Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Psychometric Properties and Factorial Analysis of the Arabic McGill-QoL Questionnaire in Breast Cancer
Authors Omar MTA , Alnahdi AH
Received 19 June 2023
Accepted for publication 9 October 2023
Published 14 November 2023 Volume 2023:15 Pages 813—824
DOI https://doi.org/10.2147/BCTT.S422369
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert Clarke
Mohammed TA Omar, Ali H Alnahdi
Department of Rehabilitation Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
Correspondence: Mohammed TA Omar, Department of Rehabilitation Health Sciences, College of Applied Medical Sciences, King Saud University, P.O Box 10219, Riyadh, 11344, Saudi Arabia, Tel +966542115404, Email [email protected]
Purpose: This study aimed to assess the psychometric properties of the Arabic McGill Quality of Life Questionnaire-Revised (MQOL-R) in breast cancer survivors.
Patients and Methods: One-hundred-forty breast cancer survivors were recruited and completed the questionnaire. The construct validity was assessed using confirmatory factor analysis (CFA). MQOL-R scores were correlated with Global Health Status/QoL and functional subscales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) for convergent validity. Reliability was estimated using Cronbach’s alpha and intraclass correlation coefficients (ICC).
Results: CFA reproduced a four-factor model (ie, physical, psychological, existential, and social) with good fit indices (comparative fitting index = 0.980; root mean square error of approximation = 0.091), with all items significantly loading on their respective subscales. The total MQOL-R scores were correlated with the global health status/QoL and functional subscales of the EORTC QLQ-C30 (r = – 0.172, P < 0.01). Known-group validity was proven by different MQOL-R scores according to functional status (50.62 ± 6.35 vs 45.98 ± 7.19, P < 0.01). Reliability was supported by good internal consistency and high test-retest correlation coefficients for the Arabic MQOL-R and its subscales (ICC range, 0.83– 0.95).
Conclusion: The Arabic MQOL-R demonstrated adequate construct validity, factor structure, excellent test-retest reliability, and good internal consistency. This tool is valuable for assessing the quality of life in research and physical therapy rehabilitation settings among Arabic-speaking breast cancer survivors.
Keywords: breast cancer, quality of life, validity, reliability
Introduction
Breast cancer (BC) is the most prevalent cancer among women worldwide.1,2 The incidence of BC has significantly increased in Arab-speaking countries, including Saudi Arabia, over the past 15 years.3–5
The advancement of medical treatment has decreased overall BC mortality rates, resulting in a growing number of survivors.6 However, BC survivors still experience many physical, emotional, and psychosocial challenges, including fatigue, pain, depression, anxiety, and social isolation. These challenges often lead to functional declines, limiting social engagement and negatively impacting the health-related quality of life (HRQoL).6–8 Consequently, BC is no longer considered an acute, incurable condition but rather a chronic condition characterized by periods of remission and symptom flare-ups.8,9
Recent research conducted in Arab countries has emphasized assessing the HRQoL of BC survivors throughout their continuum of care. The objective is to gather structured information on patients’ perceptions and self-reported disabilities and evaluate the impact of the disease and its treatment.10,11 Consequently, there is a strong recommendation to develop and validate tools that can systematically incorporate HRQoL screening into oncology clinical practice and rehabilitation.12–14 In response to this need, several patient-reported outcome measures (PROMs) have been validated in Arabic, such as the European Organization for Research and Treatment of Cancer (EORTC) Breast Cancer-Specific Quality of Life Questionnaire-23 item (QLQ-BR23), the Functional Assessment of Cancer Therapy-Breast (FACT-B), the EORTC Quality of Life Questionnaire, Version 3.0 (EORTC QLQ-C30), and the FACT-G.15–19 However, these instruments are lengthy, primarily focus on physical characteristics and symptoms, and fail to consider existential or spiritual attributes.20–22 Furthermore, these tools solely concentrate on the negative aspects of quality of life, despite both positive and negative factors influencing it.23,24
The McGill Quality of Life-Revised (MQOL-R) Questionnaire consists of 15 items that assess physical, psychological, existential, and social aspects, including a single item to measure the overall quality of life (QoL).22 Thus, it is considered a concise instrument that effectively measures overall QoL. Additionally, it recognizes the universal attribute of spirituality present in every human being, irrespective of religion or denomination. It measures the spiritual aspect as an existential factor, which can have a significant role in coping with cancer and identifying important contributors to HRQoL in cancer patients.20–22 Furthermore, the MQOL-R has demonstrated satisfactory psychometric properties and equivalence to the original version.22 It has also been successfully adapted and validated in Polish,25 Brazilian,26 Italian,27 and Korean28 samples of patients with cancer and/or undergoing palliative care. Although the MQOL-R has been validated into Arabic cancer patients,29 the previous study included various types of cancer patients and did not conduct a structural validity analysis. Establishing the structural validity of a multi-item PROM is crucial to ensure that the scale score, whether the total score or individual subscales, accurately reflects the constructs being measured.22,25–28 Therefore, the present study aimed to examine the psychometric properties, including the structural validity, of the Arabic MQOL-R among BC survivors.
Materials and Methods
Study Design and Participants
A cross-sectional study was conducted from September 2021 to February 2022, utilizing a convenience sampling method to recruit BC patients from King Abdullah Medical City in Mecca and King Abdulaziz Specialty Hospital in Taif, Saudi Arabia. The study protocol underwent review and approval by the Research Ethics Committee of King Saud Medical City (reference number E-21-5818), King Abdullah Medical City in Makkah (No. 21–789), and King Abdulaziz Specialist Hospital in Taif (No. 543), Saudi Arabia. The study adhered to the Declaration of Helsinki 1975 standards for human experimentation. Ethical approval was obtained from these institutions due to their status as major specialized cancer centers, each with a capacity of more than 1500 beds, from which the participants were recruited.
The study had specific inclusion and exclusion criteria. Inclusion criteria included individuals aged 18 years or older with a confirmed diagnosis of BC, completion of all treatments, absence of metastatic disease, fluency in reading and speaking Arabic, and a willingness to participate. Participants were excluded if they experienced lymphedema (inter-limb differences ≥ 10%),30 had bilateral BC, a current infection, a history of local or systemic disorders leading to impairment or disability, or had mental or cognitive impairment that hindered their understanding. Before being included in the study, all BC survivors provided informed consent by signing consent forms.
The study aimed to include a sample size of 5 to 10 participants per item for factor analysis. Given that the MQOL-R questionnaire comprises 14 items, a total sample size of 140 participants was deemed adequate for this study.31
Instruments and Procedure
Socio-Demographic and Cancer-Related Data
Eligible BC survivors were approached for face-to-face interviews and provided with a verbal overview of the study procedures. They were then asked to complete a self-reported questionnaire that gathered detailed demographic and cancer-related information. The socio-demographic data incorporated age, education, employment status, and marital status. Cancer-related variables comprised tumor stage, surgical site, and cancer treatment (radiation and/or chemotherapy). Participants used the Arabic versions of the MQOL-R and EORTC QLQ-C30 questionnaires to assess their QoL, while therapists evaluated their performance status using the ECOG PS (Eastern Cooperative Oncology Group Performance Status). To assess reliability, a subset of 30 participants completed a retest within a one-week interval.
Arabic McGill Quality of Life Questionnaire-Revised
The current study utilized the validated and reliable Arabic MQOL-R questionnaire.29 The MQOL-R consists of 15 items distributed across five domains: Physical (3 items), Psychological (4 items), Existential (4 items), and Social (3 items). Additionally, it includes a Single Item Scale to assess global QoL. Each item is scored on a scale of 0 (not at all) to 10 (extremely), with higher values indicating more positive responses. However, for items 1, 3, 4, 5, 6, 7, and 8, the scores are transformed by subtracting the initial result from 10 so that “0” represents the worst situation and “10” represents the best situation. Subscale scores are obtained by calculating the average value of the relevant items (ranging from 0 to 10), while the MQOL-R total score is derived from the average of the four subscale scores (range = 0–10).22
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (Version 3.0)
The EORTC QLQ-C30 scale is widely employed to assess the QoL in cancer patients, and its validity has been extensively established.32 It comprises five multi-item functional scales (physical, role, cognitive, emotional, and social), global health subscales, and symptom subscales (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial impact). Response choices for most scales range from 1 (not at all) to 4 (very much), except the global QoL scale, which ranges from 1 (very poor) to 7 (excellent). All scale scores are linearly transformed to a 0–100 scale. Higher scores on the functioning scales and global QoL scale indicate a higher level of functioning or QoL, while for the symptom scales, a higher score reflects greater symptom severity. The Arabic version of the EORTC QLQ-C30 has previously been validated for BC patients.14,18 The current study utilized the functional scale and global health subscales.
Eastern Cooperative Oncology Group Performance Status Rating (ECOG PS)
The Eastern Cooperative Oncology Group Performance Status Rating (ECOG PS) was employed to assess the physical status of the participants. The ECOG PS utilizes a five-level rating system ranging from zero to five. Level 0 represents fully active, Level 4 indicates an inability to carry out any self-care and being completely bedridden, and Level 5 signifies death. In this study, good functional status was defined by ECOG PS scores of 0–2, while poor functional status was defined by ECOG PS scores of 3–4.33 The ECOG PS is widely recognized as a reliable and valid measure of a patient’s health status particularly in cancer patients.33,34
Statistical Analysis
Data analysis was conducted using IBM SPSS Statistics 26.0 and Amos 23.0 software packages (IBM Corp., Armonk, NY, USA). Descriptive statistics were utilized to summarize demographic and clinical data, with continuous variables presented as means and standard deviations and categorical variables presented as frequencies. The psychometric properties of the MQOL-R questionnaire were assessed, including internal consistency, test-retest reliability, structural validity, convergence, and known-group validity.
Reliability
To evaluate the internal consistency of the MQOL-R scores, Cronbach’s alpha was calculated for both the total scale and each subscale. Acceptable internal consistency was defined as Cronbach’s alpha values of ≥ 0.7.35 Test-retest reliability was assessed using a two-way random model Intraclass Correlation Coefficient (ICC 2.1) with 95% confidence intervals (CI). ICC values below 0.5 indicated poor reliability, values between 0.5 and 0.75 were considered moderate, values between > 0.75 and ≤ 0.9 were considered good, and values above 0.9 were considered excellent.36
Measurement error was evaluated by calculating the standard error of measurement (SEM) and the minimum detectable change (MDC) for both the total scale and the four subscales of the MQOL-R. The SEM was determined using the equation SEM = SD12 √ (1 - ICC), where SD represents the standard deviation based on the average of the two scores.37 Additionally, the MDC95 was calculated as MDC95 = 1.96 X √2 X SEM, which provides an estimate of the MDC with 95% confidence.37
Convergent Validity
Consistent with previous literature,38,39 we hypothesized that the MQOL-R scales (physical, psychological, social, existential) would demonstrate convergent validity by exhibiting moderate (r = 0.3–0.5) to strong (r > 0.5) positive correlations with the EORTC QLQ-C30 subscales measuring functional status and global health status/QoL. Pearson’s correlation coefficients were computed for variables with a normal distribution, while Spearman’s rank correlation coefficients were used for variables without a normal distribution.40,41
Structural Validity
To examine the factor structure, exploratory factor analysis (EFA) was conducted using principal component extraction and varimax rotation. Before the EFA, the Kaiser-Meyer-Olkin (KMO) coefficient and Bartlett’s test were performed to assess the degree of sphericity. A KMO value of 0.5 or higher and a significant Bartlett’s test of sphericity (p < 0.05) were considered appropriate for conducting EFA. Factors with eigenvalues greater than 1.0 were generally retained. Additionally, items with factor loadings exceeding 0.4 were considered indicative of a meaningful contribution to a specific factor.42–45
Confirmatory Factor Analysis (CFA) was conducted using the generalized least squares method to evaluate the structural factors of the Arabic MQOL-R. Common goodness-of-fit indices were employed to assess the model fit in the CFA. The following metrics were considered for evaluating the goodness-of-fit: comparative fit index (CFI; ≥ 0.9), incremental fit index (IFI; > 0.9), Tucker-Lewis index (TLI; ≥ 0.9), root mean square error of approximation (RMSEA; ≤ 0.08), and standardized root mean square residual (SRMR; ≤ 0.08).45–47
Known Group Validity
Known-group validity was assessed by comparing BC survivors with high-performance status (ECOG PS score 0–2) to those with low-performance status (ECOG PS score 3–4). Independent t-tests (or Mann–Whitney U-test for non-normally distributed variables) were employed to examine group differences and effect sizes were calculated. Effect sizes of 0.2 were considered small, 0.5 moderate, and 0.8 large, following Cohen’s guidelines.41 Based on our hypotheses, we expected that BC survivors with high-performance status (ECOG PS scores 0–2) would score significantly higher on the MQOL-R subscales and total score than those with poor performance status (ECOG PS scores 3–4).
Results
Socio-Demographic and Cancer-Related Data
One-hundred-forty women with BC participated in this study, with a mean age of 53.52 ± 4.48 years. Most participants (82.90%) were married, 45% had a higher education level, and 60% were unemployed. Regarding cancer-related characteristics, 69% were diagnosed with stage I/II BC. The average duration of cancer was 28.14 ± 7.28 months, and more than half of the participants (80%) had been diagnosed with cancer for ≤ 36 months. Table 1 provides an overview of the participants’ demographics and clinical information.
 |
Table 1 Demographic and Clinical Characteristic of Participants (n =140) |
Reliability
The total MQOL-R questionnaire exhibited high internal consistency, with an alpha coefficient of 0.92. The alpha coefficients for the four subscales ranged from 0.83 to 0.93 (Table 2). The test-retest reliability of the MQOL-R total score, as measured by ICC2,1, was excellent: 0.95 with a 95% CI (0.91 to 0.98; p ≤ 0.001). The subscales also demonstrated excellent test-retest reliability values (ICC: 0.93 to 0.95), except for the physical subscale, which showed good test-retest reliability (ICC = 0.87). For the MQOL-R total score, the SEM was 0.38, and the minimal detectable change (MDC) was 1.05. The SEM for the four subscales ranged from 0.52 to 0.63, and the MDC ranged from 1.36 to 1.76 (Table 2).
 |
Table 2 Internal Consistency, Test-Retest Reliability, SEM and MDC and Their Corresponding 95% CI for the Arabic MQOL-R. (n=140) |
Convergent Validity
The convergent validity of the MQOL-R in BC survivors was confirmed by computing correlations between MQOL-R scores and EORTC QLQ-C30 functioning scale scores. The overall MQOL-R subscale scores exhibited significant correlations with EORTC QLQ-C30 functioning scale scores. Specifically, the global health status/QoL domain demonstrated strong correlation with all subscales of the MQOL-R (r = 0.44–0.64; p < 0.01). All subscales of the MQOL-R demonstrated moderate to strong convergent validity (Table 3).
 |
Table 3 Correlation Between EORTC QLQ-C30 (Functioning Scales), and Global Health Perception and the Arabic Version McGill Quality of Life Questionnaire-Revised (n=140) |
Structural Validity
A KMO test for sampling adequacy (KMO = 0.87) and Bartlett’s test of sphericity were conducted to verify the suitability of the data for EFA and CFA. These results indicated a good fit for the EFA data. Initially, the EFA extracted four factors with eigenvalues ≥ 1.0, which accounted for 78.115% of the total variance. Factor loadings ranged from 0.78 to 0.901 after rotating the four factors. The factors are named based on their original scale. The MQOL-R questionnaire consists of four factors: psychological (factor 1, items 4–7; factor loadings ranged from 0.797 to 0.811), existential (factor 2, items 8–11; factor loadings ranged from 0.734 to 0.866), physical (factor 3, items 1–3; factor loadings ranged from 0.768 to 0.844), and social (factor 4, items 12–14; factor loadings ranged from 0.735 to 0.911) (Table 4).
 |
Table 4 Exploratory Factor Analysis for Causal Items of the MQOL-R (n=140) |
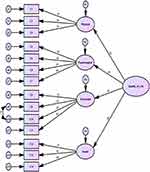
CFA confirmed the goodness-of-fit for the four-factor structural model of the Arabic MQOL-R (Table 5, Figure 1). All items of the Arabic MQOL-R were assigned to the same four factors as the original structure. The model confirmed a good fit to the data (Table 5) with the following indices: χ2 test (CMIN = 166.15; df = 79; p = 0.001), TLI = 0.926, RMSEA = 0.012, SRMR = 0.041, and IFI = 0.908. Additionally, all items exhibited significant loadings (p < 0.001) on their respective subscales: Physical (0.78–0.87), Psychological (0.81–0.90), Existential (0.79–0.82), and Social (0.78–0.82), as depicted in Figure 1.
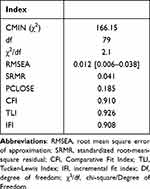 |
Table 5 Fit Indices for Confirmatory Factors Analysis of the Arabic MQOL-R |
Known Group Validity
The results revealed significant differences between the good performance status group (ECOG PS 0–2) and the poor performance status group (ECOG PS 3–4) across various measures. Statistically significant differences were observed in the total MQOL-R scale (p < 0.001), physical subscale (p < 0.001), psychological subscale (p < 0.001), existential subscale (p < 0.001), and social subscale (p < 0.001). The effect size was largest in the psychological domain (ES = 0.83) and the total MQOL-R scale (ES = 0.94), indicating substantial differences. The effect size was moderate in the physical (ES = 0.76), social (ES = 0.67), and existential (ES = 0.63) domains, indicating moderate-to-large effects (Table 6).
 |
Table 6 Discriminant Validity of Arabic MQOL-R Questionnaire (n=140) |
Discussion
This study aimed to assess the psychometric properties of the Arabic MQOL-R questionnaire in BC survivors. The study’s findings indicated that the Arabic MQOL-R demonstrated satisfactory construct validity and reliability in measuring the QoL of BC survivors. Moreover, the results also confirm the four-factor structure of the MQOL-R, consisting of 14 items, as evidenced by the model fit indices.
Overall, both the total Arabic MQOL-R and its subscales demonstrated high internal consistency values. The Cronbach’s alpha coefficient for the MQOL-R was 0.92, which was like the original version of the MQOL-R (0.94),22 the Arabic MQOL-R (0.91),29 and the Dutch version of the MQO (0.96).38 However, it was higher than the Korean MQOL-R (0.86),28 Polish version (0.89),25 Brazilian version (0.85),26 and Italian version (0.81).27 Nevertheless, all these values indicated satisfactory internal consistency reliability.
The internal consistency of the psychological and social subscales was consistent with those of the original MQOL-R22 and were comparable to previous studies. The Cronbach’s alpha coefficient for the Brazilian, Korean, Italian, Polish, and Arabic versions ranged from 0.79 to 0.89.25–29 Additionally, the internal consistency of the physical subscale (0.83) was comparable to those of the Brazilian, Polish, Arabic, and Dutch versions of the MQOL (0.83–0.83).25,26,29,39 However, they were more reliable than the original English version (0.66)22 and the Italian MQOL-R (0.60)27 but lower than the Korean MQOL-R (0.96).28 The variability in internal consistency for the physical subscales can be attributed to the fact that this domain measures different aspects of physical health, such as physical symptoms, physical well-being, and physical function, which may not necessarily correlate consistently.20–22 Furthermore, the variability in sample characteristics, as observed in the Italian study, included end-of-life patients with neurooncological conditions that directly impact physical health.
The test-retest reliability (ICC) for the total scale of the Arabic MQOL-R was found to be 0.95, while for the subdomains, they ranged from 0.87 to 0.93. These results are consistent with those reported in a previous study validating the MQOL-R among Arabic cancer patients29 and the Dutch MQOL among participants with BC.38 This indicates that the Arabic MQOL-R yields consistent results over time. Additionally, the minimal detectable change (MDC) obtained suggests that a true change in overall QoL can be reflected by a total score difference of 1.05 points, with a 95% confidence level, when using the Arabic MQOL-R.
The study provided substantial evidence of adequate construct validity through the use of EFA, CFA, convergent validity, and known-group validity statistical approaches. The initial EFA confirmed the presence of the original four subdomains. The CFA results demonstrated that the scale had an acceptable model fit, as indicated by the meeting of the criteria for model fit indices. The CFI value (0.91) for the subfactors in the current study was similar to that of the original MQOL-R (0.94), as well as the Korean (0.92),28 Polish, and Brazilian versions (both 0.93).25,26
Our results confirmed the presence of four significant factors: psychological, existential, physical, and social, which align with the original MQOL-R version22 as well as the Polish,25 Brazilian,26 and Italian versions.27 Moreover, the CFA indicated that all items of the four factors had factor loadings greater than 0.4. The factor loadings for each item in the MQOL-R ranged from 0.78 to 0.90, which is consistent with results obtained from the original version22 as well as the Polish, Italian, and Brazilian versions of the MQOL-R.25–27 These findings suggest that all items within each factor converge to form a cohesive construct.
Additionally, our findings support the notion that QoL is a multidimensional construct that extends beyond physical well-being to encompass psychological, existential, and interpersonal dimensions. Based on these results, the factor structure of the Arabic MQOL-R closely resembles that of the original MQOL-R. Consequently, the 14 items of the MQOL-R are considered representative of universal QoL characteristics and may be applicable worldwide.
The results of the convergent validation tests provided additional support for the construct validity of the scale by examining the associations between the total and subdomain scores of the Arabic MQOL-R and the functional subscales of the EORTC QLQ-C30. As anticipated, strong positive correlations were observed between both the total scale and the four subscales of the Arabic MQOL-R and the global health status/QoL of the EORTC QLQ-C30, confirming robust convergent validity. Similar correlations were found in the validation of the MQOL-R in Polish,25 Arabic,29 Korean MQOL,39 and Dutch versions.38
Of particular interest, the physical subscale of the Arabic MQOL-R exhibited the strongest correlations with the physical and role physical dimensions of the functional scale of the EORTC QLQ-C30. This finding aligns with the validation results of the MQOL-R in Polish,25 Korean,39 Dutch,38 and Arabic29 versions. These consistent associations suggest that physical conditions and functional limitations hold significant importance for individuals with BC.
Furthermore, the existential subscale of the Arabic MQOL-R demonstrated its strongest correlation with the emotional function dimension of the EORTC QLQ-C30. This pattern was also observed in the validation studies of the MQOL-R in Polish,25 Korean,39 Dutch,38 and Arabic29 versions. These results indicate that existential concerns and emotional well-being are closely linked for BC survivors. Furthermore, it is often valuable to also measure QOL with a single global item that is context-free, to obtain the respondent’s perception of overall QOL rather than what is selected to be measured or not measured in the rest of the QOL instrument. Consequently, we believe that the global item should be presented before and separately from any other items, as it is in MQOL-R.20–22
In summary, the convergent validation findings substantiate the construct validity of the Arabic MQOL-R, highlighting the meaningful relationships between its scores and the functional dimensions of the EORTC QLQ-C30. The outcomes emphasize the importance of addressing physical conditions, functional limitations, existential issues, and emotional well-being in individuals affected by BC.
The MQOL-R subscales and total scores exhibited excellent known-group validity, effectively distinguishing patients with different performance statuses. Specifically, patients with a high Eastern Cooperative Oncology Group Performance Status (ECOG PS) reported lower MQOL-R scores than those with a low ECOG PS. This finding aligns with expectations and indicates a clinically meaningful difference between the two groups in terms of their performance status. The effect sizes were moderate-to-large, further supporting the significance of these differences. These results not only validate the clinical utility of the instrument among Arabic-speaking BC participants but also align with the findings of our previous study.29
Our study has several limitations that should be acknowledged. First, the participants in our study were non-randomly selected from BC patients attending follow-up visits at tertiary hospitals. Therefore, selection bias is possible, and it is unclear whether these findings can be generalized to hospitalized patients or other patient populations. Further research is needed to explore the applicability of the Arabic MQOL-R in different patient groups, including terminally ill cancer patients. However, it is worth noting that our sample size was sufficiently large to perform factor analyses, maintaining a favorable 10:1 ratio between the number of participants and the number of items on the scale.
Additionally, invariance analyses should be conducted to examine the stability of the four-factor structure across various demographic and clinical variables. This would help determine whether the structure holds consistently across different subgroups. Furthermore, while our study successfully replicated the four-factor structure of the original MQOL-R, it would be valuable to conduct CFA in another cancer population to further validate the structure of this tool in the Arabic language.
Conclusion
The Arabic version of the MQOL-R demonstrated acceptable construct validity, structural validity, excellent test-retest reliability, and good internal consistency. This instrument is a valuable tool for assessing the QoL of Arabic-speaking BC survivors, serving as a valuable resource in research and physical therapy rehabilitation settings.
Data Sharing Statement
The data is available and can be requested from the corresponding author.
Author Contributions
Each author played a significant role in the work reported, whether in the conception, design, execution, data collection, analysis, and interpretation; contributed to the draft, revision, or critical review of the article; approved the final version; agreed on the journal to which the article will be submitted; and accepted responsibility for every aspect of the article.
Funding
The authors extend their appreciation to the Deputyship for research and innovation “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-114-3).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: cancer Today. 2018; World Health Organization: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today.
2. Centers for Disease Control and Prevention. Breast Cancer 2021. Available from: https://www.cdc.gov/cancer/breast/basic_info/index.htm.
3. Almutlaq BA, Almuazzi RF, Almuhayfir AA, et al. Breast cancer in Saudi Arabia and its possible risk factors. J Cancer Policy. 2017;12:83–89. doi:10.1016/j.jcpo.2017.03.004
4. Alotaibi RM, Rezk HR, Juliana CI, et al. Breast cancer mortality in Saudi Arabia: modelling observed and unobserved factors. PLoS One. 2018;13(10):e0206148. doi:10.1371/journal.pone.0206148
5. Alqahtani WS, Almufareh NA, Domiaty DM, et al. Epidemiology of cancer in Saudi Arabia thru 2010–2019: a systematic review with constrained meta-analysis. AIMS Public Health. 2020;7(3):679–696. doi:10.3934/publichealth.2020053
6. Mokhtari-Hessari P, Montazeri A. Health-related quality of life in breast cancer patients: review of reviews from 2008 to 2018. Health Qual Life Outcomes. 2020;18(1):1–25. doi:10.1186/s12955-020-01591-x
7. Suwankhong D, Liamputtong P. Physical and emotional experiences of chemotherapy: a qualitative study among women with breast cancer in Southern Thailand. Asian Pac J Cancer Prev. 2018;19(2):521–528. doi:10.22034/APJCP.2018.19.2.521
8. Hassan M, Barakat Z, Fares Y, Abou-Abbas L. Cognitive functioning in women with breast cancer: psychometric properties of the Arabic version of the functional assessment of cancer therapy-cognitive function tool. Health Qual Life Outcomes. 2023;21(1):1–10. doi:10.1186/s12955-023-02095-0
9. Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48–79. doi:10.7812/TPP/14-241
10. Salas M, Mordin M, Castro C, Islam Z, Tu N, Hackshaw MD. Health-related quality of life in women with breast cancer: a review of measures. BMC Cancer. 2022;22(1):1–20. doi:10.1186/s12885-021-09157-w
11. Haddou Rahou B, El Rhazi K, Ouasmani F, et al. Quality of life in Arab women with breast cancer: a review of the literature. Health Qual Life Outcomes. 2016;14(1):64–74. doi:10.1186/s12955-016-0468-9
12. Rowsell A, Sodergren SC, Vassiliou V, et al. Systematic review of health-related quality of life (HRQoL) issues associated with gastric cancer: capturing cross-cultural differences. Gastric Cancer. 2022;25(4):665–677. doi:10.1007/s10120-022-01309-6
13. Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27(1):1–31.
14. Hu X, Zhang C, Zhang Y. BC-PROM: validation of a patient-reported outcomes measure for patients with breast cancer. Medicine. 2017;96(17):e6781. doi:10.1097/MD.0000000000006781
15. Alawneh A, Yasin H, Khirfan G, et al. Psychometric properties of the Arabic version of EORTC QLQ-C15-PAL among cancer patients in Jordan. Support Care Cancer. 2016;24(6):2455–2462. doi:10.1007/s00520-015-3018-9
16. Huijer HAS, Sagherian K, Tamim H. Validation of the Arabic version of the EORTC quality of life questionnaire among cancer patients in Lebanon. Qual Life Res. 2013;22(6):1473–1481. doi:10.1007/s11136-012-0261-0
17. Al-Hoqail HA, Omar MTA, Al-Marwani MM, Al-Eisa ES. Psychometric performance of the Arabic versions of the functional assessment of cancer therapy-breast plus arm morbidity (FACT-B + 4) in patients with breast cancer related lymphedema: cross-sectional study. BMC Women's Health. 2022;22(1):207.
18. Bener A, Alsulaiman R, Doodson L, El Ayoubi HR. An assessment of reliability and validity of the European organization for research and treatment of cancer quality of life questionnaire C30 among breast cancer patients in Qatar. J Family Med Prim Care. 2017;6(4):824–831. doi:10.4103/jfmpc.jfmpc_17_17
19. Alawadhi SA, Ohaeri JU. Validity and reliability of the European Organization for research and treatment in cancer quality of life questionnaire (EORTC QLQ): experience from Kuwait using a sample of women with breast cancer. Ann Saudi Med. 2010;30(5):390–396. doi:10.4103/0256-4947.67083
20. Abdelhafeez AA, Makady NF, Hafez O, et al. Reliability and validity of the Arabic translation of the palliative performance scale. Palliat Support Care. 2020;18(5):575–579. doi:10.1017/S1478951519000889
21. Cohen SR, Mount BM, Strobel MG, Bui F. The McGill quality of life questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliative Med. 1995;9(3):207–219. doi:10.1177/026921639500900306
22. Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill quality of life questionnaire in the palliative care setting: a multicenter Canadian study demonstrating the importance of the existential domain. Palliative Med. 1997;11(1):3–20.
23. Cohen SR, Sawatzky R, Russell LB, et al. Measuring the quality of life of people at the end of life: the McGill quality of life questionnaire-revised. Palliat Med. 2017;31(2):120–129. doi:10.1177/0269216316659603
24. Van Roij J, Zijlstra M, Ham L, et al. Prospective cohort study of patients with advanced cancer and their relatives on the experienced quality of care and life (eQuiPe study): a study protocol. BMC Palliat Care. 2020;19(1):139. doi:10.1186/s12904-020-00642-w
25. Rowlands IJ, Lee C, Janda M, et al. Predicting positive and negative impacts of cancer among long-term endometrial cancer survivors. Psycho Oncology. 2013;22(9):1963–1971. doi:10.1002/pon.3236
26. Rybarski R, Zarzycka B, Bernat A. Measuring the quality of life of people with life-threatening illnesses: the internal structure of the polish adaptation of the McGill quality of life questionnaire - revised. Contemp Oncol. 2018;22(4):252–259.
27. Serrano PV, Serrano GB, Torres ILS, et al. The McGill Quality of Life Questionnaire-Revised (MQOL-R). Psychometric properties and validation of a Brazilian version on palliative care patients: a cross-sectional study. Health Qual Life Outcomes. 2020;18(1):368. doi:10.1186/s12955-020-01621-8
28. Aiello EN, Pain D, Radici A, et al. The Italian McGill Quality of Life Questionnaire-Revised (MQoL-R): psychometrics in Neurological and Neoplastic Populations. J Palliat Care. 2023;38(3):295–298. doi:10.1177/08258597221123454
29. Kang KA, Lee MN. Cross-cultural validation of the McGill Quality of Life Questionnaire-Revised (MQOL-R), Korean Version; A focus on people at the end of life. Korean J Hosp Palliat Care. 2022;25(3):110–120. doi:10.14475/jhpc.2022.25.3.110
30. Omar MT, Al-Malki MH, Bindawas SM, et al. Cross-cultural adaptation and validation of the Arabic version of McGill quality of life: revised questionnaire in the patients with cancer. Disabil Rehabil. 2023;30:1. doi:10.1080/09638288.2023.2207220
31. Schmitz KH, Ahmed RL, Troxel A. Weight lifting in women with breast-cancer related lymphedema. N. Engl. J. Med. 2009;361(7):664–673. doi:10.1056/NEJMoa0810118
32. Mundfrom DJ, Shaw DG, Ke TL. Minimum sample size recommendations for conducting factor analyses. Int J Test. 2005;5(2):159–168. doi:10.1207/s15327574ijt0502_4
33. Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
34. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655. doi:10.1097/00000421-198212000-00014
35. Azam F, Latif MF, Farooq A, et al. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12(3):728–736. doi:10.1159/000503095
36. Taber KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Edu. 2018;48(6):1273–1296. doi:10.1007/s11165-016-9602-2
37. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
38. Mokkink LB, de Vet HCW, Prinsen CAC. COSMIN risk of Bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1171–1179. doi:10.1007/s11136-017-1765-4
39. De Vrieze T, Coeck D, Verbelen H, et al. Cross-cultural psychometric evaluation of the Dutch McGill-QoL questionnaire for breast cancer patients. Facts Views Vis Obgyn. 2016;8(4):205–209.
40. Kim SH, u SK, Yun YH, et al. Validation study of the Korean version of the McGill Quality of Life Questionnaire. Palliat Med. 2007;21(5):441–447. doi:10.1177/0269216307079816
41. Md Yusof K, Mahmud R, Abdullah M, Avery-Kiejda KA, Rosli R. Cross-cultural adaptation of the Functional Assessment of Cancer Therapy-Breast (FACT-B) in Malaysian breast cancer survivors. Asian Pac J Cancer Prev. 2021;22(4):1055–1061. doi:10.31557/APJCP.2021.22.4.1055
42. Cohen J. Statistical power analysis for the behavioural sciences. In: ‘Differences Between Correlation Coefficients’, Lawrence Erlbaum Associates.
43. Hair J, Anderson R, Tatham R, Black W. Multivariate Data Analysis with Reading. Upper Saddle River, NJ: Prentice-Hall; 1995.
44. Williams B, Onsman A, Brown T. Exploratory factor analysis: a five-step guide for novices. Australis J Paramed. 2010;8(3):1–13. doi:10.33151/ajp.8.3.93
45. Nunnally JC, Bernstein IH. Psychometric Theory.
46. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):
47. Xia Y, Yang Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: the story they tell depends on the estimation methods. Behav Res Methods. 2019;51(1):409–428. doi:10.3758/s13428-018-1055-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.