Back to Journals » Integrated Pharmacy Research and Practice » Volume 10
Real-Life Active Surveillance of a Naphazoline/ Hypromellose Fixed Combination’s Safety Profile in Peruvian Population
Authors Contreras-Salinas H , Barajas-Hernández M , Baiza-Durán LM, Orozco-Ceja V, Rodríguez-Herrera LY
Received 5 August 2021
Accepted for publication 27 September 2021
Published 16 October 2021 Volume 2021:10 Pages 127—133
DOI https://doi.org/10.2147/IPRP.S332421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mudassar Iqbal Arain
Homero Contreras-Salinas, Mariana Barajas-Hernández, Leopoldo Martín Baiza-Durán, Vanessa Orozco-Ceja, Lourdes Yolotzin Rodríguez-Herrera
Pharmacovigilance Department, Laboratorios Sophia S.A. de C.V., Zapopan, Jalisco, México
Correspondence: Lourdes Yolotzin Rodríguez-Herrera Tel +52 3001 4200 ext 1188
Email [email protected]
Objective: Identifying the adverse reactions and the possible risks associated with the use of naphazoline 0.1% + hypromellose 0.5% (NAPH), thereby evaluating its tolerability and safety profile.
Methods: A total of 236 Peruvian patients were included in an active pharmacovigilance study drug event monitoring consisting in 2 phone calls conducted in order to register adverse drug reactions (ADRs), the product’s tolerability and to assess the risk concerning specific clinical and demographic characteristics using a binary logistic regression model.
Results: A total of 54 ADRs (one per patient) were reported after the use of NAPH; classified (according to the Medical Dictionary for Regulatory Activities) into two groups of System Organ Class (SOC): eye disorders and nervous system disorders; and four groups of preferred term (PT): eye irritation, vision blurred, eye pruritus and headache. All ADRs were expected, mild and not serious. No risk factors related to the clinical and demographic characteristics of the patients were identified.
Conclusion: The low incidence of ADRs, their short recovery time, and their categorization as “mild” and “not serious” demonstrates the high tolerability in the studied population; therefore, according to the study, the safety profile for NAPH seems to be adequate, with a suitable tolerability.
Keywords: pharmacovigilance, naphazoline, hypromellose, adverse drug reaction, drug event monitoring
Introduction
Drug approval requires strict efficacy and safety tests through randomized clinical trials (RCTs); however, these may not be sufficient to identify all adverse drug reactions (ADRs).1,2 Therefore, RCTs should be complemented with post-marketing follow-up studies in order to identify infrequent or rare ADRs.3 Obtaining new information on both benefits and risks of drugs must be an ongoing task in the post-marketing Phase3,4 since this surveillance has led to triggering multiple risk minimization activities, such as labeling changes, prescription restrictions, and recalls.5,6
Post-marketing information is usually obtained through spontaneous notification systems (SNS, providing basic information for the safety evaluation of medicines. However, due to the nature of this system, some ADRs may be ignored on account of patient and healthcare professional underreporting, as well as variable data quality and lack of information on drug interactions. Hence, the SNS has certain limitations for which other methods would be helpful.3
Active pharmacovigilance is a widely recognized complement to SNS; which exponentially increases the detection rate of ADRs, calculates ADRs’ incidences based on a population, and identifies risk factors associated with drugs; thereby promoting risk minimization actions.7,8
Naphazoline is a first-generation imidazole derivative ophthalmic decongestant with 2:1 activity of α2:α1 adrenergic receptors, unlike α2 selective drugs, impacts arterioles and venules as well;9 eye lubricants as hypromellose, relief symptoms of eye discomfort like burning, foreign body sensation, and dry eye by increasing the stability of the tear film, improving the ocular microenvironment.10,11
Since the use of concentrations of Naphazoline at 0.1% is over the counter in most Latin American countries,12–16 it is essential to identify adverse drug reactions and possible risks associated with the use of Naphacel Ofteno®; a fixed combination of naphazoline 0.1% + hypromellose 0.5%, Laboratorios Sophia, SA de CV, Mexico (NAPH), during its post-marketing period and thereby evaluating its tolerability and safety profile.
Methods
Study Design
An active pharmacovigilance study through Drug Event Monitoring was carried out in Peruvian population by means of 2 telephone calls taking place within a 14-days period. This study was performed from February 27, 2018 (first enrolled patient) to April 18, 2020 (last completed patient). The study was conducted according to the Declaration of Helsinki and the protocol and its corresponding informed consent form were reviewed and approved by an ethics committee (see Ethics approval section). Patients who were prescribed NAPH by an ophthalmologist (under his/her medical criteria) were referred to a pharmacist employed by Sophia based in Perú, who informed them about the selection process and in case they agreed to participate, the informed consent was signed. For patients under 18 years old (yo), the parent or legal guardian signed this document. A total of 380 patients were recruited.
Initial contact call: It was performed on day three after the patient signed the informed consent. Patients were contacted and questioned on personal data (age, sex, pregnancy or lactation), characteristics of the drug and its prescription (medical indication, dose, route of administration) and clinical history data (comorbidities, concomitant medications). This first interrogation was also carried out to identify the ADRs (start date, intensity, duration, re-administration of the suspected drug [when applicable], existence of other causes different from the application of drugs that may have explained the ADRs). Second call: 14 days after the informed consent signature process. This interrogation was aimed at identifying ADRs as mentioned above.
All data were sent to the pharmacovigilance unit of Laboratorios Sophia, S.A. de C.V (México) for data management.
Data Management
The data obtained in each call were compiled in an excel document (Microsoft Office® 365 ProPlus., Washington, Redmond, USA).
Categorization
The patients were classified as children (0–12 yo), adolescents (> 12–18 yo), adults (> 18–60 yo) or geriatrics (> 60 yo).
The ADRs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) v23 in System Organ Class (SOC) and Preferred Term (PT). The classification of ADRs related to incidence was expressed according to the Council for International Organizations of Medical Sciences (CIOMS) as “very common” (≥1/10), “common or frequent” (1/100<1/10), “uncommon or infrequent” (≥1 /1000<1/100), “rare” (≥1/10,000 <1/1000), or “very rare” (<1/10,000).17 The expected/unexpected ADR encoding was performed in accordance with information from clinical studies and routine pharmacovigilance information of the product (data not shown). Once the information from the ADRs was obtained, the severity was evaluated using the Severity Assessment Scale of the ADRs (modified Hartwig and Siegel),18 and the causalities were classified under Naranjo algorithm (”definite”, ”probable”, ”possible”, ”doubtful”, and “not assessable”). Finally, seriousness (serious/non-serious) was evaluated by its likeliness to cause permanent damage, life-threatening, or death.
Concomitant medications were classified according to the Anatomical Therapeutic Classification (ATC) 2020, World Health Organization and comorbidities according to PT from MedDRA v23.
Tolerability
The tolerability of NAPH was evaluated considering the following parameters: ADR severity, seriousness, and duration.
ADRs’ Risk According to Patient Clinical and Demographic Characteristics
Binary logistic regression was performed considering multiple variables (age group, gender, prescription, dose and concomitant medication) to observe if any of these independent variables showed an association to the presence of ADRs (dependent variable).
Statistical Analysis
The quantitative variables were described as frequencies and percentages. A binary logistic regression model was performed to adjust the associated variables with ADR and the following were used as covariates: age group (adult and geriatric); gender (male and female); prescription (red-eye, ocular inflammation, post-surgical, eye allergy, pterygium and others); dose (1 drop/4 h, 1 drop/6 h, 1 drop/8 h, 1 drop/12 h, 1 drop/24 h); and concomitant medication (yes and no);. All analyses were performed with SPSS version 25 software for Mac (IBM, Chicago, IL, USA).
Results
Characteristics of the Patients
A total of 380 patients signed the informed consent; however, 11.8% (n = 45) were not contacted due to the following reasons: unanswered call 66.7% (n = 30), inactive number 15.6% (n = 7), wrong number 17.7% (n = 8).The patients who were successfully contacted amounted to a total of 335; 190 women (adolescents: n = 3; 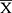 = 15 ± 1.7 yo, adults: n = 138;
= 15 ± 1.7 yo, adults: n = 138;  = 41 ± 12 yo, geriatrics: n = 49;
= 41 ± 12 yo, geriatrics: n = 49; 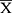 = 69 ± 6.8 yo) and 145 men (children n = 1; 12 yo, adolescents n = 1; 17 yo, adults: n = 108;
= 69 ± 6.8 yo) and 145 men (children n = 1; 12 yo, adolescents n = 1; 17 yo, adults: n = 108; 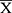 = 41.9 ± 11.1 yo, geriatrics: n = 35;
= 41.9 ± 11.1 yo, geriatrics: n = 35;  = 69.9 ± 7.6 yo) (Table 1). No NAPH exposure was identified during pregnancy, however 5 exposed patients were breastfeeding. No ADRs were reported in the newborns of treated patients.
= 69.9 ± 7.6 yo) (Table 1). No NAPH exposure was identified during pregnancy, however 5 exposed patients were breastfeeding. No ADRs were reported in the newborns of treated patients.
 |
Table 1 Clinical and Demographic Characteristics |
The main reason for prescribing NAPH was red eye 66.9% (n = 224), followed by post-surgical 8.1% (n = 27) (Table 1). One hundred and twenty patients suffered various comorbidities, the most frequent being dry eye, with an incidence of 52.5% (n = 63), followed by increased intraocular pressure in 12.5% (n = 15), hypertension (n = 9) 7.5%, eye infection (n = 7) 5.8%, hypothyroidism (n = 7) 5.8%, diabetes mellitus (n = 6) 5.0%, dyslipidaemia 3.3% (n = 4) and others in 7.5% (n = 9). Concomitant medications during treatment with NAPH were used in 27.8% (n=93) of the patients (Table 2), while the remaining 72.2% did not use any concomitant therapy.
 |
Table 2 Medicines Used Concomitantly with NAPH |
Adverse Drug Reactions
A total of 54 ADRs (one per patient) were reported after the use of NAPH in 335 patients (0.16 ADR/patient). They were classified into 2 SOC groups and 4 PT groups. The most frequent SOC group was eye disorders (98.1%) and the most frequent PT was eye irritation (92.6%). All the ADRs in the study were expected (n = 54) (Table 3). The most frequent causality was “probable” in 89% of the cases, followed by “possible” in 5%, “defined” in 4% and “not assessable” in 2%. All ADRs were classified as mild and not serious. A total of 85.2% of the ADRs were resolved in two minutes or less after the product’s instillation. The longest-lasting ADR was a headache with a recovery time of 20 min.
 |
Table 3 Incidence of Adverse Drug Reactions |
A binary logistic regression model was applied to the clinical and demographic characteristics shown in Table 1 as part of the risk assessment for NAPH. This analysis did not show any statistical significance; however, an increase ADRs was observed in the adult age group (adjusted OR = 2.352, p = 0.060, 95% CI 0.964–5.736) compared to geriatric patients. Also, the incidence of ADRs increased when the drug was administered every 4 hours (adjusted OR = 4.443, p = 0.096, 95% CI 0.767–25.748) and 6 hours (adjusted OR = 3.425, p = 0.070, 95% CI 0.904–12.974) in comparison to the 24 hours administration scheme (Table 4).
 |
Table 4 Binary Logistic Regression of Variables Possibly Associated with the Incidence of ADRs |
Discussion
Even though the adverse reactions reported for ophthalmic naphazoline present a low incidence, various publications stress the risk of possible systemic severe ADRs associated with it.19–21 However, these are usually caused by overdose or prolonged use.21,22 All ADRs reported in the study were mild and not-serious; nevertheless, appropriate use of the drug is essential to limit the risk of severe ADRs.
The study included five breastfeeding patients, and although the maternal use of topical ophthalmic products generally carries a lower risk than systemic agents, naphazoline can be absorbed after topical administration;20 for this reason, the use in these circumstances must be evaluated by the prescribing physician. However, no ADRs were reported in newborns of patients treated with NAPH; despite these findings, there is not enough information to ensure that the drug is safe while breastfeeding.
Various references mention eye irritation, vision blurred, administration site discomfort, intraocular pressure increased, and headache as ophthalmic naphazoline’s most frequent ADR.20,23,24 Meanwhile, eye irritation and vision blurred have been described after hypromellose topical application.25,26 In this study, 93% of ADRs were classified according to PT as “eye irritation,” with a 15% incidence, classified as “Very common” according to the CIOMS (Table 3).17 This coincides with the literature, since “eye irritation” is mentioned as the most frequent ADR for both active principles; and even though both active principles list vision blurred as one of their most frequent adverse reactions; it is a characteristic of artificial tears such hypromellose to be associated to a high incidence this type of ADRs.10,25,26
Unlike the previously addressed ADRs, headache is not reported in the literature as a known ADR for hypromellose; however, this reaction is not associated with the drug due to the null absorption at the ocular surface.27 On the other hand, different sources have described that the vasoconstriction caused by naphazoline may trigger headaches in susceptible patients.28,29
This study’s presented results are similar to the naphazoline’s and hypromellose’s safety profile reported in available literature, since ophthalmic preparations with either or both active ingredients have been described as tolerable and safe,9,11,30,31 and their associated ADRs are transient and mild on severity.
The risk assessment of the different study variables was not statistically significant; however, specific trends towards an increase in ADR incidence are observed with certain variables; such is the case of adult patients in comparison to geriatrics. It is worth mentioning that various studies have reported the loss of corneal sensitivity in geriatric patients,32–34 to which a decrease in the presence of discomfort related to instillation could be attributed in these patients. Similarly, an increase in the incidence of ADRs was observed in patients who instilled the drug every 4 and 6 hours compared to those who did every 24 hours. This may be due to sensation of a foreign substance on the ocular surface, rather than to the drug “per se”, this results in a short instillation frequency increasing the probability of presenting local ADRs.
The limitations of our study were that the telephonic follow-up restricts the identification of ADRs that require the evaluation of an ophthalmologist to be identified and graded, causing a limited detection of such reactions. Nevertheless, it presents some advantages, such as individualized pharmacovigilance, continuous monitoring and more detailed information on adverse events from a large number of patients.
Conclusion
The low incidence of ADRs, a short ADR recovery period, and the fact they were mild and not serious demonstrates the high tolerability in the studied population; furthermore, no increased risk was found concerning the patients’ clinical and demographic characteristics. Regardless, the correct use of the drug is essential to maintain its safety profile. At this point, according to this study, the safety profile for NAPH seems to be adequate and its tolerability satisfactory.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Ethics Approval
This study was approved by the “Comité Institucional de Bioética (CIB), Vía libre”, located in Lima, Perú (Approval Number 4204, Dic-18-2018). This study was conducted in compliance with international guidelines and in accordance with strictest international ethical regulations for research.
Acknowledgments
This study was sponsored by Laboratorios Sophia, S.A. de C.V. (Zapopan, Jalisco, México). The sponsor provided support in the form of salaries for authors (HCS, MBH, LMBD, VOC and LYRH), but did not have any additional role in the data collection. The authors thank Alejandra Sánchez Rios, MD for the medical writing support.
Disclosure
Homero Contreras-Salinas, Mariana Barajas-Hernández, Leopoldo Martín Baiza-Durán, Vanessa Orozco-Ceja, Lourdes Yolotzin Rodríguez-Herrera are employees of Laboratorios Sophia S.A. de C.V. The authors report no other conflicts of interest in this work.
References
1. Caster O, Juhlin K, Watson S, Norén GN. Improved statistical signal detection in pharmacovigilance by combining multiple strength-of-evidence aspects in vigiRank. Drug Saf. 2014;37(8):617–628. doi:10.1007/s40264-014-0204-5
2. Leslie WD, Schousboe JT. Pharmacovigilance in the real world. Ann Intern Med. 2019;170(3):201–202. doi:10.7326/M18-3550
3. Moses C, Celi LA, Marshall J. Pharmacovigilance: an active surveillance system to proactively identify risks for adverse events. Popul Health Manag. 2013;16(3):147–149. doi:10.1089/pop.2012.0100
4. Pitts PJ, Le Louet H, Moride Y, Conti RM. 21st century pharmacovigilance: efforts, roles, and responsibilities. Lancet Oncol. 2016;17(11):e486–e492. doi:10.1016/S1470-2045(16)30312-6
5. Meyboom RHB, Egberts ACG, Gribnau FWJ, Hekster YA. Pharmacovigilance in perspective. Drug Saf. 1999;21(6):429–447. doi:10.2165/00002018-199921060-00001
6. Foppiano M, Lombardo G. Worldwide pharmacovigilance systems and tolrestat withdrawal. Lancet. 1997;349(9049):399–400. doi:10.1016/S0140-6736(97)80018-9
7. Li X, Li H, Deng J, et al. Active pharmacovigilance in China: recent development and future perspectives. Eur J Clin Pharmacol. 2018;74(7):863–871. doi:10.1007/s00228-018-2455-z
8. Mann M, Mengistu A, Gaeseb J, et al. Active surveillance versus spontaneous reporting for first-line antiretroviral medicines in Namibia: a cost–utility analysis. Drug Saf. 2016;39(9):859–872. doi:10.1007/s40264-016-0432-y
9. Hosten LO, Snyder C. Over-the-counter ocular decongestants in the United States - mechanisms of action and clinical utility for management of ocular redness. Clin Optom. 2020;12:95–105. doi:10.2147/OPTO.S259398
10. Contreras-Salinas H, Barajas-Hernández M, Baiza-Durán LM, Orozco-Ceja V, Rodríguez-Herrera LY. Ophthalmic solution safety profile: active surveillance of a sodium hyaluronate/chondroitin sulfate combination in Peruvian population. Drug Healthc Patient Saf. 2021;13:117–123. doi:10.2147/DHPS.S311817
11. Tauber J. Efficacy, tolerability and comfort of a 0.3% hypromellose gel ophthalmic lubricant in the treatment of patients with moderate to severe dry eye syndrome. Curr Med Res Opin. 2007;23(11):2629–2636. doi:10.1185/030079907x233197
12. ARCSA. Agencia nacional de regulación, control y vigilancia sanitaria. Available from: http://permisosfuncionamiento.controlsanitario.gob.ec/consulta/index.php.
13. INVIMA. Instituto nacional de vigilancia de medicamentos y alimentos. Available from: https://www.invima.gov.co/consultas-registros-y-documentos-asociados.
14. DGDF. Dirección general de drogas y farmacia. Available from: https://www.msp.gob.do/web/?page_id=2558.
15. COFEPRIS. Comisión Federal para la Protección Contra Riesgos Sanitarios; 2021. Available from: https://tramiteselectronicos02.cofepris.gob.mx/BuscadorPublicoRegistrosSanitarios/BusquedaRegistroSanitario.aspx.
16. ISPCH. Instituto de salud pública Chile. Available from: https://registrosanitario.ispch.gob.cl/.
17. Council for International Organization of Medical Sciences (CIOMS). Working group IV benefit-risk balance for marketed drugs: evaluating safety signals. 1 edition; 1998.
18. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232. doi:10.1093/ajhp/49.9.2229
19. Kuzminov B, Turkina V, Кuzminov Y. Rationale for naphazoline effects in-depth study. Curr Issues Pharm Medical Sci. 2018;31(1):29–33. doi:10.1515/cipms-2018-0007
20. Naphazoline. Micromedex; 2021. Available from: https://www.micromedexsolutions.com/.
21. Costantino G, Ceriani E, Sandrone G, Montano N. Ischemic stroke in a man with naphazoline abuse history. Am J Emerg Med. 2007;25(8):
22. Alizadeh S, Mohebbi N, Gholami K, Jabbarvand M. Adverse drug events leading to emergency department visits at an eye hospital: a brief report. J Curr Ophthalmol. 2017;29(2):139–141. doi:10.1016/j.joco.2017.01.007
23. Hurwitz P, Thompson JM. Uses of naphazoline (privine(R)) in ophthalmology. Arch Ophthalmol. 1950;43(4):712–717. doi:10.1001/archopht.1950.00910010723008
24. Di Lorenzo C, Coppola G, la Salvia V, Pierelli F. Nasal decongestant and chronic headache: a case of naphazoline overuse headache? F1000Res. 2013;2(237):1–9. doi:10.12688/f1000research.2-237.v1
25. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
26. Lievens C, Berdy G, Douglass D, et al. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: a multicenter, double-masked, randomized 30-day study. Cont Lens Anterior Eye. 2019;42(4):443–449. doi:10.1016/j.clae.2018.12.003
27. Ergun R, Guo J, Huebner-Keese B. Cellulose. Elsevier; 2016:694–702. doi:10.1016/B978-0-12-384947-2.00127-6.
28. Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20(10):907–918. doi:10.1046/j.1468-2982.2000.00146.x
29. Willems EW, Valdivia LF, Villalón CM, Saxena PR. Possible role of α-adrenoceptor subtypes in acute migraine therapy. Cephalalgia. 2003;23(4):245–257. doi:10.1046/j.1468-2982.2003.00547.x
30. Uncini A, de Nicola G, Di Muzio A, et al. Topical naphazoline in treatment of myopathic ptosis. Acta Neurol Scand. 1993;87(4):322–324. doi:10.1111/j.1600-0404.1993.tb05516.x
31. Thomseth V, Cejvanovic V, Jimenez-Solem E, Poulsen HE, Utheim TP, Andersen JT. Exposure to antazoline-naphazoline eye drops during pregnancy and the risk of congenital malformations: a Danish nationwide cohort study. Acta Ophthalmol. 2019;97(5):505–509. doi:10.1111/aos.13980
32. Roszkowska AM, Colosi P, Ferreri FMB, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218(5):350–355. doi:10.1159/000079478
33. Yang AY, Chow J, Liu J. Corneal innervation and sensation: the eye and beyond. Yale J Biol Med. 2018;91(1):13–21.
34. Millodot M. The influence of age on the sensitivity of the cornea. Invest Ophthalmol Vis Sci. 1977;16(3):240–242.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
