P=0.9440, Figure 5N), white blood cell count (P=0.6201, Figure 5J), lymphocyte count (P=0.8396, Figure 5K), and neutrophil count (P=0.5272, Figure 5L) exhibited no discernible statistical significance. Employing logistic regression analysis, an exploration of the viability of ddPCR bacterial load as a diagnostic tool for predicting BSI outcomes was undertaken. The outcomes revealed the diagnostic efficacy of both ddPCR (OR=1.718, [95% CI]1.044–2.828, P=0.033) and IL-6 (OR=1.575, [95% CI]1.000–2.481, P=0.050) as prognostic indicators of substantive value. The formulation of a predictive model transpired through the construction of the regression formula. The ROC curve analysis for ddPCR as an isolated diagnostic entity yielded an AUC of 0.6877 ([95% CI]0.5471–0.8284, P=0.0176, Figure 5Q) for prognosticating BSI clinical outcomes. Upon combination with IL-6 (AUC=0.7352, [95% CI]0.6012–0.8691, P=0.0030), ddPCR evinced a sensitivity of 56.67% and specificity of 91.3% (Figure 5R). On a parallel note, exclusive employment of IL-6 (AUC=0.6746, [95% CI]0.5340–0.908151, P=0.0273) yielded a sensitivity of 63.33% and specificity of 86.96% (Figure 5P). The strategic employment of ddPCR in conjunction with IL-6 specificity.
The Value of ddPCR in the Dynamic Monitoring of Medication for BSI
In this study, selected 100 patients with clinical BSI were divided into two groups based on their previous antibiotic therapy. Group P consisted of 21 patients who received effective antimicrobial therapy for 3 days or more before sample collection, while group N consisted of 79 patients who failed to receive effective antimicrobial therapy. The detection rates of ddPCR and blood culture were compared between the two groups, with ddPCR showing a higher detection rate in group N (49.4%) compared to group P (5%) ([95% CI]38.3% to 60.5% and −5.5% to 15.5%). The sensitivity and specificity of ddPCR were found to be 85.1% ([95% CI]74.5% to 95.7%) and 97.0% ([95% CI]90.8% to 103.1%), while blood culture had a lower sensitivity of 36.2% ([95% CI]21.9% to 50.4%) and specificity of 52.4% ([95% CI]39.7% to 65.1%). The correct use of antibiotics in previous empiric treatment significantly affected the sensitivity and specificity of ddPCR detection (P<0.0001). To observe the dynamic monitoring role of ddPCR during BSI and negative conversion, repeated ddPCR tests were performed on five cases, with the results showing that ddPCR became negative in parallel with a decrease in leukocytes (Figure 6A), neutrophils (Figure 6B), and PCT values (Figure 6C). Additionally, a patient with effective anti-Klebsiella infection treatment was monitored over time, with the copy number detected by blood ddPCR decreasing over time, and the results for WBC, neutrophils, and PCT also showed a decrease in the same direction of change (Figure 6D). These findings suggest that ddPCR may be useful for monitoring the efficacy of antimicrobial therapy and predicting negative conversion during BSI.
Clinical Utility of ddPCR in Viremia Detection
The present study undertook a comprehensive assessment of the diagnostic potential of ddPCR in detecting viremia. The investigation centered on 176 patients clinically diagnosed with BSI, delving into the presence of Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Herpes simplex virus (HSV), and Varicella-zoster virus (VZV) within this cohort. The detected frequencies encompassed 69 occurrences of EBV (39.20%), 22 occurrences of CMV (12.5%), 14 occurrences of HSV (7.95%), and 1 occurrence of VZV (0.568%) as graphically depicted in Figure 7A. Of particular note, a noteworthy 47.72% (84/176) of ddPCR analyses revealed viral positivity, with 20.45% (36/176) of these instanced indicating concurrent bacterial or fungal infections. Delving deeper, a discerning pattern emerged wherein 31 out of 60 ddPCR-positive patients exhibited positive virus detection, a proportion significantly greater than the 53 out of 116 ddPCR-negative patients manifesting the same phenomenon. This observation substantiated a substantially elevated probability of virus infection among the positive patient cohort. Intriguingly, a mere 25.00% (21/84, [95% CI]15.5 to 34.5%) of positively identified patients received antiviral therapy, predominantly featuring Ganciclovir as the prominent choice (Figure 7B). Furthermore, a comparative analysis of ddPCR virus-positive and -negative patients in the population that tested negative for BSI revealed nuanced differences. Particularly, the mean SOFA score among ddPCR virus-positive patients stood at 6.58, juxtaposed with 4.45 for their ddPCR virus-negative counterparts. Statistical exploration revealed that ddPCR virus-positive patients exhibited significantly lower absolute lymphocyte counts (P=0.0003, Figure 7D) compared to negative. Conversely, parameters such as white blood cell count (P=0.2714, Figure 7C), neutrophil count (P=0.3191, Figure 7E), IL6 (P=0.4615, Figure 7F), PCT levels (P=0.1016, Figure 7G), and CRP levels (P=0.7741, Figure 7H) did not demonstrate statistically significant variations. Furthermore, an intriguing observation materialized regarding BSI patients presenting a mixed bacterial-viral infection scenario. The PCT levels (P=0.0485, Figure 7M) of such patients were significantly lower than negative. While white blood cell count (P= 0.2197, Figure 7I), lymphocyte count (P=0.9672, Figure 7J), neutrophil count (P=0.1691, Figure 7K), IL-6 (P=0.0616, Figure 7L), and CRP levels (P=0.5126, Figure 7N) demonstrated no statistically significant differences. Evidently, patients with diminished immune capacity displayed an augmented likelihood of concurrent viral infections. This substantiates the need for vigilant consideration of clinical co-infection involving blood-borne bacteria and viruses, thereby underscoring the necessity for proactive antiviral interventions by clinicians.
Discussion
ddPCR is a technique that partitions the reaction mixture into numerous tiny individual reaction chambers within a small volume. Each reaction chamber is designed to contain only one or a few target molecules, if present. These small-scale reactions are then amplified individually, and the resulting products are detected by fluorescence, with positive reactions indicating the presence of the target sequence and negative reactions indicating its absence. Using Poisson statistical analysis of the number of positive and negative reactions, absolute quantification of the target sequence can be determined. This study found that ddPCR demonstrated superior performance for pathogen detection and clinical management, surpassing that of blood culture. In addition, ddPCR provided valuable clinical information for over one-third of patients with suspected BSI. Despite the existence of multiple rapid bacterial detection programs, their sensitivity and specificity are still suboptimal, underscoring the potential of ddPCR as a promising alternative for accurate and efficient diagnosis of BSI.21,22 This study has provided further evidence to support the positive predictive ability of ddPCR in predicting disease outcomes. The rapid feedback and high specificity can improve clinical decision-making for sepsis against BSI, thereby reducing mortality rates. Moreover, the quantitative load data of pathogens can accurately reflect the severity of a patient’s current infection and inflammation, allowing clinicians to monitor the patient’s condition in real-time and make informed decisions. While numerous studies have demonstrated the potential advantages of ddPCR over blood culture, this study provides additional support for its effectiveness in clinical settings.
Blood culture remains the preferred method for diagnosing clinical BSI. Relying solely on the results of ddPCR testing may result in radical clinical treatment plans and increased economic burdens. Therefore, it is essential to consider the technical advantages of ddPCR and explore its potential applications in clinical practice. Our analysis reveals that ddPCR has several advantages over blood culture, including its ability to detect a wider range of pathogens through nucleic acid analysis. This includes viral infections that may be undetectable by blood cultures and fungal infections that are challenging to culture. Additionally, ddPCR can provide early information on antibiotic resistance, which is crucial in determining effective treatment strategies. Our study found that EB virus had the highest carrier infection rate, with many individuals becoming infected due to COVID-19 reactivation. Therefore, it is crucial to consider ddPCR’s potential advantages in improving the accuracy of pathogen detection and diagnosis, which may lead to more effective and efficient clinical management of infectious diseases.23 The impact of CMV and HSV infections on the clinical management of sepsis cannot be overlooked, as evidence indicates that these infections are associated with poorer patient outcomes.24 This is especially true for kidney transplant patients, who are often prescribed immunosuppressive drugs that increase the risk of viral infections in the bloodstream, leading to transplant rejection.25 In such cases, clinicians must be vigilant of concomitant viral infections and implement active antiviral regimens, such as the use of ganciclovir.26 Additionally, detecting fungal infections remains a significant challenge. Fungal BSIs are more dangerous and difficult to culture than bacterial infections, and often affect individuals with extremely low immunity, such as those with HIV. Currently, various rapid detection schemes based on fungal nucleic acid analysis have emerged, but ddPCR remains advantageous due to its broad-spectrum and pathogen-specific detection capabilities.27 Furthermore, providing information on Drug-resistance genes along with pathogen diagnosis reports to physicians can improve the accuracy of diagnosis and inform effective treatment strategies.28,29
In this study, we proposed a novel approach that combines IL-6, PCT and ddPCR as a more effective indicator for predicting the outcome of BSI in clinical patients, which has significant innovative implications. Our research highlights the clinical importance of considering the combined use of ddPCR and existing clinical inflammatory indicators for optimal results. We stratified sepsis patients according to their SOFA scores and found that patients with higher scores were associated with higher viral loads. This suggests that our quantitative ddPCR results may be influenced by existing protocols for inflammation assessment, as ddPCR provides more accurate information on pathogen proliferation in the bloodstream. In contrast, existing inflammatory indicators are likely to be influenced by the patient’s immune system and immune drugs. Higher pathogen loads indicate more severe infections and require more aggressive anti-infection regimens.11,30 To test our hypothesis, we found that ddPCR pathogenic loads were correlated with blood levels of IL-6, CRP, and PCT. Furthermore, we demonstrated that changes in pathogenic loads detected by ddPCR were consistent with concurrent changes in blood inflammation markers, especially PCT, in paired patients who converted from positive to negative. This suggests that pathogen load is closely related to the severity of BSI.
When admitting febrile patients with suspected bloodstream infections, clinicians should promptly collect peripheral blood samples for examination. A complete peripheral blood cell count will be conducted on a whole blood sample to assess potential neutrophilic granulocyte elevation indicative of bacterial infection. Serum or plasma samples will be analyzed for routine Inflammation indicators. Notably, IL-6 level typically surge within the first hour, followed by subsequent increases in PCT after 2 hours, and the peak of CRP after 6h. Consequently, a comprehensive assessment involving multiple indicators is essential. For ddPCR bacterial and viral load testing, a whole blood sample is collected. It’s advisable for medical practitioners to combine ddPCR load data with PCT to better evaluate the likelihood of sepsis development in patients with BSI. Incorporating findings from ddPCR drug resistance gene detection can guide proactive antibiotic and hormone therapy. Decisions regarding antiviral regimens should be based on thorough analysis of individual patient’s clinical conditions. Combining ddPCR load data with IL-6 offers an effective means to predict disease progression. Simultaneous high levels of both indicators may necessitate transferring the patient to an intensive care unit for vigilant monitoring and treatment. Additionally, a whole blood sample should be sent to the microbiology laboratory for bacterial and fungal cultures for 72 to 144h. The ultimate treatment strategy is determined based on the results of these cultures. This protocol introduces the potential to substantially reduce mortality among BSI patients, yet its precise impact requires further validation in subsequent stages of research and testing (Figure 8).
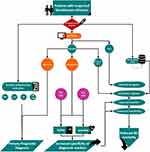 |
Figure 8 Recommendations for clinical application of ddPCR qualitative and quantitative results. |
Although ddPCR has higher sensitivity and specificity, the equipment and reagents are still expensive. Moreover, compared with the traditional infection markers, the operation is more complex and the detection time is longer. Moreover, the types of pathogens detected by ddPCR are still limited. Large-throughput and broad-spectrum ddPCR still need more in-depth research.31
Our study is limited by its retrospective design. This was also a single-center study and thus had its known drawbacks. Moreover, this result may not be reflected in other developed or developing countries. Advances are being made in the treatment of bloodstream infections, including the use of new antibiotics, immunotherapeutic agents, and various hormones. There may therefore be minor differences in the clinical application of this strategy. However, our center is a tertiary class A hospital with a large number of units, so our data are also a valid reference for advancing research on the diagnosis of BSI. In the future, there may be opportunities to initiate multi-center studies to promote the combined application of multiple infection indicators in clinical practice.
Conclusion
The combination of quantitative ddPCR results and IL-6 can predict clinical outcomes in patients with BSI. ddPCR and PCT can make a more accurate prediction of the progression of sepsis SOFA. Nevertheless, further investigations are needed to explore the full clinical significance of using ddPCR for the diagnosis of multiple BSI.
Abbreviations
BSI, Bloodstream infection; ddPCR, Droplet digital polymerase chain reaction; IL-6, Interleukin-6; PCT, Procalcitonin; AUC, Area under the curve; SOFA, Sequential Organ Failure Assessment; TAT, Turnaround time; CRP, C-reactive protein; AMR, Antimicrobial resistance; ICU, Intensive care unit; PCR, Polymerase chain reaction; ROC, Receiver operating characteristic; EBV, Epstein-Barr virus; CMV, Cytomegalovirus; HSV, Herpes simplex virus; VZV, Varicella-zoster virus.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and informed consent
This was a single-center prospective cohort study conducted at a teaching hospital in Changsha, China. The study protocol was reviewed and approved by the Hospital Ethics Committee (Ethical Review Committee of the Second Xiangya Hospital of Central South University, Changsha, China, No. 2022xyeyy051). The study enrolled hospitalized patients over 18 years old with suspected BSI from November 2022 to February 2023.
Consent for Publication
All participants consented to the publication of the raw data from the assay. This study has protected patient personal information. The patient number and other information involved are replaced by the sample number.
Acknowledgments
We would like to express our gratitude to Pilot Gene Technology [Hangzhou] Co., Ltd., Hangzhou, China for providing us with technical support and guidance during the course of this study. We express our gratitude to the Molecular Biology Department, Microbiology Department, and Clinical Blood Department at the Second Xiangya Hospital of Central South University for providing their invaluable support to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Hunan Provincial Department of Finance Project (Grant No. 202065), the Natural Science Foundation of China (Grant No. 82270840), Changsha Natural Science Foundation (Grant NO. kq2202414), and the Fundamental Research Funds for the Central Universities of Central South University (Grant NO. 1053320212719).
Disclosure
All the authors have no conflict of interest.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi:10.1164/rccm.201504-0781OC
3. Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536–1551. doi:10.1007/s00134-020-06106-2
4. Lamy B, Sundqvist M, Idelevich EA. Bloodstream infections - Standard and progress in pathogen diagnostics. Clin Microbiol Infect. 2020;26(2):142–150. doi:10.1016/j.cmi.2019.11.017
5. Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–2417. doi:10.1097/CCM.0000000000000509
6. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9(1):107. doi:10.1186/1741-7015-9-107
7. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409. doi:10.1038/nrrheum.2017.83
8. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
9. Tziolos N, Giamarellos-Bourboulis EJ. Contemporary approaches to the rapid molecular diagnosis of sepsis. Expert Rev Mol Diagn. 2016;16(11):1201–1207. doi:10.1080/14737159.2016.1246958
10. Zhou F, Sun S, Sun X, Chen Y, Yang X. Rapid and sensitive identification of pleural and peritoneal infections by droplet digital PCR. Folia microbiologica. 2021;66(2):213–219. doi:10.1007/s12223-020-00834-0
11. Lin K, Zhao Y, Xu B, et al. Clinical diagnostic performance of droplet digital PCR for suspected bloodstream infections. Microbiol Spectr. 2023;11(1):e0137822. doi:10.1128/spectrum.01378-22
12. Zheng Y, Jin J, Shao Z, et al. Development and clinical validation of a droplet digital PCR assay for detecting Acinetobacter baumannii and Klebsiella pneumoniae in patients with suspected bloodstream infections. MicrobiologyOpen. 2021;10(6):e1247. doi:10.1002/mbo3.1247
13. Hu B, Tao Y, Shao Z, et al. A comparison of blood pathogen detection among droplet digital PCR, metagenomic next-generation sequencing, and blood culture in critically ill patients with suspected bloodstream infections. Front Microbiol. 2021;12:641202. doi:10.3389/fmicb.2021.641202
14. Wu J, Tang B, Qiu Y, et al. Clinical validation of a multiplex droplet digital PCR for diagnosing suspected bloodstream infections in ICU practice: a promising diagnostic tool. Crit Care. 2022;26(1):243. doi:10.1186/s13054-022-04116-8
15. Liu W, Wang C, Pan F, et al. Clinical application of a multiplex droplet digital PCR in the rapid diagnosis of children with suspected bloodstream infections. Pathogens. 2023;12(5):719.
16. Wouters Y, Dalloyaux D, Christenhusz A, et al. Droplet digital polymerase chain reaction for rapid broad-spectrum detection of bloodstream infections. Microb Biotechnol. 2020;13(3):657–668. doi:10.1111/1751-7915.13491
17. Shao Z, Zhu J, Wei Y, et al. Pathogen load and species monitored by droplet digital PCR in patients with bloodstream infections: a prospective case series study. BMC Infect Dis. 2022;22(1):771. doi:10.1186/s12879-022-07751-2
18. Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–674. doi:10.1038/s41564-018-0349-6
19. Pohl G, Shih Ie M. Principle and applications of digital PCR. Expert Rev Mol Diagn. 2004;4(1):41–47. doi:10.1586/14737159.4.1.41
20. Fayed M, Patel N, Angappan S, et al. Sequential organ failure assessment (SOFA) score and mortality prediction in patients with severe respiratory distress secondary to COVID-19. Cureus. 2022;14(7):e26911.
21. Hogan CA, Yang S, Garner OB, et al. Clinical impact of metagenomic next-generation sequencing of plasma Cell-Free DNA for the diagnosis of infectious diseases: a multicenter retrospective cohort study. Clin Infect Dis. 2021;72(2):239–245. doi:10.1093/cid/ciaa035
22. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14(1):319–338. doi:10.1146/annurev-pathmechdis-012418-012751
23. Naendrup JH, Garcia Borrega J, Eichenauer DA, Shimabukuro-Vornhagen A, Kochanek M, Böll B. Reactivation of EBV and CMV in Severe COVID-19-Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients. J Intensive Care. 2022;37(9):1152–1158. doi:10.1177/08850666211053990
24. Imlay H, Limaye AP. Current understanding of cytomegalovirus reactivation in critical illness. J Infect Dis. 2020;221(Suppl 1):S94–S102. doi:10.1093/infdis/jiz638
25. Le J, Durand CM, Agha I, Brennan DC. Epstein-Barr virus and renal transplantation. Transplant Rev. 2017;31(1):55–60. doi:10.1016/j.trre.2016.12.001
26. Ishiyama K, Arakawa-Hoyt J, Aguilar OA, et al. Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients. Proc Natl Acad Sci U S A. 2022;119(8). doi:10.1073/pnas.2116588119
27. Martinez RM, Wolk DM. Bloodstream Infections. Microbiol Spectr. 2016;4(4). doi:10.1128/microbiolspec.DMIH2-0031-2016
28. Mancini N, Infurnari L, Ghidoli N, et al. Potential impact of a microarray-based nucleic acid assay for rapid detection of Gram-negative bacteria and resistance markers in positive blood cultures. J Clin Microbiol. 2014;52(4):1242–1245. doi:10.1128/JCM.00142-14
29. Abram TJ, Cherukury H, Ou CY, et al. Rapid bacterial detection and antibiotic susceptibility testing in whole blood using one-step, high throughput blood digital PCR. Lab on a Chip. 2020;20(3):477–489. doi:10.1039/C9LC01212E
30. Fuchs A, Tufa TB, Hörner J, et al. Clinical and microbiological characterization of sepsis and evaluation of sepsis scores. PLoS One. 2021;16(3):e0247646. doi:10.1371/journal.pone.0247646
31. Abellan-Schneyder I, Schusser AJ, Neuhaus K. ddPCR allows 16S rRNA gene amplicon sequencing of very small DNA amounts from low-biomass samples. BMC Microbiol. 2021;21(1):349. doi:10.1186/s12866-021-02391-z
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.