Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Prevalence and Predictors of Remission and Sustained Remission in Patients with Rheumatoid Arthritis from the United Arab Emirates: A Two-Year Prospective Study
Authors Al-Saleh J , Almarzooqi A , Negm AA
Received 21 February 2023
Accepted for publication 5 May 2023
Published 10 May 2023 Volume 2023:15 Pages 51—63
DOI https://doi.org/10.2147/OARRR.S408894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Jamal Al-Saleh,1 Ahlam Almarzooqi,2 Ahmed A Negm1
1Rheumatology, Dubai Hospital, Dubai Health Authority, Dubai, United Arab Emirates; 2Rheumatology, Al Qassimi Hospital, Emirates Health Services, Sharjah, United Arab Emirates
Correspondence: Jamal Al-Saleh, Rheumatology, Dubai Hospital, Dubai Health Authority, P.O. 7272, Dubai, United Arab Emirates, Tel +9714-219 5506, Fax +97142195788, Email [email protected]
Aim: To estimate the prevalence of remission and sustained remission for more than 12 months in a cohort of patients with rheumatoid arthritis in the United Arab Emirates and explore predictors of remission and sustained remission in these patients.
Methods: A two-year prospective study conducted in Dubai Hospital (January 1, 2018-December 31, 2019) included all consecutive patients with rheumatoid arthritis attending the rheumatology clinic. Patients with a Simplified Disease Activity Index ≤ 3.3 and/or Clinical Disease Activity Index ≤ 2.8 in December 2018 were considered in remission and followed until December 2019. Those who maintained remission through 2019 were considered in sustained remission.
Results: In this study, a total of 444 patients were followed for a 12-months period. The percentage of remission achieved in RA patients was 30.4% according to the Clinical Disease Activity Index, 31.1% according to Simplified Disease Activity Index, and 50.9% according to the Value of Disease Activity Score 28 (DAS28) remission criteria. The 12-months sustained remission rates ranged from 38.3% for the ACR-EULAR to 69.3% for the DAS28. Male gender, shorter disease duration, better functioning as evaluated by the Health Assessment Questionnaire Disability Index (lower HAQ scores), and higher compliance rates are among sustained remission predictors.
Conclusion: Establishing “real-world” data and understanding local predictors to sustained remission is principal for implementing timely and appropriate patient-tailored strategies. These strategies include early detection, close monitoring, and enhancing treatment adherence among UAE patients.
Keywords: predictors, remission, rheumatoid arthritis, sustained remission, United Arab Emirates
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory rheumatic disease associated with progressive disability, lower daily physical functioning/activities, lower quality of life, shortened life expectancy, and increased socioeconomic costs. Hence, the gold standard outcomes for RA patients include achieving clinical remission (absence of any sign and symptom of significant inflammatory disease) or low disease activity (LDA) being an alternative goal in patients with long-standing disease.1–3
Numerous studies from around the world have reported an increasing proportion of patients in remission over the years. This has been attributed to the use of novel therapies, early initiation of treatment, and the adoption of treat-to-target (T2T) approaches in clinical practice, as recommended by the international task force American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR).4–8 Although remission rates have been relatively high in the last decade, a recent systematic review with meta-analysis highlighted that more efforts are required to maintain remission and achieve higher sustained remission rates. Thus, understanding and identifying predictors of remission/sustained remission would allow the implementation of adequate targeted interventions for better management and quality of care.3,9
Several predictors for remission have been identified, including sociodemographic characteristics, such as gender (male vs female), younger age, smoking status (non-smoker vs current or previous smoker), a higher level of education, and disease-related factors, eg, late age of disease onset, lower disease activity at baseline, lower functional status at baseline, shorter disease duration, and treatment options.10–18 Similarly, biomarkers, including baseline levels of inflammatory and specific markers, eg, Rheumatoid factor (RF), anti-citrullinated protein/peptide antibody (ACPA), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and serum IL-2, have also been with disease remission.3,19 It is noteworthy that these studies reported conflicting results due to the heterogeneity of the disease presentations (mainly different stages), differences in definitions of remission/sustained, patient characteristics, and treatment regimens. Moreover, some studies evaluated remission at a single point in time, making the results less representative and reliable than those derived from prospective studies.1,18
Thus, establishing “real-world” data and understanding regional and local predictors of sustained remission is paramount to allow implementing patient-tailored strategies, early detection, and better management of RA. In that context, very few studies have addressed remission and sustained remission in the Arab region.20,21 A team of rheumatologists from Africa and the Middle East highlighted the need to design and implement longitudinal epidemiological studies to accurately evaluate the disease prevalence and burden.20 Therefore, this two-year prospective study was performed to estimate the prevalence of remission and sustained remission for more than 12 months in a cohort of RA patients in the United Arab Emirates (UAE) and explore predictors of remission and sustained remission in these patients.
Methods
Study Design
A prospective study was conducted in Dubai Hospital, a secondary care hospital serving the city area of the Emirate, between January 1, 2018, and December 31, 2019, including all consecutive patients with RA attending the rheumatology clinic.
Patient’s Selection
In December 2018, 5,607,981 patients were actively registered with the Dubai Health Authority. The majority of patients were residents of the Emirate of Dubai; others lived in neighboring Emirates. The rheumatology care in Dubai Health Authority is unified under one service line that serves the urban and rural areas in two hospital settings, Dubai Hospital and Hatta Hospital, respectively. During the two-year study period, the rheumatology department in Dubai Hospital has completed an average of 14,000 consultation visits per year.
To ensure the accuracy of the data, the lead investigator and one of the co-investigators validated the gathered data extracted independently from the electronic medical record, with an excellent correlation rate between the investigators.
Inclusion and Non-Inclusion Criteria
All patients meeting the inclusion criteria were recruited as of January 1, 2018. Inclusion criteria consisted of adults aged 18 and above, who fulfilled the 2010 ACR/ EULAR classification criteria for rheumatoid arthritis22 and consented to enroll in the study during routine clinical visits.
Non-inclusion criteria consisted of patients who missed the follow-up visit during 2018 (defined as patients who did not attend the rheumatology clinic or for whom the disease activity was not measured for six months or more), and those who were lost to follow-up in 2019 (defined as patients for whom disease activity was not measured by any of the activity measurement indices in four months since the last visit).
Patient’s Sociodemographic and Clinical Information
Demographic characteristics and clinical information were retrieved from electronic medical records and validated through interviews with the patients attending the clinics. These data included age, age at the time of diagnosis, gender, ethnicity, smoking status, weight, and height (to calculate the body mass index - BMI), educational background, insurance coverage for biological products, and access to medications. Disease-related variables were also recorded, including disease duration and whether the initial presentation to the clinic was less than 42 days. Additional laboratory results were collected, including ESR, CRP, and the status of ACPA, and RF.
Outcomes and Clinical Assessments
Several assessments and evaluations were performed at each visit, ie, the number of missed follow-up visits, the number of swollen joints/number of tender joints, the Charlsons’ Comorbidities Index (CCI), and the atherosclerotic cardiovascular disease risk (ASCVD). A general assessment of the patient was also done using specific scales, ie, Value of Disease Activity Score 28 (DAS28),23 Clinical Disease Activity Index (CDAI),23 Simplified Disease Activity Index (SDAI),23 Health Assessment Questionnaire Disability Index (HAQ),24 and if the patients were lost to follow-up. The CDAI/SDAI was chosen over the DAS28 since they are more stringent than the DAS28 in assessing clinical remission.25,26 Moreover, CDAI/SDAI correlates better with patients reported outcomes than DA28 in patients with RA.27
HAQ is a 41-item scale measuring functional status in RA and yielding a total score ranging from 0 to 3.0 (in 0.125 increments). Higher scores indicate worse functioning, with 0 = no functional impairment and 3 = complete impairment.24
Remission was defined according to the CDAI/SDAI criteria:28 patients with an SDAI ≤3.3 and CDAI ≤2.8 at any visit during 2018 were considered in remission. All patients who reached remission in December 2018 were followed until December 31, 2019. Patients were subsequently classified into two groups: 1) patients with sustained remission for 12 months (maintained CDAI/SDAI criteria definition of remission throughout 2019) and 2) patients who relapsed during follow-up of the same year (did not achieve the SDAI & CDAI remission).
The primary outcome was to estimate the prevalence of remission and sustained remission for more than 12 months in this cohort of RA patients. Secondary outcomes were to explore predictors of remission and sustained remission.
Treatment and Compliance
All previous and current conventional treatments were noted, including steroids, traditional/conventional disease-modifying anti-rheumatic drugs (DMARDs, including methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide), biologic disease-modifying anti-rheumatic drug (bDMARDs, including abatacept, anti-TNF drugs [adalimumab, certolizumab, etanercept], IL-6 inhibitors [tocilizumab], anti-CD20 inhibitors) and targeted synthetic disease-modifying anti-rheumatic drugs (tsDMARDs), Janus kinase inhibitors (JAK inhibitors, ie, tofacitinib and baricitinib), both referred to as biologics.16
Patients were asked to give a subjective estimation of their treatment compliance. Compliance was then defined as follows: 100% (almost always taking the prescribed medicine), 75% (usually missing 25% of the scheduled dose), 50% (usually missing 50% of the scheduled dose), and 25% (taking less than 25% of the scheduled dose or not taking the active treatment at all).
Data and Statistical Analysis
Descriptive statistics, Student’s t-test, Mann–Whitney U-test, Chi-square (X2), and Fisher’s exact test were used for statistical analysis as appropriate. Demographic data and disease and treatment characteristics were described as median and the 25th–75th interquartile range (IQR).
The Relative Risk (RR) and confidence interval (CI) were calculated using 2×2 tables of different demographic and clinical variables to compare patients with sustained remission and those with relapse. Chi-square (X2) and Fisher’s exact test were used to compare percentages between groups, and Student’s t-test and Mann–Whitney U-test for continuous variables.
Multiple regression analyses were performed to investigate the impact of different factors at baseline on remission and sustained remission. Variables to be included in the different models were selected based on their statistical significance in the univariate analysis (variables with p-values <0.1) and their clinical relevance. Significant variables were isolated using stepwise forward selection described as t-value: the coefficient divided by the standard error. Statistical analysis was performed using Minitab version 18.1 software. All statistical tests were two-sided; a p-value less than 0.05 was considered statistically significant.
Results
Patient’s Selection and Sociodemographic Features
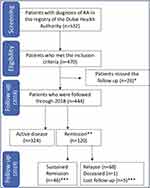
Among the 470 patients who were screened at the Dubai Health Authority Registry and met the inclusion criteria, 26 (5.53%) missed the follow-up (defined as patients who did not attend the rheumatology clinic or for whom the disease activity was not measured for six months or more), yielding a total of 444 patients to be followed during 2018 (Figure 1).
The majority of patients included in the study were Arabs 86.3% (n = 383). Other nationalities include 12.9% (n = 53) from the Indian subcontinent, 1.6% (n = 7) non-Arab Middle Eastern, 0.2% (n = 1) from South East Asia. Almost half of the patients had a secondary level of education (49.8%; n = 221), while almost 43% (n = 190) had a university level and only 7.4% a primary educational level. Disease activity scores were measured in four consecutive visits during 2018 in 87.4% SDAI, 91.7% CDAI, and 84.7% DAS28.
Remission (2018): Prevalence and Associated Factors
Remission in 2018 was reached by 30.4% according to the CDAI classification, 31.1% according to SDAI, while 226 (50.9%) achieved DAS28 remission (disease activity was assessed every 3 months on four consecutive visits during 2018). More details related to remission rates and RA disease activities are shown in Table 1.
 |
Table 1 Rheumatoid Arthritis Disease Activity Scores and Remission (n = 444; Last Quarter – Q4 – of 2018) |
Comparative assessment of baseline factors associated with remission versus active disease is displayed in Table 2. Patients from the remission group had significantly lower age (mean age of 51 years versus 54 in the active group; p-value=0.01), lower disease duration (median of 5 years versus 7 for the active disease group; p-value = 0.001) and lower HAQ scores, and thus better functioning than the active disease group (median HAQ score of 0.65 in the remission group versus 0.94 in the disease group; p-value=0.0001). Moreover, the number of patients switching to another treatment consisting of a biological agent (including anti-TNF drugs, IL-6 inhibitors or abatacept) was significantly higher in the active group as compared to the remission group (p-value=0.001). In fact, none of the patients from the remission group switched to other biologics. All other demographical, clinical, and biological factors did not reach significance.
 |
Table 2 Comparative Assessment of Patients with Remission versus Active Disease (Quarter 4 2018) |
Sustained Remission (2019): Prevalence and Associated Factors
Among the 120 patients who achieved remission defined by (ACR/EULAR remission criteria: SDAI ≤3.3 and CDAI ≤2.8) in 2018 and were followed in 2019, 46 (38.3%) reached a sustained remission over 12 months (based on the ACR/EULAR remission: SDAI ≤3.3 and CDAI ≤2.8 criteria), 1 patient died (0.8%) during the third quartile of 2019, and 5 (4.2%) were lost to follow-up (Figure 1).
More than half of the patients, 68 (56.7%), had an active disease in 2019, with 55 (45.8%) exhibiting low disease activity, 11 (9.2%) moderate disease activity, and only 2 (1.7%) high disease activity.
The Kaplan-Meier for sustained remission using the three different measurement indices is shown in Figure 2.
Patients in the sustained remission group were younger (mean age 51 years versus 54 in the relapsed/active group; p-value=0.006), had lower disease duration (mean of five years versus seven years for the relapsed/active disease group; p-value=0.001), better functioning as evaluated by the HAQ scores (lower scores; p-value=0.004), a lower frequency of double-positive biological markers (RF+ and ACPA+), (30.9% versus 46.6% in the relapse/active group; p-value=0.03), and higher compliance rates than patients in the relapsed/active disease group (100% versus 80%, p-value=0.01) (Table 3).
 |
Table 3 Comparative Assessment Between Patients with Sustained Remission and Relapsed/Active Disease (2019) |
Predictors of Remission: Multivariable Analysis
Multiple regression analysis taking remission in 2018 as the dependent variable showed that the HAQ score is inversely associated with remission (- 0.1243; p = 0.003) (Table 4; Model 1).
 |
Table 4 Regression Analysis Taking Remission or Sustained Remission (in 2019) as Dependent Variables |
Predictors of Sustained Remission: Multivariable Analyses
Predictors of sustained remission in 2019 (as compared to relapse/active disease) as the dependent variable showed significantly higher sustained remission rates in male gender (versus female, −0.1436; p = 0.013), shorter disease duration (−0.00511; p = 0.035), HAQ (lower HAQ scores, −0.0907; p = 0.001), and higher compliance rates (0.00787; p = 0.012) (Table 4; Model 2).
However, when considering patients with sustained remission in 2019 as dependent variable compared to those who relapsed only, age was the only variable identified as significantly associated with sustained remission rates: higher rates being noted in younger patients versus older ones (−0.01799; p-value=0.004) (Table 4; Model 3).
Discussion
This study aimed to assess the prevalence and the predictors of remission and sustained remission rates in a sample of patients with RA. Real-world data evidenced that remission in RA patients is a relatively achievable goal in clinical practice; sustained remission, however, is harder to maintain over time.1,3 Achieving sustained remission remains a challenging issue for health-care professionals, as disease state and relapse have a detrimental impact on the quality of life of patients and families. Thus, identifying predictors for sustained remission is paramount for implementing patient-tailored strategies for better clinical outcome, and cost-effective approach.1,3
In this population-based study, the percentage of remission achieved in RA patients was around 31% according to ACR/EULAR and 50.9% according to the DAS28 remission criteria. The 12-months sustained remission rates ranged from 38.3% for the ACR-EULAR to 69.3% for the DAS28. This result was expected because of the less stringent criteria of the DAS28. Comparing remission and sustained remission rates among studies is difficult, partly due to the wide range of available definitions and criteria, disease stages (early RA or established RA), patient characteristics, and treatment regimen.1,10 Despite these potential discrepancies, the numbers reported in this study are similar to what was reported in another study with a 6-month follow-up18 (45.6% and 44%, respectively, according to the CDAI and SDAI criteria). However, these sustained remission rates are relatively higher than what was previously reported elsewhere,10,13–15,17 which can be attributed to the lower follow-up period (6 months18 and 12 months in the current study) as compared to other studies (reporting sustained remission outcomes in patients from 3 to 8 years).13,14,29 Indeed, it is always a challenge to maintain large proportion of patients with RA in sustained remission over a long time. Hence, rheumatologist in daily practice should regularly continue to measure disease activity on regular intervals and optimize patients management to achieve the common quest of sustained remission.14 Several other factors could explain such high remission and sustained remission rates, including the early detection strategies adopted in the UAE (encompassing nationwide support groups and awareness programs), efficient referral systems, good health coverage of treatment costs (including the use of DMARDs at an early stage), and access to specialized physicians.20,30 It is noteworthy that the sustained remission rates are somehow notable despite the relatively low prescription of biologics in the present study (less than 30% of our patients).
Interestingly, the only factor for sustained remission among patients who achieved remission during the first year was age: younger patients reported higher sustained remission rates than older ones. These results are in line with previous reports identifying that older age, especially at symptoms onset, was associated with more disability and worse HAQ scores.31,32
Furthermore, male gender, shorter disease duration, lower HAQ scores, and higher compliance rates are among sustained remission predictors. Several studies from early and established RA cohorts reported that male is an independent predictor for sustained remission.3,11,13,14,17,29,33 Different hypotheses have been suggested to explain, such as observation, including the role of hormone fluctuations, genetic differences, differences in immunological and psychological responses, and drug dosing differences.10,13
Expectedly, shorter disease duration was identified as a predictor of sustained remission, similarly to other studies.11,12,17,18 Such finding highlights the importance of an early and timely diagnosis and intervention before any functional disability develops in patients with RA. Thus, rheumatologists in the UAE should be diligent in the early detection of low levels of clinical disease activity.11 Meticulous monitoring and optimizing treatment to achieve remission should be the standard in real-world practice to improve patients’ quality of life and reduce long-term cost.
In this study, patients in the remission and sustained remission groups had higher functioning scores as evaluated by the HAQ (the only variable identified as the predictor for both remission and sustained remission), in line with other findings.10,14,15,33 Studies have shown that HAQ is one of the strongest predictors of long-term outcomes;34 it is also a predictor of remission and functional outcomes,15 mortality,35 and treatment response.24,36 Hence, patients with higher HAQ scores at baseline, probably reflecting the cumulative effects of the disease and later onset of diagnosis, might be offered more aggressive therapies since they are less likely to achieve remission than other patients.
Regarding pharmacological treatment, conventional DMARDs were the most prescribed medications in our population (around 76%), and to a lesser extent biologics, or the association of several therapeutic classes, as previously described in UAE cohorts.20,37 However, these treatments were not significantly different between the remission/sustained remission group and active/relapsed disease patients. Irrespective of the treatments, patient adherence to therapy has been identified as an independent predictor of sustained remission, where patients from the remission group had a 100% median subjective compliance rate compared to 80% with the other group. Compliance to treatment showed to be suboptimal among RA patients in clinical settings, ranging from 11% to 80%.38–40 Poor adherence was associated with detrimental outcomes, including increased disease flares, lower remission and sustained remission rates, poor quality of life, and higher economic health-care costs.38,41–44 Treatment adherence is paramount in RA patients, particularly in the early phases of the disease, since aggressive treatment during this phase has been shown to prevent structural damage and result in higher remission rates.31,45
Numerous factors could induce poor adherence to treatment, including patient beliefs, medication side effects and costs, and disease-related psychological distress (such as anxiety and depression), leading to detrimental outcomes.38,43,46 Educational material/visual aids can be prepared and offered to patients while considering their literacy level.20,38,43
Limitations and Strengths
This study has some limitations mainly related to the data coming from one center compared to other international cohorts, which have larger sample size. However, it is the largest in the UAE, enrolling “real-world” patients from the Dubai Health Authority Registry; the data included several sociodemographic, biological, and clinical/treatment features. Some data and variables were missing, such as compliance at remission, which could have been interesting to evaluate. Furthermore, compliance with treatment was subjectively evaluated, with possible recall bias related to the retrospective evaluation; it would have been valuable to assess it using validated questionnaires for medication adherence or calculate the medication possession ratio. Finally, the follow-up period was relatively short (two years), and it would be interesting to extend this follow-up, especially in patients with sustained remission, to evaluate their disease status over time.
Despite all these limitations, this study has several strengths. Three different commonly used remission criteria were considered to define remission (DAS28, SDAI, CDAI), and all patients who met the ACR/EULAR inclusion criteria for RA were included, regardless of whether they have an early RA or an established disease, which might better reflect current practice. Moreover, since the study is prospective and not retrospective, remission and sustained remission were evaluated over time. Hence, the two-year follow-up period allowed the identification of the baseline predictors of both remission and sustained remission rather than an evaluation at a single point in time, making our results more reliable and of higher clinical relevance.
Future Perspectives
It is paramount to follow up with the patients (38.3%) who could maintain the 12-month sustained remission during the consecutive year and check their remission status after two years. Factors associated with sustained remission, including telemedicine services, should be evaluated in RA management during the COVID-19 pandemic. Such services have been reported to be a successful alternative to face-to-face visits in rheumatology clinics, with considerable satisfaction to both patient and physician.47,48
Conclusion
Our findings demonstrated the importance of conducting regional studies to elucidate the specific predictors for sustained remission in rheumatologic diseases such as RA. The results presented in this article are of great relevance as some of the factors could be avoidable or modifiable, suggesting the need for implementing timely and appropriate T2T strategies. Hence, early detection and close monitoring of patients in the UAE are crucial to achieving remission, improving quality of life, and reducing management costs. Clinicians should identify specific barriers to non-compliance in the UAE and promote the culture of treatment adherence among their patients.
Abbreviations
ACR, American College of Rheumatology; ACPA, Anti-Citrullinated Protein/Peptide Antibody; ASCVD, Atherosclerotic Cardiovascular Disease; CRP, C-Reactive Protein; CCI, Charlsons’ Comorbidities Index; CDAI, Clinical Disease Activity Index; CI, Confidence interval; DAS28, Disease Activity Score 28; ESR, Erythrocyte Sedimentation Rate; EULAR, European League Against Rheumatism; HAQ, Health Assessment Questionnaire Disability Index; IQR, Interquartile Range; LDA, Low Disease Activity; RR, Relative Risk; RA, Rheumatoid Arthritis; RF, Rheumatoid Factor; SDAI, Simplified Disease Activity Index; T2T, Treat-to-Target; UAE, United Arab Emirates.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This work has been performed in accordance with the ethical standards of the Declaration of Helsinki. The study received the Institutional Review Board (IRB) from the Dubai Scientific Research Ethics Committee (DSREC), Dubai Health Authority (ethics committee number: DSREC-11/2018.04), and all patients signed written informed consent for data collection and research use.
Acknowledgments
The authors would like to thank the Emirates Society for Rheumatology for supporting this work, and Science PRO sarl for conducting critical review and editing of the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare they have no conflicts of interest.
References
1. Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017;9(10):249–262. doi:10.1177/1759720X17720366
2. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi:10.1038/nrdp.2018.1
3. Yu C, Jin S, Wang Y, et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol. 2019;38(3):727–738. doi:10.1007/s10067-018-4340-7
4. Aga AB, Lie E, Uhlig T, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann Rheum Dis. 2015;74(2):381–388. doi:10.1136/annrheumdis-2013-204020
5. Overman CL, Jurgens MS, Bossema ER, Jacobs JW, Bijlsma JW, Geenen R. Change of psychological distress and physical disability in patients with rheumatoid arthritis over the last two decades. Arthritis Care Res. 2014;66(5):671–678. doi:10.1002/acr.22211
6. England BR, Tiong BK, Bergman MJ, et al. 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res. 2019;71(12):1540–1555. doi:10.1002/acr.24042
7. Littlejohn G, Roberts L, Bird P, et al. Patients with rheumatoid arthritis in the Australian OPAL Cohort show significant improvement in disease activity over 5 years: a multicenter observational study. J Rheumatol. 2015;42(9):1603–1609. doi:10.3899/jrheum.141575
8. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
9. Schett G, Tanaka Y, Isaacs JD. Why remission is not enough: underlying disease mechanisms in RA that prevent cure. Nat Rev Rheumatol. 2021;17(3):135–144. doi:10.1038/s41584-020-00543-5
10. Cook MJ, Diffin J, Scire CA, et al. Predictors and outcomes of sustained, intermittent or never achieving remission in patients with recent onset inflammatory polyarthritis: results from the Norfolk Arthritis Register. Rheumatology. 2016;55(9):1601–1609. doi:10.1093/rheumatology/kew210
11. Furst DE, Pangan AL, Harrold LR, et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res. 2011;63(6):856–864. doi:10.1002/acr.20452
12. Gremese E, Salaffi F, Bosello SL, et al. Very early rheumatoid arthritis as a predictor of remission: a multicentre real life prospective study. Ann Rheum Dis. 2013;72(6):858–862. doi:10.1136/annrheumdis-2012-201456
13. Jawaheer D, Messing S, Reed G, et al. Significance of sex in achieving sustained remission in the consortium of rheumatology researchers of North America cohort of rheumatoid arthritis patients. Arthritis Care Res. 2012;64(12):1811–1818. doi:10.1002/acr.21762
14. Jayakumar K, Norton S, Dixey J, et al. Sustained clinical remission in rheumatoid arthritis: prevalence and prognostic factors in an inception cohort of patients treated with conventional DMARDS. Rheumatology. 2012;51(1):169–175. doi:10.1093/rheumatology/ker250
15. Lee KE, Choi SE, Xu H, Kang JH, Park DJ, Lee SS. HAQ score is an independent predictor of sustained remission in patients with rheumatoid arthritis. Rheumatol Int. 2017;37(12):2027–2034. doi:10.1007/s00296-017-3833-z
16. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68(1):1–25. doi:10.1002/acr.22783
17. Sung YK, Yoshida K, Prince FHM, et al. Prevalence and predictors for sustained remission in rheumatoid arthritis. PLoS One. 2019;14(4):e0214981. doi:10.1371/journal.pone.0214981
18. Xie W, Li J, Zhang X, Sun X, Zhang Z. Sustained clinical remission of rheumatoid arthritis and its predictive factors in an unselected adult Chinese population from 2009 to 2018. Int J Rheum Dis. 2019;22(9):1670–1678. doi:10.1111/1756-185X.13651
19. Pope JE, Movahedi M, Rampakakis E, et al. ACPA and RF as predictors of sustained clinical remission in patients with rheumatoid arthritis: data from the Ontario Best practices Research Initiative (OBRI). RMD Open. 2018;4(2):e000738. doi:10.1136/rmdopen-2018-000738
20. Halabi H, Alarfaj A, Alawneh K, et al. Challenges and opportunities in the early diagnosis and optimal management of rheumatoid arthritis in Africa and the Middle East. Int J Rheum Dis. 2015;18(3):268–275. doi:10.1111/1756-185X.12320
21. Sun X, Li R, Cai Y, et al. Clinical remission of rheumatoid arthritis in a multicenter real-world study in Asia-Pacific region. Lancet Reg Health West Pac. 2021;15:100240. doi:10.1016/j.lanwpc.2021.100240
22. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
23. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, Disease Activity Score (DAS) and disease activity score with 28-joint counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(Suppl 11):S14–36. doi:10.1002/acr.20621
24. Maska L, Anderson J, Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. 2011;63(Suppl 11):S4–13. doi:10.1002/acr.20620
25. Takanashi S, Kaneko Y, Takeuchi T. CDAI and DAS28 in the management of rheumatoid arthritis in clinical practice. Ann Rheum Dis. 2020;79(5):671–674. doi:10.1136/annrheumdis-2019-216607
26. Balsa A, de Miguel E, Castillo C, Peiteado D, Martin-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology. 2010;49(4):683–690. doi:10.1093/rheumatology/kep442
27. Salaffi F, Di Carlo M, Farah S, Marotto D, Atzeni F, Sarzi-Puttini P. Rheumatoid Arthritis disease activity assessment in routine care: performance of the most widely used composite disease activity indices and patient-reported outcome measures. Acta Biomed. 2021;92(4):e2021238. doi:10.23750/abm.v92i4.10831
28. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–586. doi:10.1002/art.30129
29. Acosta-Merida A, Naranjo A, Rodriguez-Lozano C. Prognostic factors for sustained remission in a “real life” cohort of rheumatoid arthritis patients. Reumatol Clin. 2020;16(5 Pt 2):405–409. doi:10.1016/j.reuma.2018.10.002
30. Zafar S, Badsha H, Mofti A, et al. Efforts to increase public awareness may result in more timely diagnosis of rheumatoid arthritis. J Clin Rheumatol. 2012;18(6):279–282. doi:10.1097/RHU.0b013e3182676975
31. Contreras-Yanez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: how persistence with therapy matters. Arthritis Res Ther. 2015;17:177. doi:10.1186/s13075-015-0697-z
32. Norton S, Fu B, Scott DL, et al. Health Assessment Questionnaire disability progression in early rheumatoid arthritis: systematic review and analysis of two inception cohorts. Semin Arthritis Rheum. 2014;44(2):131–144. doi:10.1016/j.semarthrit.2014.05.003
33. Almoallim H, Hassan R, Cheikh M, et al. Rheumatoid Arthritis Saudi Database (RASD): disease characteristics and remission rates in a tertiary care center. Open Access Rheumatol. 2020;12:139–145. doi:10.2147/OARRR.S260426
34. Einarsson JT, Geborek P, Saxne T, Kristensen LE, Kapetanovic MC. Sustained remission improves physical function in patients with established rheumatoid arthritis, and should be a treatment goal: a prospective observational cohort study from Southern Sweden. J Rheumatol. 2016;43(6):1017–1023. doi:10.3899/jrheum.150995
35. van Nies JA, de Jong Z, van der Helm-van Mil AH, Knevel R, Le Cessie S, Huizinga TW. Improved treatment strategies reduce the increased mortality risk in early RA patients. Rheumatology. 2010;49(11):2210–2216. doi:10.1093/rheumatology/keq250
36. Mohammed RH, Farahat F, Kewan HH, Bukhari MA. Predictors of European League Against Rheumatism (EULAR) good response, DAS-28 remission and sustained responses to TNF-inhibitors in rheumatoid arthritis: a prospective study in refractory disease. Springerplus. 2015;4:207. doi:10.1186/s40064-015-0979-6
37. Namas R, Joshi A, Ali Z, Al Saleh J, Abuzakouk M. Demographic and clinical patterns of rheumatoid arthritis in an Emirati Cohort from United Arab Emirates. Int J Rheumatol. 2019;2019:3057578. doi:10.1155/2019/3057578
38. Wong PK. Medication adherence in patients with rheumatoid arthritis: why do patients not take what we prescribe? Rheumatol Int. 2016;36(11):1535–1542. doi:10.1007/s00296-016-3566-4
39. van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi:10.1586/eci.12.23
40. Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol. 2016;43(11):1997–2009. doi:10.3899/jrheum.151212
41. De Vera MA, Mailman J, Galo JS. Economics of non-adherence to biologic therapies in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(11):460. doi:10.1007/s11926-014-0460-5
42. Heidari P, Cross W, Weller C, Nazarinia M, Crawford K. Medication adherence and cost-related medication non-adherence in patients with rheumatoid arthritis: a cross-sectional study. Int J Rheum Dis. 2019;22(4):555–566. doi:10.1111/1756-185X.13549
43. Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology. 2021;60(8):3778–3788. doi:10.1093/rheumatology/keaa860
44. Pascual-Ramos V, Contreras-Yanez I, Villa AR, Cabiedes J, Rull-Gabayet M. Medication persistence over 2 years of follow-up in a cohort of early rheumatoid arthritis patients: associated factors and relationship with disease activity and with disability. Arthritis Res Ther. 2009;11(1):R26. doi:10.1186/ar2620
45. Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43(7):906–914. doi:10.1093/rheumatology/keh199
46. Matcham F, Davies R, Hotopf M, et al. The relationship between depression and biologic treatment response in rheumatoid arthritis: an analysis of the British Society for Rheumatology Biologics Register. Rheumatology. 2018;57(5):835–843. doi:10.1093/rheumatology/kex528
47. Tornero-Molina J, Sanchez-Alonso F, Fernandez-Prada M, Bris-Ochaita ML, Sifuentes-Giraldo A, Vidal-Fuentes J. Tele-rheumatology during the COVID-19 pandemic. Reumatol Clin. 2022;18(3):157–163. doi:10.1016/j.reuma.2020.10.004
48. Chevallard M, Belloli L, Ughi N, et al. Use of telemedicine during the COVID-19 pandemic in patients with inflammatory arthritis: a retrospective study on feasibility and impact on patient-reported outcomes in a real-life setting. Rheumatol Int. 2021;41(7):1253–1261. doi:10.1007/s00296-021-04863-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.