Back to Journals » Patient Preference and Adherence » Volume 18
Real-Time Experience of Abrocitinib for the Treatment of Mucous Membrane Pemphigoid: A Case Report
Authors Teng Y , Ren M , Yang X, Lu W, Tao X
Received 23 November 2023
Accepted for publication 16 February 2024
Published 23 February 2024 Volume 2024:18 Pages 503—506
DOI https://doi.org/10.2147/PPA.S451007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Qizhi (Cathy) Yao
Yan Teng, Mingyang Ren, Xianhong Yang, Wei Lu, Xiaohua Tao
Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China
Correspondence: Xiaohua Tao, Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China, Tel +86-13505811700, Email [email protected]
Background: Mucous membrane pemphigoid (MMP), a rare autoimmune vesiculous and erosive disorder, may affect multiple mucous membranes, with the oral cavity being the most commonly affected site. Its treatment depends on the site(s) of mucosal involvement and disease severity.
Patients and Methods: A 62-year-old female patient with MMP that predominantly involved the oral cavity strongly rejected systemic corticosteroid or immunosuppressive agents and was successfully treated with abrocitinib, a highly selective JAK-1 inhibitor with a good safety profile.
Results: The case demonstrated good efficacy and safety profile of abrocitinib for the treatment of MMP with predominant oral involvement.
Conclusion: Abrocitinib is a promising agent for the treatment of MMP with oral involvement.
Keywords: abrocitinib, mucous membrane pemphigoid, JAK inhibitor
Introduction
Mucous membrane pemphigoid (MMP), a rare autoimmune, vesiculous, erosive disease, is characterized by post-bullous erosion of the mucous membranes, including that of the oral, ocular, laryngeal, and skin,1 with the oral cavity being the most commonly affected site. Limited cutaneous involvement, typically localized to the head, neck, or upper trunk, is also observed. Patients with extensive oral involvement or those who do not respond adequately to local therapy are good candidates for systemic treatment. Long-term systemic corticosteroids or immunosuppressive agents are first prescribed. However, these agents have their limitations due to long-term side effects. Herein, we reported a case of MMP with predominant oral involvement that was successfully treated by abrocitinib, which is a highly selective JAK-1 inhibitor.
Case Representation
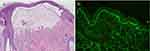
A 62-year-old female was referred to our dermatological department with a 6 months history of progressive oral erosion and skin erythema and blisters. The severe oral pain caused feeding disorders, leading to weight loss. Physical examination revealed that post-blistering erosions were mainly present on the left palatal mucosa (Figure 1a) and several small fluid-filled blisters and erythema were scattered on the scalp, trunk, and limbs. Histopathology of a blister on the scalp revealed that there was a cleavage between the epidermis and dermis with a few eosinophils and lymphocyte infiltrations (Figure 2a). Direct immunofluorescence testing found linear immunoglobulin G (IgG) and C3 deposits along the dermoepidermal junction (Figure 2b). A serological examination revealed elevated anti-BP180 levels (81.16 U/mL) and normal BP230 levels on enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Medizinische Labordiagnostika AG, Germany). Other laboratory findings, including routine blood analysis; urine analysis; liver, kidney, and thyroid function tests; erythrocyte sedimentation rate; antinuclear antibodies; and Rheumatoid factor were within the normal range. Based on these findings, a final diagnosis of MMP was made.
For 2 months, the patient was placed on a high-potency topical corticosteroid and topical tacrolimus; however, no significant improvements in the symptoms were noted. The patient refused to receive the treatment of systemic corticosteroid or immunosuppressive agents for their long-term adverse events and she also refused the dupilumab therapy for its subcutaneous injection administration.
After a routine blood examination, biochemistry analysis, coagulation function tests, and chest computed tomography (CT) to rule out severe infection, coagulation dysfunction, hepatic failure, renal disorder, and tuberculosis, she was prescribed 100 mg of abrocitinib per day. After 3 days of abrocitinib therapy, she reported significant improvement in pain. Two weeks later, the oral erosions had mostly subsided (Figure 1b); 4 weeks later, they had disappeared without any accompanying pain and discomfort (Figure 1c), and the skin blisters were mostly dry. The anti-BP180 levels had decreased to within the normal range. The abrocitinib was reduced to 100 mg every other day and discontinued 2 months later. No relapse or adverse events were observed during the treatment (median, 3 months) or follow-up period (median, 3 months).
Discussion
Based on the typical clinical, histological, and immunological findings of our case, a diagnosis of MMP was made. Although both oral mucous membrane and skin lesions were present, the pain induced by the progressive oral erosion severely impaired her quality of life and required active and effective therapy. Moreover, the skin lesions were extremely small and could even be ignored. In our case, topical corticosteroids and tacrolimus were discontinued due to their poor efficacy and compliance issues. Systemic therapy is necessary to alleviate the patient’s severe pain and discomfort. The patient strongly resisted the systemic corticosteroid and immunosuppressive agents due to their long-term adverse events. Biologics and JAK inhibitors were both suggested for the patient. Dupilumab is a novel therapy for bullous pemphigoid;2 however, the patient refused it due to its subcutaneous route of administration.
The Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, a crucial intracellular signaling pathway, regulates many important processes including apoptosis, immunology, and inflammation.3,4 Abrocitinib, a highly selective JAK1 inhibitor has been approved for the treatment of moderate-severe AD, inhibits many key cytokine signaling pathways, including those mediated by interferon (IFN)-γ and interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-17.5 Recent case studies have reported that abrocitinib is effective in the treatment of refractory bullous pemphigoid.6 Moreover, previous studies have also reported good efficacy and safety profiles of other types of JAK inhibitors, including baricitinib, a JAK1/2 inhibitor, and tofacitinib, a JAK1/3 inhibitor, to treat the ocular MMP successfully.7,8 However, real-life data on abrocitinib for the treatment of MMP are scarce. The JAK-STAT signaling pathway has been confirmed to participate in the pathogenesis of bullous pemphigoid.9 It has been proposed that abrocitinib might be involved in the loss of tolerance to BP180 through the regulation of Th2-type responses.10 The clear mechanism of abrocitinib for the treatment of pemphigoid, particularly MMP, remains unclear. Additionally, the patient in the present case was over 60 years old and demonstrated a good safety profile of abrocitinib therapy. Complete examinations are crucial to evaluate the adverse events that occur during the course of the treatment. However, this is only a single case report and detailed clinical data that would substantiate the observation to prove its efficacy and safety in the real world are essential.
Conclusion
We used abrocitinib to successfully alleviate the skin and oral lesions in a patient with MMP and achieved complete pain relief, presenting a novel strategy for the therapy of MMP. Further studies are required to investigate the precise mechanisms involved and to thoroughly evaluate the long-term efficacy and safety of abrocitinib in treating MMP.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Statement
The publication of images was included in the patient’s consent for publication of the case. The Hospital Ethics Committees of the Affiliated People’s Hospital of Hangzhou Medical College approved publishing the case details.
Consent Statement
The authors have obtained the patient’s consent to publish photographs. The patient in this manuscript has given written informed consent to the publication of his case details.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
The research was supported by Young Talents Project of Zhejiang Medicine and Health Science and Technology Project (2022KY049).
Disclosure
The authors declare that they have no conflicts of interest and no payment for expert testimony for this work.
References
1. Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370–379.
2. Abdat R, Waldman RA, de Bedout V, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52.
3. Napolitano M, Fabbrocini G, Genco L, et al. Rapid improvement in pruritus in atopic dermatitis patients treated with upadacitinib: a real-life experience. J Eur Acad Dermatol Venereol. 2022;36(9):1497–1498.
4. Martora F, Scalvenzi M, Ruggiero A, et al. Hidradenitis suppurativa and JAK inhibitors: a review of the published literature. Medicina. 2023;59(4):1.
5. Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165–1173.
6. Jiang W, Ma X, Guo T,et al. Abrocitinib-A promising option for patients with refractory bullous pemphigoid. J Eur Acad Dermatol Venereol. 2023;38(1):e119–21.
7. Burningham KM, Cao J, Dominguez AR. Successful treatment of recalcitrant mucous membrane pemphigoid with multisystem involvement with baricitinib and methotrexate. JAAD Case Rep. 2022;27:67–69.
8. James H, Paley GL, Brasington R, et al. Tofacitinib for refractory ocular mucous membrane pemphigoid. Am J Ophthalmol Case Rep. 2021;22:101104.
9. Juczynska K, Wozniacka A, Waszczykowska E, et al. Expression of the JAK/STAT signaling pathway in bullous pemphigoid and dermatitis herpetiformis. Med Inflamm. 2017;2017:6716419.
10. Ciechanowicz P, Rakowska A, Sikora M, et al. JAK-inhibitors in dermatology: current evidence and future applications. J DermatolTreat. 2019;30(7):648–658.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.