Back to Journals » Infection and Drug Resistance » Volume 17
Prevalence of Smear-Positive, Rifampicin-Resistant Mycobacterium tuberculosis and Related Factors Among Residents with Cough in Northern Ethiopian Refugee Health Facilities
Authors Mezgebe H, Gebrecherkos T, Hagos DG, Muthupandian S
Received 27 December 2023
Accepted for publication 13 March 2024
Published 20 March 2024 Volume 2024:17 Pages 1135—1145
DOI https://doi.org/10.2147/IDR.S453306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Hailemariam Mezgebe,1 Teklay Gebrecherkos,2 Dawit Gebreegziabiher Hagos,2 Saravanan Muthupandian3
1Department of Medical Microbiology, College of Health Sciences, Aksum University, Aksum, Tigray, Ethiopia; 2Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle University, Mekelle, Tigray, Ethiopia; 3AMR and Nanotherapeutics Lab, Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, Tamil Nadu, 600077, India
Correspondence: Teklay Gebrecherkos, Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle University, P.O. Box 1871, Mekelle, Tigray, Ethiopia, Email [email protected]
Purpose: To ascertain the prevalence of Mycobacterium tuberculosis (M.tb) among refugees suspected of tuberculosis (TB) and related risk factors, including smear-positive and Rifampicin-resistant M.tb.
Methods: A cross-sectional study was conducted between January 2020 and May 2020 among 384 refugees in four refugee camps in Northwest Tigray, Ethiopia. Socio-demographic and clinical data were collected from refugees with a history of cough for more than two weeks prospectively. Spot-spot sputum samples were collected and transported in an ice box to the Shire Suhul Hospital Microbiology laboratory; and then examined using a Fluorescent Microscope. All smear-positive samples were further processed by GeneXpert to detect Rifampicin-resistant MTB. Data were analyzed using SPSS version 21 and a p-value < 0.05 was considered statistically significant.
Results: The overall prevalence of smear-positive PTB infection was 5.5% (21/384), but No TB case was resistant to Rifampicin detected by GeneXpert MTB/RIF assay. About 70% of the smear-positive pulmonary TB identified were females. Five (23.8%) of the smear-positive pulmonary tuberculosis cases were co-infected by HIV. Sharing of drink and food materials (AOR = 4.36, 95% CI = 1.19– 15.89), active TB contact (AOR 7.24, 95% CI = 1.62– 32.125), BMI (AOR = 5.23, 95% CI = 1.28– 21.29), opening window practice (AOR = 4.32, 95% CI = 1.02– 18.30) and HIV status (AOR = 9.36, 95% CI = 1.64– 53.35) were statistically significant predisposing factors.
Conclusion: The prevalence of smear-positive pulmonary TB among northwest Tigray refugee camps was still high. The prevalence of TB/HIV co-infection was also high. Minimizing close contact with active TB cases, reducing malnutrition, rapid TB/HIV screening, and establishing a ventilation system can reduce the transmission of TB among refugees.
Keywords: M. tuberculosis, Rifampicin resistant, refugees, risk factors, HIV
Introduction
Mycobacterium tuberculosis (M.tb) is the causative agent of tuberculosis (TB), an infectious disease that primarily affects the lungs. Tuberculosis (TB) can manifest as a wide range of symptoms, from an infection with no symptoms to a potentially fatal illness.1,2 In 2021, tuberculosis ranks as the second most fatal disease after COVID-193 and the 13th leading cause of death worldwide.3 An estimated 1.6 million people died from tuberculosis (TB), and half a million developed drug-resistant TB; of these, 187, 000 also had HIV infections.3,4 Additionally, 8.6% of the glove’s TB cases had HIV/AIDS, with 72% of those cases occurring in Africa and more than half of those cases coming from southern Africa.4,5
In migrants, refugees, and internally displaced people, the disease is more severe due to unjust temporary housing and living conditions, poor health and food insecurity, overcrowding, and limited access to TB treatment and prevention.6–8 People who are outside of their country of origin and are unable to return because of a legitimate fear of persecution due to their race, religion, nationality, involvement in a particular conflict, political beliefs, or membership in a specific social group are classified as refugees.9 On the other hand, migrants are individuals and/or family members who relocate to another nation in search of a better life or to increase their chances of success or those of their families.10
As of 2017, Ethiopia was the country that had taken in the greatest number of African immigrants, with 728,113 coming from Sudan, South Sudan, Somalia, and Eritrea. This is because the nation is bordered by the most unstable and conflict-ridden nations on the list.11,12 According to reports from sub-Saharan Africa, the prevalence of multidrug-resistant tuberculosis (MDR-TB) is estimated to be extremely high at 14% of all cases. Ethiopia is the third-highest tuberculosis burden country in Africa and the eighth-highest in the world. Every year, more than 5000 suspected cases of multidrug-resistant tuberculosis are reported in Ethiopia.13
TB is still a major public health concern, and in countries with limited resources, there is a high risk of developing an active disease due to several factors including poverty, the HIV pandemic, a high number of displaced people, and the emergency of MDR-TB strains.14 Globally, China (170/100,000 people) has been reported to have a higher prevalence of tuberculosis in refugee camps,15 followed by California (39/571 people),16 and Malaysia (12.8% from three refugee schools).17
In camps for African refugees, tuberculosis is frequently a serious public health issue. The incidence of TB among new cases per 100,000 population in Somalia, Djibouti, Ethiopia, Kenya, Uganda, South Africa, and Yemen was 274, 378, 192, 233, 202, 843, and 48, according to the WHO 2016 TB profile by the country for asylums. The prevalence of MDR-TB among new cases in these countries was 5.2, 4.3, 2.7, 1.3, 1.6, 3.5, and 2.3, respectively.18
One of the high-burden African nations where the dual burden of HIV/TB co-infection is most severely impacted is Ethiopia. Numerous studies have revealed that because of the large-scale relocation of people into camps, refugees are especially vulnerable to contracting active tuberculosis. The immune systems of many refugees are severely compromised by their poor nutritional status, co-occurring illnesses, and HIV infection, which increases their susceptibility to tuberculosis. Additionally, the overcrowding in most refugee camps facilitates the easy spread of tuberculosis from infectious patients.
One major factor contributing to life-threatening infections that need quick identification and the start of early treatment is tuberculosis infection in refugee camps. Regarding the current prevalence of MTB and Rifampicin-resistant TB in northern Ethiopian refugee camps, not much information is currently available. This research will help implement refugee-focused interventions, early screening and detection of the most infectious diseases (AIDS and TB) for displaced people, particularly before they enter into camps, and treatment of TB/HIV-positive individuals. Thus, the purpose of the study is to ascertain the prevalence of MTB that is both smear-positive and resistant to Rifampicin in the refugee camps located in North Western Tigray, Ethiopia.
Methods
Study Design, Study Area, and Study Period
This study was conducted in the refugee camps of the Tigray regional state, situated in the North Western Tigray region, Northern Ethiopia (Figure 1). Four refugee camps, namely Adi Harush, Shimelba, Mai-Aini, and Hitsats, were included in the study. According to the Administration for Refugees Returnees Affairs (ARRA) data from 2018, these camps were home to a total of 44,683 refugees, with 9783, 13,900, 10,000, and 11,000 refugees residing in Adi-harsh, Shimelba, May-Ayni, and Hitsats, respectively. An institution-based cross-sectional study was conducted from January to May 2020.
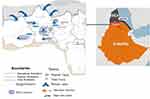 |
Figure 1 A map showing the locations of refugee camps and the major entry routes for Eritrean immigrants into the Tigray region, northern Ethiopia. |
Inclusion and Exclusion Criteria
The study included refugees who were willing to participate and had a cough lasting for at least two weeks. However, refugees who had a cough lasting for at least two weeks but were unable to produce sputum, as well as refugees who were receiving anti-TB treatment or provided incomplete information, were not included in the study.
Sample Size and sampling Technique
The sample size was determined using the following single population proportion formula: N = z2 p (1-p)/w2, where N = TB suspected individuals, Z = standard normal distribution value at 95% CI; 1.96, P = the prevalence of TB (0.5%, since there is no previous in the study area), W = the margin of error, taken as 5%. Accordingly, the sample size calculated was 384 and all TB-suspected cases with a cough history of two weeks and above were selected using a convenience sampling technique until we secured the allocated sample size. 84, 120, 86, and 94 cases from Adi Harush, Shimelba, Mai-Aini, and Hitsats, respectively were included using proportionate allocation formulas. To identify PTB suspects, a mass screening approach was used to ensure equal chances of selecting eligible cases and reduce the possibility of losing PTB suspects. Initially, questions about the occurrence of coughs were asked of all refugees collectively, and questions regarding the length of coughs were asked of each suspected case individually. In the study, refugees who had a cough of two weeks and above were included.
Data and Sputum Collection Process
Using a structured questionnaire, we gather data on behavioral and living practice aspects, clinical factors, and sociodemographic variables. Age, sex, marital status, level of education, monthly income, history of tuberculosis, contact with TB patients or families, pregnancy, history of diabetes mellitus, body mass index, HIV status, history of incarceration, cigarette smoking, chewing tobacco, alcohol consumption, number of bedrooms in the home, window opening habits, and sharing of food and beverages were all included in the information. Sputum specimens, with a volume ranging from 2 to 5 milliliters, were obtained from suspected cases utilizing labeled sputum cups. The standard spot-spot method was followed to take two samples, one hour apart. Specimens were kept in an ice box for transportation if the delay was longer than 24 hours, and at a maximum temperature of 35 °C before processing.
Laboratory Analysis
Sputum samples were taken using 30mL screw-capped, transparent, leakproof, dry, and clean plastic containers. The investigators moved these samples to the Suhul Hospital Microbiology Laboratory so that standard operating procedures could be followed for processing and analysis. A portion of the purulent sputum was placed on glass slides, allowed to air dry, and then heated through the flame passage two or three times. The auramine O Staining Procedure was used to stain each smear. To reduce non-specific fluorescence, a 0.1% Auramine O solution was applied to the smears, left to stain for 20 minutes, then rinsed with tap water and decolorized with a 0.5% potassium permanganate solution for one minute. After that, the slides were air-directed and subjected to Acid Fast Bacilli (AFB) fluorescence microscopy (FM) using Primo Star iLED LED at 20X and 40X magnifications. The AFB was visible as vivid yellow buildings set against a deep black background.
GeneXpert MTB/RIF Assay
To detect rifampicin (RIF) resistance, all acid-fast bacilli-positive sputum samples were subjected to additional processing using the GeneXpert MTB/RIF (Cepheid Gene Xpert system), following national guidelines and manufacturer’s instructions.
HIV screening: Informed consent and assent were obtained from all enrolled participants for an HIV screening at the Providing Initiative Counselling and Testing (PICT) clinics located within the refugee camps. The National Tuberculosis and Leprosy Control Programme (NTLCP) guidelines advised refugees with positive sputum smears to begin anti-TB treatment, while patients without a positive sputum smear were given a ten-day course of broad-spectrum antibiotic treatment.
The flowchart of sampling techniques and laboratory methods is summarized in Figure 2.
 |
Figure 2 Schematic illustration of the sampling techniques and laboratory methods. |
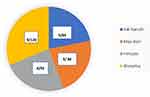 |
Figure 3 Distribution of study participants and smear positivity in four refugee camps. |
Quality Control
To ensure data quality, we maintained the questionnaire’s quality by translating it from English to Tigrigna and conducting a pre-test to assess its appropriateness and completeness before data collection. During the laboratory work, we implemented measures for quality control. Sputum specimens were handled, collected, and processed following standard operating procedures to ensure their quality. We utilized both positive and negative controls in the process.
Statistical Analysis
After being examined and cleaned, the data were exported to version 21 of the Statistical Package for the Social Sciences (SPSS) for analysis. Standard descriptive statistics, which include mean values ± standard deviation for continuous variables, were used to summarize data on sociodemographic and clinical characteristics.
First, binary logistic regression was used to evaluate each variable’s relationship to smear positivity. To evaluate the relationship between the predisposing factors and the outcome variable, variables that in the above model demonstrated statistically significant association with smear positivity were included in the multivariate analysis. A statistically significant p-value was defined as one that was less than 0.05.
Results
Socio-Demographic Characteristics of the Study Participants in Refugee Camps
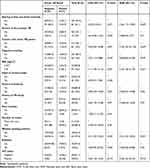
A total of 384 refugees who had coughs of two weeks or more were included in the study. Out of the overall study participants, the number of pulmonary TB suspected cases identified from the health facilities were 84 from the Adi-harsh clinic, 120 from the Shimelba clinic, 86 from the May-ayni clinic, and 94 from the Hitsats clinic. The age of participants ranged between 6 and 72 years, with a mean age of 22 (Mean ± SD=13.39) and the majority (38%) of the refugees were <15 years old. Most of the participants (58.3%) were single. Among the participants, more than half (56.3%) with a family size of 4–7 (75%) were unemployed. Almost half of the study participants (49%) do not attend school. About 79.7% of study participants have reported low monthly income (<500 Ethiopian birr/month), of these 16/21(76.2%) become positive for smear-positive pulmonary tuberculosis cases (Table 1).
 |
Table 1 Demographic Characters of M. Tuberculosis Suspected Refugees (n = 384) with Smear-Positive PTB Prevalence in Tigray Refugee Camp, North Western Tigray (January–May, 2020) |
Prevalence of Smear-Positive PTB and Rifamycin Resistance-Tuberculosis Study Participants in Refugee Camps
The total prevalence of smear-positive PTB was 21 (5.5%) among refugees. The prevalence of smear-positive PTB among tuberculosis-suspected refugees in the refugee camp of Shimelba (the camp with the largest number of refugees, n = 13,900 with 120 tuberculosis suspected cases) was 7.5%, but 3.5% for Adiharush refugee camp had the least number of refugees (n = 9783) and tuberculosis suspected cases (n = 84) (Figure 3). Fifteen of the smear-positive pulmonary TB identified were females. In this study, no PTB case was resistant to Rifampicin detected by GeneXpert MTB/RIF assay.
Five out of the 21 smear-positive pulmonary tuberculosis cases were in the age group of 15–24, 25–34, and 35–44 years old. More than half of the smear-positive TB cases (66.7%; n = 14) were unemployed and unable to read, and eight out of twenty-one were with a family size of 4–7 and above seven. Furthermore, 5/21(23.8%) of the smear-positive pulmonary tuberculosis cases were co-infected by HIV (Table 2).
Factors Associated with Smear-Positive Tuberculosis in Refugee Camps
Univariable binary logistic regression was applied for the following variables: history of previous TB, history of contact with TB family/patient, diabetes mellitus, body mass index, HIV status, cigarette smoking, cigarette smoking, history of incarceration, window opening practice, sharing food and drinks material. Variables that had a p-value less than 0.2 were further analyzed by multivariate binary logistic regression. Finally, there were notable correlations found between smear-positive pulmonary tuberculosis and the following factors: sharing food and drink materials, window opening practices, body mass index, HIV status, and contact with TB patients.
Participants who have a history of contact with active PTB patients have a risk of developing PTB by 7. Twenty-four more than those with no TB contact (AOR = 7.24, 95% CI = 1.62–32.12). Refugee participants who had the habit of sharing food and drink material were 4.36 times more likely to develop PTB than those who did not have the habit (AOR = 4.36, 95% CI = 1.19–15.89).
Refugees with a body mass index of less than 18.5 were 5.23 times more likely to develop PTB than those who had greater than 18.5 (AOR = 5.23, 95% CI = 1.28–21.29). Participants who did not have the habit of window opening practice were 4.32 times more likely to develop PTB than those who had the habit (AOR = 4.32, 95% CI = 1.02–18.30). Furthermore, refugees with HIV seropositivity were 9.36 times more at risk of developing PTB than HIV seronegative patients (AOR = 9.36 95% CI = 1.64–53.35) (Table 2).
Discussion
Tuberculosis in refugee camps has been associated with various factors, such as psychological stress, limited access to essential resources leading to malnutrition, insufficient ventilation, and a fragile health system. These factors have the potential to exacerbate the situation and hinder efforts to prevent and control tuberculosis. Considering these factors, it is noteworthy that the estimated prevalence of pulmonary tuberculosis (PTB) infection among refugees tends to be significantly higher than in the general population.19
Our study revealed that the overall prevalence of smear-positive pulmonary tuberculosis among TB-suspected refugees was 5.5%, resulting in a point prevalence of 384.4 cases per 100,000 refugee populations. This finding is consistent with similar studies conducted in Poland, South Korea, and Ghana which reported prevalence rates of 4.13%, 5.8%, and 5%, respectively.20–22 In contrast, our study’s prevalence is higher than that found among homeless people in Iran (3.4%), marginalized immigrant individuals in Italy (2.7%), and migrants in Rome (0.33%).23–25 These disparities could be attributed to differences in study design, sample size, healthcare facilities available in refugee camps, living conditions, and the level of TB awareness among refugees.
However, it is worth noting that the prevalence of smear-positive pulmonary tuberculosis in refugee camps of Northwest Tigray was lower than reported in the previous studies from Syrian refugees in Canada (9%),26 Colombia (7.9%),27 and two retrospective studies from Gambell region, Ethiopia, which reported 57.6%, and 71.2% smear-positive pulmonary tuberculosis.28,29 These variations could be attributed to differences in study design and setting, sample size, and laboratory diagnosis method used. Notably, the two retrospective cohort studies indicated a heightened risk of TB transmission, which could potentially lead to an outbreak in refugee camps of Northern Ethiopia unless immediate measures are taken.
Furthermore, disparities in the prevalence of smear-positive pulmonary tuberculosis among different countries may be linked to variations in socioeconomic status and low overall TB prevalence within each country. Groups like refugees, who often live in underprivileged conditions marked by malnutrition, poverty, and overcrowded unhygienic environments, may bear a disproportionate burden of TB due to limited access to health care.30
Our study did not detect any case of rifampicin (RIF)-resistant TB. This aligns with studies conducted among prison inmates of the North Gondar zone,31 Northwest Ethiopia,30 and spiritual holy water sites of Northwest Ethiopia.32 However, high rates of RIF-resistant TB have been reported in studies conducted in Korea (11.5%),30 London at (6.5%),33 Uganda (1.9%),34 and Tanzania (1.3%).34 The absence of RIF-resistant TB in our study may be attributed to the small number of smear-positive cases included.
We also found significant associations between smear-positive pulmonary tuberculosis and certain factors. Participants with a history of contact with active TB cases and those who shared drink and food materials had a higher risk of acquiring smear-positive TB. This is likely because Mycobacterium tuberculosis circulates in close surroundings, increasing the risk of TB transmission. These findings are consistent with studies conducted in the North Gondar Zone among prisoners, Northwest Ethiopia’s spiritual holy water sites, and WHO Report 2019.35
Moreover, individuals with body mass index (BMI) of less than 18.5 Kg/m2 were 5.23 times more likely to develop smear-positive TB compared to those with a BMI greater than and equal to 18.5 Kg/m2 (p-value = 0.021). Malnutrition is adversely affecting the immune status of individuals and makes individuals more susceptible to TB infection and the progression of active TB disease.36 This is supported by the WHO report of 2019,35 a study conducted in Rome37 and north Gondar zone prison inmates in northwest Ethiopia.31
Additionally, the lack of window-opening practices was significantly associated with smear-positive PTB (p-value=0.021). This finding aligns with previous reports, including a study in Lima, Peru, which found a strong association between window opening and the risk of TB acquisition38 and one study from northwest Ethiopia.31 This truly indicates that refugee housing cells in the study area were poorly ventilated and overcrowded.
Conversely, 23.8% of the smear-positive pulmonary tuberculosis cases were co-infected by HIV, and HIV positivity was significantly associated with PTB development (p-value = 0.012). HIV infection is known to increase the risk of TB through reactivation or increased susceptibility to a new M. tuberculosis infection. Individuals living with HIV have a significantly higher lifetime risk of tuberculosis compared to those without HIV, highlighting the influential role of HIV in driving the transmission of tuberculosis.35
In our study, participants who smoked cigarettes were not significantly associated with the development of smear-positive pulmonary TB (p-value=0.079). However, the well-established role of smoking in increasing susceptibility to new infections or the risk of active TB development should not be overlooked.28,39
Conclusions
Although there is still a high prevalence of smear-positive pulmonary tuberculosis among refugees in northwest Tigray, no Rifampicin-resistant M. tuberculosis was found in the samples of sputum that tested positive for the disease. Sharing food and drink items, prior TB contact, opening windows frequently, body mass index, and HIV were found to be significantly linked to PTB in the study.
Thus, it is crucial to develop and put into practice specific TB prevention and control strategies. To reduce the spread of TB among refugees, these tactics should include treating infected cases, utilizing separated food and drink materials, quick HIV testing, preventing malnutrition, setting up a ventilation system, and avoiding close contact with patients who have active TB. To lower the illness, it is advised to increase health education, regularly evaluate PTB suspects, identify cases as soon as possible, and provide treatment. Targeted programmatic screening of refugees in the Tigray refugee camp is also necessary, with an emphasis on putting into practice an all-encompassing epidemic control strategy that would lower the number of new tuberculosis cases to levels below which the disease would be eliminated. Additionally, early detection, timely treatment, and disease prevention depend on rapid screening.
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the Ethical Review Committee (ERC) of the College of Health Science, Mekelle University and the ERC number is 1503/2020. We were granted permission to enter the designated refugee camps by the Tigray Health Bureau and the Refugees Returnees Affairs (ARRA) administration, based on the letter of support we obtained from Mekelle University. Above all, before data collection, the objective of the study was explained to each parent/caregiver of the study participants and informed consent and assent were collected. Every detail about the participants was kept private. Any positive findings were communicated to the concerned participants. Patients positive for TB and/or HIV were linked to the refugee clinic for treatment.
The study did not use any personal patient information, and all data were kept confidential following the revised Declaration of Helsinki.
Acknowledgments
We would like to thank the study participants for their collaboration to participate in the study. We would also like to thank the Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle University, and Aksum University for providing funds and Laboratory work to finalize this study. Our sincere thanks also go to the Refugees Returnees Affairs (ARRA) office and refugee clinic staff.
Funding
This study was funded by Mekelle University. Aksum University also participated in providing the reagents required to perform the respective laboratory investigations. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no competing interests in this work.
References
1. Esmail H, Barry C, Young D, Wilkinson R. The ongoing challenge of latent tuberculosis. Philos Trans R Soc. 2014;369(1645):20130437. doi:10.1098/rstb.2013.0437
2. Van Soolingen D. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Int Med. 2001;249(1):1–26. doi:10.1046/j.1365-2796.2001.00772.x
3. World Health Organization. Global tuberculosis report 2021: supplementary material. 2022.
4. World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization;2019; 2020. License: CC BY-NC-SA 3.0 IGO.
5. Floyd K, Glaziou P, Houben R, et al. Global tuberculosis targets and milestones set for 2016–2035: definition and rationale. IJTLD. 2018;22(7):723–730. doi:10.5588/ijtld.17.0835
6. Njuki C, Abera W. Forced displacement and mixed migration challenges in the IGAD region. GREAT Insights Magazine. 2018;7(1).
7. Dhavan P, Dias H, Creswell J, Weil D. An overview of tuberculosis and migration. IJTLD. 2017;21(6):610–623. doi:10.5588/ijtld.16.0917
8. Rendon A, Centis R, Zellweger J-P, et al. Migration, TB control and elimination: whom to screen and treat. Pulmonology. 2018;24(2):99–105. doi:10.1016/j.rppnen.2017.11.007
9. Jackson IC. The 1951 Convention Relating to the Status of Refugees: a universal basis for protection. Int’L J Refugee L. 1991;3(3):403. doi:10.1093/ijrl/3.3.403
10. Urquia ML, Gagnon AJ. Glossary: migration and health. J Epidemiol Community Health. 2011;65(5):467–472. doi:10.1136/jech.2010.109405
11. Lasater M, Woldeyes G, Le Roch K, et al. Lessons learned evaluating the baby friendly spaces program for South Sudanese refugees in Gambella, Ethiopia: strengthening research and programmatic partnerships to address maternal and child health and psychosocial needs in humanitarian emergencies. Confl Health. 2020;14(1):1–9. doi:10.1186/s13031-020-00299-5
12. Refugees Returnees Affairs (ARRA) Afrara. Climate change adaptation program. Addis Ababa Ethiopia. 2011.
13. Mekonnen F, Tessema B, Moges F, et al. Multidrug-resistant tuberculosis: prevalence and risk factors in districts of Metema and west armachiho, Northwest Ethiopia. BMC Infect Dis. 2015;15(1):1–6. doi:10.1186/s12879-015-1202-7
14. Berhane Y, Mariam DH, Kloos H. Epidemiology and Ecology of Health and Disease in. Ethiopia: Shama books; 2006.
15. Mijiti P, Yuehua L, Feng X, et al. Prevalence of pulmonary tuberculosis in western China in 2010–11: a population-based, cross-sectional survey. Lancet Glob Health. 2016;4(7):e485–e494. doi:10.1016/S2214-109X(16)30074-2
16. LoBue PA, Moser KS. Screening of immigrants and refugees for pulmonary tuberculosis in San Diego County, California. Chest. 2004;126(6):1777–1782. doi:10.1378/chest.126.6.1777
17. Wong YJ, Ng KY, Lee SWH. How can we improve latent tuberculosis infection management using behavior change wheel: a systematic review. J Public Health. 2023;fdad051.
18. World Health Organization. Tuberculosis Country Profiles. 2016.
19. Suarez I, Fuenger SM, Jung N, et al. Severe disseminated tuberculosis in HIV-negative refugees. Lancet Infect Dis. 2019;19(10):e352–e359. doi:10.1016/S1473-3099(19)30162-8
20. Romaszko J, Buciński A, Kuchta R, et al. The incidence of pulmonary tuberculosis among the homeless in north-eastern Poland. Open Med. 2013;8(2):283–285. doi:10.2478/s11536-012-0114-9
21. Lee C-H, Jeong Y-J, Heo EY, et al. Active pulmonary tuberculosis and latent tuberculosis infection among homeless people in Seoul, South Korea: a cross-sectional study. BMC Public Health. 2013;13(1):1–6. doi:10.1186/1471-2458-13-720
22. Senya BK, Anim NB, Domson BSK, Adu P. Prevalence of Asymptomatic Mycobacterium tuberculosis Infection in Charcoal Producers: a Cross-Sectional Study in Kaase, Ghana. J Pathog. 2018;2018:1–4. doi:10.1155/2018/9094803
23. Baussano I, Mercadante S, Pareek M, et al. High Rates of Mycobacterium tuberculosis among Socially Marginalized Immigrants in Low-Incidence Area, 1991–2010, Italy. Emerg Infect Diss. 2013;19(9):1437. doi:10.3201/eid1909.120200
24. Sañé Schepisi M, Gualano G, Fellus C, et al. Tuberculosis case finding based on symptom screening among immigrants, refugees, and asylum seekers in Rome. BMC Public Health. 2013;13(1):1–8. doi:10.1186/1471-2458-13-872
25. Bagheri Amiri F, Gouya MM, Saifi M, et al. Vulnerability of homeless people in Tehran, Iran, to HIV, tuberculosis, and viral hepatitis. PLoS One. 2014;9(6):e98742. doi:10.1371/journal.pone.0098742
26. Warrington P, Tyrrell G, Choy K, et al. Prevalence of latent tuberculosis infection in Syrian refugees to Canada. Can J Public Health. 2018;109(1):8–14. doi:10.17269/s41997-018-0028-7
27. Hernández Sarmiento JM, Correa N, Correa M, et al. Tuberculosis among homeless population from Medellín, Colombia: associated mental disorders and socio-demographic characteristics. J Immigr Minor Health. 2013;15(4):693–699. doi:10.1007/s10903-013-9776-x
28. Ejeta E, Beyene G, Balay G, et al. Factors associated with unsuccessful treatment outcome in tuberculosis patients among refugees and their surrounding communities in Gambella Regional State, Ethiopia. PLoS One. 2018;13(10):e0205468. doi:10.1371/journal.pone.0205468
29. Legesse T, Admenur G, Gebregzabher S, et al. Tuberculosis (TB) in the refugee camps in Ethiopia: trends of case notification, profile, and treatment outcomes, 2014 to 2017. BMC Infect Diss. 2021;21(1):1–12.
30. Heo D-J, Min HG, Lee HH. The clinical characteristics and predictors of treatment success of pulmonary tuberculosis in homeless persons at a public hospital in Busan. Korean J Fam Med. 2012;33(6):372. doi:10.4082/kjfm.2012.33.6.372
31. Gebrecherkos T, Gelaw B, Tessema B. Smear-positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BMC Public Health. 2016;16(1):1–10. doi:10.1186/s12889-016-3761-y
32. Derseh D, Moges F, Tessema B. Smear positive pulmonary tuberculosis and associated risk factors among tuberculosis suspects attending spiritual holy water sites in Northwest Ethiopia. BMC Infect Dis. 2017;17(1):1–8. doi:10.1186/s12879-017-2211-5
33. Story A, Murad S, Roberts W, et al. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. 2007;62(8):667–671. doi:10.1136/thx.2006.065409
34. Matee M, Mfinanga S, Holm‐hansen C. Anti‐TB drug resistance levels and patterns among Mycobacterium tuberculosis isolated from newly diagnosed cases of pulmonary tuberculosis in Dar es Salaam, Tanzania. Apmis. 2009;117(4):263–267. doi:10.1111/j.1600-0463.2008.02429.x
35. World Health Organization (2019) Global Tuberculosis Report 2019. 2019.
36. Kim H, Lee C, Shin S, et al. The impact of nutritional deficit on mortality of in-patients with pulmonary tuberculosis. IJTLD. 2010;14(1):79–85.
37. Laurenti P, Bruno S, Quaranta G, et al. Tuberculosis in the sheltered homeless population of Rome: an integrated model of recruitment for risk management. Sci World J. 2012;2012:1–7. doi:10.1100/2012/396302
38. Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4(2):e68. doi:10.1371/journal.pmed.0040068
39. Dogar OF, Shah SK, Chughtai AA, Qadeer E. Gender disparity in tuberculosis cases in eastern and western provinces of Pakistan. BMC Infect Dis. 2012;12(1):1–7. doi:10.1186/1471-2334-12-244
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.