Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Pretreatment Circulating Albumin, Platelet, and RDW-SD Associated with Worse Disease-Free Survival in Patients with Breast Cancer
Authors Chen CC , Tang WH, Wu CC, Lee TL, Tsai IT, Hsuan CF, Wang CP, Chung FM, Lee YJ, Yu TH, Wei CT
Received 5 October 2023
Accepted for publication 9 January 2024
Published 16 January 2024 Volume 2024:16 Pages 23—39
DOI https://doi.org/10.2147/BCTT.S443292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Pooja Advani
Chia‐Chi Chen,1– 4 Wei-Hua Tang,5,6 Cheng-Ching Wu,2,7,8 Thung-Lip Lee,7,9 I-Ting Tsai,2,10 Chin-Feng Hsuan,2,7,11 Chao-Ping Wang,7,9 Fu-Mei Chung,7 Yau-Jiunn Lee,12 Teng-Hung Yu,2,7,* Ching-Ting Wei4,13,*
1Department of Pathology, E-Da Hospital, I-Shou University, Kaohsiung, 82445, Taiwan; 2School of Medicine, College of Medicine, I-Shou University, Kaohsiung, 82445, Taiwan; 3Department of Physical Therapy, I-Shou University, Kaohsiung, 82445, Taiwan; 4The School of Chinese Medicine for Post Baccalaureate, College of Medicine, I-Shou University, Kaohsiung, 82445, Taiwan; 5Division of Cardiology, Department of Internal Medicine, Taipei Veterans General Hospital, Yuli Branch, Hualien, 98142, Taiwan; 6Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, 112304, Taiwan; 7Division of Cardiology, Department of Internal Medicine, E-Da Hospital, I-Shou University, Kaohsiung, 82445, Taiwan; 8Division of Cardiology, Department of Internal Medicine, E-Da Cancer Hospital, I-Shou, University, Kaohsiung, 82445, Taiwan; 9School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, 82445, Taiwan; 10Department of Emergency, E-Da Hospital, I-Shou University, Kaohsiung, 82445, Taiwan; 11Division of Cardiology, Department of Internal Medicine, E-Da Dachang Hospital, I-Shou University, Kaohsiung, Taiwan; 12Lee’s Endocrinologic Clinic, Pingtung, 90000, Taiwan; 13Division of General Surgery, Department of Surgery, E-Da Hospital, I-Shou University, Kaohsiung, 82445, Taiwan
*These authors contributed equally to this work
Correspondence: Teng-Hung Yu; Ching-Ting Wei, E-Da Hospital, I-Shou University, No. 1, Yi-Da Road, Jiau-Shu Village, Yan-Chao District, Kaohsiung, 82445, Taiwan, Tel +886-7-615-1100 ext. 5914 or 5018, Email [email protected]; [email protected]
Objective: Breast cancer is the second most common malignancy globally and a leading cause of cancer death in women. Analysis of factors related to disease-free survival (DFS) has improved understanding of the disease and characteristics related to recurrence. The aim of this study was to investigate the predictors of DFS in patients with breast cancer to enable the identification of patients at high risk who may benefit from prevention interventions.
Methods: We retrospectively analyzed 559 women with breast cancer who underwent treatment between 2004 and 2022. The study endpoint was DFS. Recurrence was defined as local recurrence, regional recurrence, distant metastases, contralateral breast cancer, other second primary cancer, and death. Baseline tumor-related characteristics, treatment-related characteristics, sociodemographic and biochemical data were analyzed using Cox proportional hazards analysis.
Results: The median DFS was 45 months (range, 2 to 225 months). Breast cancer recurred in 86 patients (15.4%), of whom 10 had local recurrence, 10 had regional recurrence, 17 had contralateral breast cancer, 29 had distant metastases, 10 had second primary cancer, and 10 patients died. Multivariate forward stepwise Cox regression analysis showed that AJCC stage III, Ki67 ≥ 14%, albumin, platelet, and red cell distribution width-standard deviation (RDW-SD) were predictors of worse DFS. In addition, the effects of albumin, platelet, and RDW-SD on disease recurrence were confirmed by structural equation model (SEM) analysis.
Conclusion: In addition to the traditional predictors of worse DFS such as AJCC stage III and Ki67 ≥ 14%, lower pretreatment circulating albumin, higher pretreatment circulating platelet count and RDW-SD could significantly predict worse DFS in this study, and SEM delineated possible causal pathways and inter-relationships of albumin, platelet, and RDW-SD contributing to the disease recurrence among Chinese women with breast cancer.
Keywords: breast cancer, albumin, platelet, RDW-SD, disease-free survival
Introduction
Breast cancer is a major global health problem including in Taiwan, and the leading cause of cancer-related death in women.1 In Taiwan, the mean breast cancer detection rates were 4.76 and 4.08 cancers per 1000 screening mammograms in the earlier period (2004–2009) and the latter period (2010–2020), respectively. The 10-year survival rate increased from 89.68% in the early period to 97.33% in the latter period.2 In patients with newly diagnosed breast cancer, 61% have localized disease confined to the primary site, 32% have tumor spread to regional lymph nodes, and 5% have metastatic disease.3 Approximately one third of all breast cancer patients suffer local recurrence within 10 years of diagnosis, and most cases occur within 5 years.4 Therefore, how to evaluate and determine the prognosis or recurrence rate of breast cancer after the diagnosis and treatment is important as this may influence the treatment plan and the patient’s quality of life.5
The pathology of breast cancer is heterogeneous, and the disease course is affected by factors related to the patient, socioeconomic status, molecular factors,6,7 and the tumor. Specifically, the factors associated with disease progression include younger age, obesity, ethnicity, social factors, early diagnosis, histological and biological tumor characteristics, inflammatory breast cancer, and staging.8–11 Furthermore, previous studies have shown that factors related to a shorter disease-free survival (DFS) include lymph node involvement, tumors larger than 2 cm, and triple negative hormone receptor status.12–14 Moreover, therapeutic modalities for breast cancer may also significantly affect DFS. Bundred et al found that involved or close pathological margins after breast conserving surgery for early-stage invasive breast cancer were associated with increased distant recurrence and local recurrence.15 In addition, Ma et al reported that delayed initiation of radiation therapy was associated with inferior outcomes among patients who underwent breast-conserving surgery, especially among patients in the hormone receptor-negative subgroup.16 Moreover, Collin et al reported that the early discontinuation of adjuvant endocrine therapy affected breast cancer recurrence in a population-based cohort of premenopausal women diagnosed with breast cancer.17 Therefore, the purpose of this study was to evaluate whether baseline risk factors such as sociodemographic, tumor-related, treatment-related, and biochemical factors were associated with worse DFS in patients with breast cancer receiving surgery. In addition, we further assessed the effects of risk factors on disease recurrence using a structural equation model (SEM) in this study. Such findings could increase the awareness of physicians with regards to the importance of regularly screening for baseline risk factors in women with breast cancer.
Materials and Methods
Ethics
This retrospective study was approved by the Human Research Ethics Committee of Kaohsiung E-Da Hospital (KEDH), Taiwan. The ethical approval code is No. EMRP-110-104. The approval date: 08-25-2022. All participants provided written informed consent before their data were collected.
Study Participants
We retrospectively analyzed the medical records of 559 women with pathologically proven breast cancer who were treated at KEDH between November 2004 and August 2021. All patients underwent breast cancer surgery including breast- conserving surgery, total mastectomy, partial mastectomy, and modified radical mastectomy. If the patient had not visited KEDH in the last 6 months, they were contacted by telephone and their current status was recorded. At the same time, they were invited to return for follow-up. The women were identified in the electronic database of KEDH based on these inclusion criteria: (1) >18 years of age at the diagnosis of breast cancer, (2) breast cancer diagnosed by invasive needle biopsy, (3) complete clinical and follow-up data, and (4) ability to sign an informed consent form. The exclusion criteria were: (1) a diagnosis of metastatic disease, (2) incomplete records, and (3) inability to provide consent to participate in the study.
All of the women were followed up according to the standard protocol of the hospital at 3, 6 and 12 months, during which they received chest X-ray, breast ultrasonography, mammography, abdominal and pelvic ultrasonography (and a full body scan if needed), and physical examinations.
Data Collection
The diagnoses, sociodemographic factors, tumor-related factors, treatment-related factors (surgery, chemotherapy, hormone therapy, or radiation), responsiveness to treatment (remission, recurrence, or metastasis), outcome (survival or death), and anthropometric-related factors were recorded from the electronic database of KEDH. There categories of independent variables were recorded: (1) sociodemographic factors: age (<50 years and ≥50 years), smoking (grouped as non-smokers vs current and former smokers [stopped smoking for ≥1 year]), and alcohol consumption (grouped as non-drinkers vs current and former drinkers [stopped drinking for ≥1 year]); (2) tumor-related factors: tumor site (bilateral, right, and left); tumor size (<2 cm, 2–5 cm, and >5 cm), clinical tumor stage (T0-1, T2, and T3-T4), tumor grade (grades 1–3), American Joint Committee on Cancer (AJCC) stage (I–II and III), estrogen receptor (ER) and progesterone receptor (PR) (positive/negative tests were defined as ≥1% and <1% positive staining of tumor cells, respectively), HER2 (0, 1+, 2+ with fluorescence in situ hybridization (FISH) negative was defined as negative for HER2 protein expression; 3+, 2+ with FISH positive was defined as positive for HER2 expression); Ki67 status (<14% and ≥14%), histopathology (mucinous carcinoma, invasive ductal carcinoma, invasive lobular carcinoma, or others); (3) treatment-related factors: surgery type (breast-conserving surgery, total mastectomy, or others); chemotherapy, radiotherapy, and hormone therapy; and (4) anthropometric-related factors: weight and height (to 0.1 kg and 0.1 cm, respectively); body mass index (BMI, kg/m2); and blood pressure (BP, measured after 5 minutes of rest).
Laboratory Measurements
The patients’ baseline biochemical variables at the primary diagnosis were retrieved from computer files. Following overnight fasting, we measured levels of serum carcinoembryonic antigen (CEA), CA153, glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, alkaline phosphatase, albumin, and complete blood cell count as reported previously.18,19 Hemoglobin A1c (HbA1c) was measured used a high-performance liquid chromatograph (Tosoh Automated Glycohemoglobin Analyzer, HLC-723G8). Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase were also measured according to the Japan Society of Clinical Chemistry. The Jaffe method was used to measure serum creatinine level, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the estimated glomerular filtration rate (eGFR).20 Concentrations of plasma leptin were determined using a solid-phase enzyme-linked immunosorbent assay (ELISA) (Quantikine Human Leptin Immunoassay; R&D Systems, Minneapolis, MN). The dilution and standard curves were parallel. For values of 15.6–283.3 pg/mL, the intra-assay coefficients of variation ranged from 3.2–6.9%. In addition, plasma cystatin C concentrations were determined using a solid-phase ELISA (Quantikine Human Cystatin C Immunoassay; R&D Systems). The dilution and standard curves were parallel. For values of 16.2–52.6 ng/mL, the intra-assay coefficients of variation ranged from 4.6–6.6%. All measurements were made twice in a single experiment. The sarcopenia ratio = serum creatinine (mg/dl)/serum cystatin C (mg/dl).
Study Endpoints
DFS was defined as the time from the initial breast cancer diagnosis to recurrence. In this study, recurrence was defined as local recurrence, regional recurrence, distant metastases, contralateral breast cancer, second primary cancer, and death from any cause.21,22 Patients with no signs of recurrence at the end date of the study period and those who were lost to follow-up were censored.
Follow-Up
The study endpoints were assessed during follow-up visits at 3, 6 and 12 months after discharge and then every 12 months until August 2022, during which the patients received the examinations detailed in section 2.2. The patients were also contacted by telephone to collect and confirm the sociodemographic characteristics and verify their clinical condition. If the patient could not be contacted, we referred to the medical records and also contacted the patient’s mastologist. The regional death registry was also searched to identify any missing cases.
Definitions
We defined hyperlipidemia as a LDL-cholesterol level ≥130 mg/dL, and/or a total cholesterol level ≥200 mg/dL, and/or a triglyceride level ≥150 mg/dL, and/or a HDL-cholesterol level <35/39 mg/dL in men/women, and/or receiving lipid disorder treatment in accordance with the ATP III criteria.23 We defined hypertension as the use of anti-hypertensive medications or systolic/diastolic BP ≥140/90 mmHg, and diabetes mellitus (DM) as a fasting glucose level >126 mg/dl,24 or the use of anti-diabetic medications.
Statistical Analysis
All statistical analyses were conducted with JMP version 7.0 for Windows (SAS Institute). Categorical data are presented as number (%), and continuous data are presented as mean (±SD). Cox regression univariate analysis was performed. The outcome was defined as the time from the diagnosis to recurrence. Hazard ratios (HRs) from Cox regression analysis were used to identify predictors of the risk of breast cancer recurrence in all patients. We first analyzed the factors potentially related to breast cancer recurrence in univariate analysis, and then entered these variables into multivariate Cox regression models. The multivariate models were based on multivariate forward stepwise Cox regression analysis and included variables with a p-value <0.1 in univariate analysis. The optimal cutoff point of pretreatment albumin, platelet, and red cell distribution width-standard deviation (RDW-SD) as a predictor for the disease recurrence and overall predictive discrimination of the model was assessed using area under the receiver operating characteristic (ROC) curve (AUC) analysis. The Kaplan-Meier method and Log rank test were used to compare albumin, platelet, and RDW-SD categories stratified by cutoff points. In addition, we utilized IBM SPSS AMOS version 24 (Amos Development Corporation, Meadville, PA, USA) software to construct and analyze both the path model and structural equation model (SEM). We used standard criteria including standardized root mean square residual (SRMSR) <0.06, root mean square error of approximation (RMSEA) <0.08, and comparative fit index (CFI) >0.90 as indices of the statistical fit of the models to the data.25 Furthermore, we used the maximum likelihood method to estimate the fit of a model. The results are presented as standardized path coefficients with their statistical significance.
Results
From November 2004 to August 2021, 559 women were included in this study. They were followed until August 31, 2022, and the median follow-up period was 45 months (range, 2 to 225 months). The follow-up rate was 96.8% and 18 patients were lost to follow-up due to a lack of contact and outpatient follow-up records, and refusal to participate. At the end of the study, breast cancer recurrence had occurred in 86 patients (15.4%), of whom 10 had local recurrence, 10 had regional recurrence, 17 had contralateral breast cancer, 29 had distant metastases, 10 had second primary cancer, and 10 patients died. Of the 10 deaths, 6 were related to breast cancer and 4 to other reasons.
Baseline Clinical Characteristics
The baseline clinical characteristics at the primary diagnosis in the enrolled patients by disease recurrence status are shown in Table 1. The median age of the patients was 55 (range, 24–94) years. Seventy-nine (14.1%) of the patients had DM, 30 (5.4%) had hyperlipidemia, and 158 (28.3%) had hypertension. The patients with disease recurrence had higher rates of other types of surgery, T3-T4 clinical tumor stage, tumor grade 3, AJCC stage III, Ki67 ≥14%, ER-negative, PR-negative, chemotherapy, and hormone therapy, and a lower rate of breast-conserving surgery than the patients without disease recurrence. In addition, the patients with disease recurrence had a lower BMI and diastolic BP than the patients without disease recurrence (Table 1).
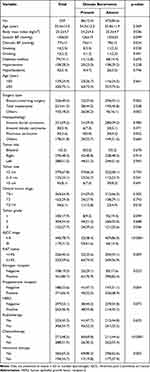 |
Table 1 Descriptive Baseline Characteristics of the Study Participants by Disease Recurrence Status |
Baseline Biochemical Characteristics at the Primary Diagnosis
The baseline biochemical data at the primary diagnosis of the patients are shown in Table 2. The patients with disease recurrence had a higher CEA, CA153, AST, white blood cell, neutrophil, monocyte, basophil, and platelet counts, red cell distribution width (RDW)-standard deviation (SD), and RDW-coefficient of variation (CV), and lower triglycerides, albumin, hemoglobin, and hematocrit than the patients without disease recurrence.
 |
Table 2 Descriptive Baseline Biochemical Data at Primary Diagnosis of the Study Participants by Disease Recurrence Status |
Associations of the Baseline Clinical and Biochemical Variables at the Primary Diagnosis with the Risk of Disease Recurrence
Univariate Cox regression analysis showed that baseline age, BMI, surgery type, T3-T4 clinical tumor stage, tumor grade 3, AJCC stage III, Ki67 ≥14%, ER-negative, PR-negative, chemotherapy, CEA, CA153, AST, eGFR, albumin, white blood cell, neutrophil, monocyte and platelet counts, hemoglobin, RDW-SD, and RDW-CV were associated with disease recurrence (Tables 3). Multivariate Cox regression analysis showed that AJCC stage III [HR 4.84 (2.88–8.22), p<0.0001], Ki67 ≥14% [HR 2.23 (1.23–4.33), p=0.007], albumin [HR 0.23 (0.13–0.45), p<0.0001], platelet count [HR 1.00 (1.00–1.01), p=0.037], and RDW-SD [HR 1.10 (1.00–1.18), p=0.041] were independently associated with disease recurrence (Table 4).
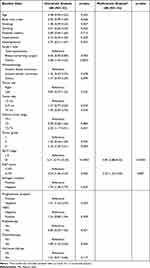 |
Table 3 Cox Proportional Hazard Model of Baseline Clinical Risk Factors for Breast Cancer Recurrence in the Whole Cohort |
 |
Table 4 Cox Proportional Hazard Model of Baseline Biochemical Risk Factors for Breast Cancer Recurrence in the Whole Cohort |
AUC Analysis
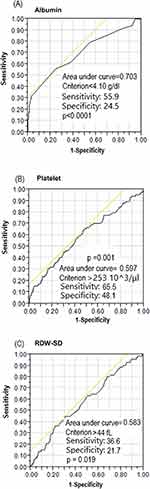
ROC curve analysis to detect the risk of disease recurrence revealed that the area under the curve (AUC) for pretreatment albumin was 0.703 (95% CI: 0.03–0.26, p<0.0001). An albumin cutoff point of 4.1 g/dl was associated with the risk of disease recurrence, with a sensitivity of 55.9% and specificity of 24.5% (Figure 1A). Furthermore, the AUC for pretreatment platelet count was 0.597 (95% CI: 1.00–1.01, p=0.001) to predict the risk of disease recurrence. A platelet cutoff point of 253 10^3/μL was associated with the risk of disease recurrence, with a sensitivity of 65.5% and specificity of 48.1% (Figure 1B). Moreover, the AUC for pretreatment RDW-SD count was 0.583 (95% CI: 1.01–1.12, p=0.019) to predict the risk of disease recurrence. A RDW-SD cutoff point of 44 fL was associated with the risk of disease recurrence, with a sensitivity of 36.6% and specificity of 21.7% (Figure 1C).
Pretreatment Circulating Albumin, Platelet, and RDW-SD Were Associated with DFS
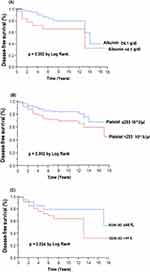
Kaplan-Meier analysis showed that albumin <4.1 g/dl (p=0.002; Figure 2A), platelet >253 10^3/μL (p=0.002; Figure 2B), RDW-SD >44 fL (p=0.034; Figure 2C) were significantly predict worse DFS in patients with breast cancer.
Effects of Pretreatment Circulating Albumin, Platelet, and RDW-SD on Disease Recurrence
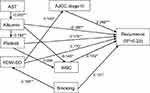
As with the Cox proportional hazard model described above (Table 4), we designed a SEM model to assess the effects of pretreatment circulating albumin, platelet, and RDW-SD on disease recurrence. The estimated model proved that the model fits well with a CFI of 1.000, an RMSEA of 0.000, and an SRMSR of 0.049 (Figure 3). AJCC stage III, platelet, RDW-SD, WBC count, and smoking had statistically significant positive direct effects on disease recurrence. Furthermore, albumin had statistically significant negative direct effect on disease recurrence. Moreover, AST (β = −0.202) indirectly affected disease recurrence through albumin. Albumin indirectly affected disease recurrence through platelet (β = −0.184) and WBC count (β = −0.145). RDW-SD (β = 0.145) indirectly affected disease recurrence through AJCC stage III. Smoking (β = 0.198) indirectly affected disease recurrence through RDW-SD. The model explained 23% of the variability in disease recurrence (Figure 3).
Discussion
The current study investigated whether baseline risk factors were associated with worse DFS in patients with breast cancer receiving surgery. There are three main findings in this study. First, multivariate forward stepwise Cox regression analysis showed that AJCC stage III, Ki67 ≥14%, albumin, platelet, and RDW-SD were predictors of worse DFS. Second, albumin<4.1 g/dl, platelet count >253 10^3/μL, and RDW-SD >44 fL were independent predictors of DFS. Third, the causal relationship of albumin, platelet, and RDW-SD on disease recurrence was confirmed by SEM analysis.
First, of the 559 women included in this study, the DFS was 15.4% after a median follow-up period of 45 months (interquartile range: 2–225 months). Similar to a previous study,26 166 patients (15.0%) developed breast cancer recurrence during the monitoring period. With regards to the biochemical risk factors after multivariate Cox regression analysis, the level of albumin, platelet count and RDW-SD were associated with breast cancer recurrence (Table 4). Albumin, a crucial biomarker for assessing nutritional status, has been found to be associated with the progression of advanced cancer. Various mechanisms contribute to elucidating the adverse prognostic impact of low albumin levels in patients with advanced cancer. A previous study demonstrated that within the tumor microenvironment, albumin is rapidly absorbed by the tumor to counteract the relative shortage of amino acids. This adaptive process allows the tumor to meet the heightened metabolic demands associated with rapid proliferation, ultimately resulting in a decrease in serum albumin levels.27 Sarett et al suggested that tissue damage and inflammation expedite the process of catabolism, thereby reducing the levels of albumin in the plasma.28 Furthermore, in previous studies on breast cancer, it has been demonstrated that albumin modulates the activation of autocrine growth regulatory factors in breast cancer cell lines, influencing cell proliferation.29 Baseline serum albumin level has also been shown to be a powerful prognostic factor for breast cancer survival.30 In the present study, we further showed that the pretreatment circulating albumin level in breast cancer patients was associated with disease recurrence.
Platelets, crucial for blood clotting, also play a role in angiogenesis (the formation of new blood vessels) and metastasis. Thrombocytosis and increased variation in red blood cells size are always found in patients with inflammation-related diseases.31,32 An elevated platelet count may indicate increased angiogenesis and the potential for cancer cells to spread to other parts of the body.33 Furthermore, platelets can interact with tumor cells, promoting their survival and dissemination, a dynamic that may contribute to the recurrence of cancer.34 RDW-SD serves as a measure of variability in the size of red blood cells. Elevated RDW may be associated with inflammation,35 poor nutritional status,36 and oxidative stress.37 Chronic inflammation in cancer can contribute to tumor progression and recurrence. Moreover, changes in the distribution of red blood cells may signify alterations in the tumor microenvironment, which plays a crucial role in supporting cancer cell survival and growth.38 While these are general considerations, the specific biological mechanisms can vary depending on the context of breast cancer recurrence. Further research should be conducted specifically for breast cancer, considering factors such as hormone receptor status, molecular subtypes, and other individual patient characteristics.
Secondly, in our report, it is noteworthy that despite the normal range values of albumin, platelet count, and RDW-SD levels in our patients (Table 2), we observed associations with disease recurrence at cutoff points of 4.1 g/dL for albumin, 253 10^3/μL for platelet count, and 44 fL for RDW-SD, as illustrated in Figures 1 and 2. Taken together, these findings indicate that although the clinical staging and appearance of the patient may be similar, the microenvironment of the body is already different in patients with a higher risk of future disease recurrence.
Third, the present study is the first of its kind to explore the causal relationship of albumin, platelet, and RDW-SD on disease recurrence in patients with breast cancer to date. However, the exact mechanism underlying the association among albumin, platelet, and RDW-SD and disease recurrence was not yet fully clarified. Our SEM analysis revealed that there were significant positive direct effects from AJCC stage III, platelet, RDW-SD, WBC count, and smoking on disease recurrence. Furthermore, albumin had statistically significant negative direct effect on disease recurrence. Besides, AST indirectly affected disease recurrence through albumin. Albumin indirectly affected disease recurrence through platelet and WBC count. RDW-SD indirectly affected disease recurrence through AJCC stage III. Smoking indirectly affected disease recurrence through RDW-SD. Previous studies demonstrated associations between albumin,29 platelet,39 RDW-SD,40 WBC count,41 and smoking42 and disease recurrence. Furthermore, Steinberg et al showed that baseline albumin and AST were significant determinants of survival.43 Similar phenomenon has found in the previous study44 that the AST levels were inversely to the serum albumin level (r = −0.418). AST response was greater in patients with a serum albumin of <3.5 g/dl (p<0.001), and suggested that in patients with hypoalbuminemia of <3.5 g/dl can be considered close monitoring the AST levels.44 Moreover, in some clinical studies found that higher preoperative platelet count and lower albumin level, and platelet-to-albumin ratio were correlated with cancer progression.45,46 Notably, serum albumin also is considered an indicator of a patient’s nutritional and inflammatory status.47 In addition, Kurtoğlu et al showed that elevated RDW found in smoker and may be a useful indicator of inflammatory activity in smokers.48 Hence, it is reasonable to suggest that albumin, platelet, and RDW-SD may be involved in common pathways contributing to disease recurrence in patients with breast cancer.
A higher stage of cancer is associated with a more advanced tumor grade, and unfavorable receptor expression status is associated with a poor prognosis and higher recurrence rate of breast cancer.49,50 Our results also indicate that a higher clinical stage, higher tumor grade, excess Ki67 expression and ER-negative and PR-negative status were related to higher disease recurrence (Table 1). In addition, with regards to the type of therapy, we found that the patients who received chemotherapy and other types of surgery had a higher disease recurrence rate. This result could not simply be explained by the stage of breast cancer, and it reflects the complexity of planning breast cancer treatment.49 In the present study, we found patients receiving hormone therapy are shown to have poor DFS (Table 1). Several factors may influence the outcomes of patients undergoing hormone therapy for breast cancer, potentially leading to inferior DFS results. These factors include: (a) resistant tumors;51 (b) de novo and acquired resistance;52 (c) genetic factors;53 (d) incomplete adherence to treatment;54 (e) tumor heterogeneity;55 (f) adverse events and side effects;56 (g) new genetic mutations or pathways.57 However, the initial observation of poor DFS in patients receiving hormone therapy disappeared in both univariate and multivariate Cox regression analyses (Table 3). Moreover, we also found that the patients who had lower BMI and diastolic BP had higher diseases recurrence. This could be because the patients who had more invasive disease status had a weaker health status.58 Considering the actual level of BMI and diastolic BP levels in these patients, we found that there was only very small difference between these two groups. This suggests that although there was no significant change in the patients’ clinical appearance, underlying progression of the disease may have already occurred and could be detected by careful observation. This phenomenon was further shown when we analyzed the biochemical data of the patients.
Inflammation has been shown to affect the development of cancer and to be involved in all stages of tumorigenesis. Therefore, the white blood cell count and differential white cell count, especially neutrophil, basophil and monocyte counts, are relatively higher in patients with recurrence.59–62 Similarly, in this study, we also found that hemoglobin level and red blood cell indices were related to disease recurrence. This is consistent with a previous study which showed that the expressions of several anemia-related proteins such ferritin, hepcidin and ferroportin were deregulated in breast cancer cells and that they had a prognostic impact in breast cancer patient; however, further studies are needed to clarify the underlying circumstances.63 In addition, albumin and triglycerides, which may represent the patient’s health status, where are relatively low in the patients with recurrence in the present study. This is also consistent with previous reports; however, the results are unusual and may be due to differences in the study subjects and design.64–66 A previous study found that pathologic stage, especially if higher than stage II, was significantly associated with disease recurrence.67 In addition, Ki67 is a cell-cycle regulated protein in breast cancer cells. A tumor with a high Ki67 expression has larger number of proliferating cells, and the overexpression of immunohistochemical staining for Ki67 in breast cancer cells has been shown to be a predictor of a poor prognosis.68–70 Therefore, it is not surprising that an advanced stage of disease and the overexpression of Ki67 were associated with disease recurrence in the present study (Table 3).
Finally, in the present study, we found that ER and PR expression were not associated with disease recurrence, even though ER-negative and PR-negative status were found to be associated with disease recurrence before multivariate Cox regression analysis (Table 3). This could be because hormone therapy is not the only treatment for breast cancer, and the latest PI3K inhibitors, Janus Kinase-signal transducer and activator of transcription (JAK-STAT) inhibitors, and HER-2 target therapy also have important roles. PI3K inhibitors, on the other hand, target the PI3K pathway, which plays a crucial role in cell growth, survival, and metabolism. Aberrations in this pathway are common in various cancers, including breast cancer. Clinical trials have been conducted to assess the efficacy of PI3K inhibitors in breast cancer treatment, especially in cases with mutations in the PIK3CA gene.71 Alpelisib is an example of a PI3K inhibitor that has gained approval for use in combination with fulvestrant for the treatment of HR+, HER2-, PIK3CA-mutant advanced breast cancer after CDK4/6i treatment.72 The JAK-STAT signaling pathway is involved in the regulation of cell growth, differentiation, and immune responses. Dysregulation of this pathway has been associated with various cancers, including breast cancer. A recent study has shown that JAK-STAT inhibitors are being investigated as potential treatments for breast cancer.73 Additionally, HER-2 targeted therapy has marked a significant advancement in the treatment of HER-2 positive breast cancer.74 Drugs such as trastuzumab, pertuzumab, and ado-trastuzumab emtansine have been employed to target and inhibit the HER-2 protein, which is overexpressed in some breast cancers. These drugs have demonstrated efficacy in improving outcomes for patients with HER-2 positive breast cancer.75 Consequently, the introduction of novel breast cancer treatments may reduce the importance of ER and PR expression in breast cancer recurrence.76,77
While our study contributes valuable insights into the disease recurrence among Chinese women with breast cancer, it is imperative to acknowledge certain limitations that may impact the generalizability of our findings. First, the retrospective nature of our study design introduces potential recall bias. Second, the reliance on a single-center approach may limit the broader applicability of our results. Third, the modest sample size warrants caution in extrapolating our findings to the entire population. In light of these limitations, there is a compelling need for broader studies that encompass diverse populations and healthcare settings. Collaborative efforts involving multiple centers and a more extensive participant pool would facilitate a more nuanced understanding of the factors influencing disease recurrence among patients with breast cancer. Such studies are essential for establishing a solid foundation for generalizability and informing clinical practice on a wider scale. In addition, despite our diligent efforts to conduct a thorough study, it is important to acknowledge a potential limitation in the duration of our follow-up, which spanned 45 months. While this timeframe allowed us to observe and analyze a significant portion of the study population, it may not fully capture late recurrence cases. Recognizing the importance of long-term studies in providing a comprehensive understanding of disease trajectories, we acknowledge that our findings may be limited in assessing the complete spectrum of recurrence patterns. Future research with extended follow-up periods is warranted to further elucidate the long-term dynamics of the disease and refine our understanding of its progression over time.
Conclusions
In addition to the traditional predictors of worse DFS such as AJCC stage III and Ki67 ≥14%, lower pretreatment circulating albumin level, higher pretreatment circulating platelet count and RDW-SD level could significantly predict worse DFS in patients with breast cancer in this study. In addition, SEM delineated inter- relationships of albumin, platelet, and RDW-SD and the potential pathways that may contribute to the development of disease recurrence among Chinese women with breast cancer.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Human Research Ethics Committee of Kaohsiung E-Da Hospital (KEDH), Taiwan and complied with the tenets of the Declaration of Helsinki. The ethical approval code is No. EMRP-110-104. The approval date: 08-25-2022. Each patient provided written informed consent before being enrolled into the study.
Consent for Publication
All authors approved the version submitted for publication.
Acknowledgments
We appreciated for all participants enrolled in the present study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
E-Da Hospital financially supported this research under Contracts EDAHP111034 and EDAHI111001.
Disclosure
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yao MM, Vpt V, Chen TH, et al. Performance measures of 8,169,869 examinations in the National Breast Cancer Screening Program in Taiwan, 2004–2020. BMC Med. 2023;21(1):497. doi:10.1186/s12916-023-03217-7
3. National Cancer Institute. SEER Stat Fact Sheets: breast cancer. Available from: http://seer.cancer.gov/statfacts/html/breast.html.
4. Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment-a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800–809. doi:10.1158/1055-9965.EPI-11-1089
5. Wang Y, Gavan SP, Steinke D, Cheung KL, Chen LC. The impact of age on health utility values for older women with early-stage breast cancer: a systematic review and meta-regression. Health Qual Life Outcomes. 2022;20(1):169. doi:10.1186/s12955-022-02067-w
6. Subramaniyan V, Fuloria S, Gupta G, et al. A review on epidermal growth factor receptor’s role in breast and non-small cell lung cancer. Chem Biol Interact. 2022;351:109735. doi:10.1016/j.cbi.2021.109735
7. Thapa R, Afzal O, Gupta G, et al. Unveiling the connection: long-chain non-coding RNAs and critical signaling pathways in breast cancer. Pathol Res Pract. 2023;249:154736. doi:10.1016/j.prp.2023.154736
8. Hirko KA, Rocque G, Reasor E, et al. The impact of race and ethnicity in breast cancer-disparities and implications for precision oncology. BMC Med. 2022;20(1):72. doi:10.1186/s12916-022-02260-0
9. Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol. 2014;5(3):412–424. doi:10.5306/wjco.v5.i3.412
10. Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015;13(1):33. doi:10.1186/s12957-014-0429-x
11. Li J, Xia Y, Wu Q, et al. Outcomes of patients with inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes: a population-based study from the SEER program. Oncotarget. 2017;8(30):49370–49379. doi:10.18632/oncotarget.17217
12. Liu J, Li Y, Zhang W, et al. The prognostic role of lymph node ratio in breast cancer patients received neoadjuvant chemotherapy: a dose-response meta-analysis. Front Surg. 2022;9:971030. doi:10.3389/fsurg.2022.971030
13. Fisher B, Slack NH, Bross ID. Cancer of the breast: size of neoplasm and prognosis. Cancer. 1969;24(5):1071–1080. doi:10.1002/1097-0142(196911)24:5<1071::AID-CNCR2820240533>3.0.CO;2-H
14. Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15(1):138. doi:10.1186/s12885-015-1121-4
15. Bundred JR, Michael S, Stuart B, et al. Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. BMJ. 2022;378:e070346. doi:10.1136/bmj-2022-070346
16. Ma X, Chen J, Ma D, et al. Delayed initiation of radiation therapy is associated with inferior outcomes for breast cancer patients with hormone receptor-negative tumors after breast-conserving surgery. Gland Surg. 2021;10(9):2631–2643. doi:10.21037/gs-20-717
17. Collin LJ, Cronin-Fenton DP, Ahern TP, et al. Early discontinuation of endocrine therapy and recurrence of breast cancer among premenopausal women. Clin Cancer Res. 2021;27(5):1421–1428. doi:10.1158/1078-0432.CCR-20-3974
18. Hung WC, Tang WH, Yu TH, et al. Low plasma growth/differentiation factor 1 levels are associated with liver fibrosis in patients with stable angina. J Clin Lab Anal. 2022;36(11):e24745. doi:10.1002/jcla.24745
19. Wei CT, Tsai IT, Wu CC, et al. Elevated plasma level of neutrophil gelatinase- associated lipocalin (NGAL) in patients with breast cancer. Int J Med Sci. 2021;18(12):2689–2696. doi:10.7150/ijms.58789
20. Kong X, Ma Y, Chen J, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2013;28(3):641–651. doi:10.1093/ndt/gfs491
21. Arriagada R, Rutqvist LE, Kramar A, Johansson H. Competing risks determining event-free survival in early breast cancer. Br J Cancer. 1992;66(5):951–957. doi:10.1038/bjc.1992.391
22. O’Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600–2608. doi:10.1200/JCO.2015.60.8620
23. Expert Panel on Detection. Evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–2497. doi:10.1001/jama.285.19.2486
24. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Supplement_1):S64–S71. doi:10.2337/dc12-s064
25. Byrne BM. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming.
26. Hsiao PC, Liu JT, Lin CL, Chou W, Lu SR. Risk of breast cancer recurrence in patients receiving manual lymphatic drainage: a hospital-based cohort study. Ther Clin Risk Manag. 2015;11:349–358. doi:10.2147/TCRM.S79118
27. Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. doi:10.1016/j.addr.2018.07.011
28. Sarett SM, Werfel TA, Lee L, et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci U S A. 2017;114(32):E6490–E6497. doi:10.1073/pnas.1621240114
29. Laursen I, Briand P, Lykkesfeldt AE. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. Anticancer Res. 1990;10(2A):343–351.
30. Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27(1):10–15. doi:10.1177/014860710302700110
31. Sonmez O, Sonmez M. Role of platelets in immune system and inflammation. Porto Biomed J. 2017;2(6):311–314. doi:10.1016/j.pbj.2017.05.005
32. Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: results from the national health and nutrition examination survey. Indian Heart J. 2012;64(4):380–387. doi:10.1016/j.ihj.2012.06.006
33. Free SR, Carraway KL. Platelets in hematogenous breast cancer metastasis: partners in crime. In: Mayrovitz HN, editor. Breast Cancer. Brisbane (AU): Exon Publications; 2022.
34. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi:10.1111/j.1538-7836.2010.04131.x
35. Demirkol S, Balta S, Cakar M, Unlu M, Arslan Z, Kucuk U. Red cell distribution width: a novel inflammatory marker in clinical practice. Cardiol J. 2013;20(2):209. doi:10.5603/CJ.2013.0037
36. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
37. Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women’s health and aging study I. Clin Nutr. 2010;29(5):600–604. doi:10.1016/j.clnu.2010.03.001
38. Yin JM, Zhu KP, Guo ZW, Yi W, He Y, Du GC. Is red cell distribution width a prognostic factor in patients with breast cancer? A meta-analysis. Front Surg. 2023;10:1000522. doi:10.3389/fsurg.2023.1000522
39. Liu S, Fang J, Jiao D, Liu Z. Elevated platelet count predicts poor prognosis in breast cancer patients with supraclavicular lymph node metastasis. Cancer Manag Res. 2020;12:6069–6075. doi:10.2147/CMAR.S257727
40. Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z. Elevated red blood cell distribution width contributes to poor prognosis in patients undergoing resection for nonmetastatic rectal cancer. Medicine. 2018;97(3):e9641. doi:10.1097/MD.0000000000009641
41. Weng M, Zhao W, Yue Y, et al. High preoperative white blood cell count determines poor prognosis and is associated with an immunosuppressive microenvironment in colorectal cancer. Front Oncol. 2022;12:943423. doi:10.3389/fonc.2022.943423
42. Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the after breast cancer pooling project. J Natl Cancer Inst. 2014;106(1):djt359. doi:10.1093/jnci/djt359
43. Steinberg J, Erlichman C, Gadalla T, Fine S, Wong A. Prognostic factors in patients with metastatic colorectal cancer receiving 5-fluorouracil and folinic acid. Eur J Cancer. 1992;28A(11):1817–1820. doi:10.1016/0959-8049(92)90011-P
44. Gitlin N. Salicylate hepatotoxicity: the potential role of hypoalbuminemia. J Clin Gastroenterol. 1980;2(3):281–285. doi:10.1097/00004836-198009000-00018
45. Giannakeas V, Kotsopoulos J, Brooks JD, et al. Platelet count and survival after cancer. Cancers. 2022;14(3):549. doi:10.3390/cancers14030549
46. Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37:787–793. doi:10.21873/anticanres.11378
47. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi:10.1111/j.0894-0959.2004.17603.x
48. Kurtoğlu E, Aktürk E, Korkmaz H, et al. Elevated red blood cell distribution width in healthy smokers. Turk Kardiyol Dern Ars. 2013;41:199–206.
49. Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi:10.6004/jnccn.2022.0030
50. Zhang W, Xu Y, Wang Y, et al. Prognostic analysis of three forms of Ki-67 in patients with breast cancer with non-pathological complete response before and after neoadjuvant systemic treatment. Cancer Med. 2023;12(8):9363–9372. doi:10.1002/cam4.5693
51. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62(1):233–247. doi:10.1146/annurev-med-070909-182917
52. Chang M. Tamoxifen resistance in breast cancer. Biomol Ther. 2012;20(3):256–267. doi:10.4062/biomolther.2012.20.3.256
53. Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–643. doi:10.1038/nrc2713
54. Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res. 2014;7(4):378–387. doi:10.1158/1940-6207.CAPR-13-0389
55. Malavasi E, Giamas G, Gagliano T. Estrogen receptor status heterogeneity in breast cancer tumor: role in response to endocrine treatment. Cancer Gene Ther. 2023;30(7):932–935. doi:10.1038/s41417-023-00618-x
56. Johnsson A, Fugl-Meyer K, Bordas P, Åhman J, Von Wachenfeldt A. Side effects and its management in adjuvant endocrine therapy for breast cancer: a matter of communication and counseling. Breast Cancer. 2023;17:11782234221145440. doi:10.1177/11782234221145440
57. Asghari A, Wall K, Gill M, et al. A novel group of genes that cause endocrine resistance in breast cancer identified by dynamic gene expression analysis. Oncotarget. 2022;13(1):600–613. doi:10.18632/oncotarget.28225
58. West HJ, Jin JO. JAMA oncology patient page. performance status in patients with cancer. JAMA Oncol. 2015;1(7):998. doi:10.1001/jamaoncol.2015.3113
59. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
60. Patysheva M, Larionova I, Stakheyeva M, et al. Effect of early-stage human breast carcinoma on monocyte programming. Front Oncol. 2022;11:800235. doi:10.3389/fonc.2021.800235
61. Marone G, Schroeder JT, Mattei F, et al. Is there a role for basophils in cancer? Front Immunol. 2020;11:2103. doi:10.3389/fimmu.2020.02103
62. Park B, Lee HS, Lee JW, Park S. Association of white blood cell count with breast cancer burden varies according to menopausal status, body mass index, and hormone receptor status: a case-control study. Sci Rep. 2019;9(1):5762. doi:10.1038/s41598-019-42234-6
63. Lamy PJ, Durigova A, Jacot W. Iron homeostasis and anemia markers in early breast cancer. Clin Chim Acta. 2014;434:34–40. doi:10.1016/j.cca.2014.04.011
64. Kumie G, Melak T, Wondifraw Baynes H. The Association of serum lipid levels with breast cancer risks among women with breast cancer at felege hiwot comprehensive specialized hospital, Northwest Ethiopia. Breast Cancer. 2020;12:279–287. doi:10.2147/BCTT.S279291
65. Abdelsalam KE, Hassan IK, Sadig IA. The role of developing breast cancer in alteration of serum lipid profile. J Res Med Sci. 2012;17(6):562–565.
66. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. doi:10.1186/1475-2891-9-69
67. Lee J, Kim SH, Kang BJ. Prognostic factors of disease recurrence in breast cancer using quantitative and qualitative Magnetic Resonance Imaging (MRI) parameters. Sci Rep. 2020;10(1):7598. doi:10.1038/s41598-020-64564-6
68. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi:10.1016/S0140-6736(20)32381-3
69. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi:10.1016/S1470-2045(09)70262-1
70. Zhu X, Chen L, Huang B, et al. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci Rep. 2020;10(1):225. doi:10.1038/s41598-019-57094-3
71. Fuso P, Muratore M, D’Angelo T, et al. PI3K inhibitors in advanced breast cancer: the past, the present, new challenges and future perspectives. Cancers. 2022;14(9):2161. doi:10.3390/cancers14092161
72. Turner S, Chia S, Kanakamedala H, et al. Effectiveness of alpelisib + fulvestrant compared with real-world standard treatment among patients with HR+, HER2–, PIK3CA -mutated breast cancer. Oncologist. 2021;26(7):1133–e1142. doi:10.1002/onco.13804
73. Rizwi FA, Abubakar M, Puppala ER, et al. Janus kinase-signal transducer and activator of transcription inhibitors for the treatment and management of cancer. J Environ Pathol Toxicol Oncol. 2023;42(4):15–29. doi:10.1615/JEnvironPatholToxicolOncol.2023045403
74. Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22(2):101–126. doi:10.1038/s41573-022-00579-0
75. Sussell JA, Press DJ, Hansen SA, Kim E, Du Toit Y, Fung A. Impact of pertuzumab and ado-trastuzumab emtansine on cumulative avoidance of recurrence among women treated for locally advanced, inflammatory, or early-stage nonmetastatic HER2-positive breast cancer in the United States. Adv Ther. 2023;40(9):3857–3874. doi:10.1007/s12325-023-02554-6
76. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, hormone receptor-Positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi:10.1056/NEJMoa1813904
77. Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi:10.1056/NEJMoa2115022
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.