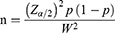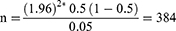Back to Journals » Integrated Pharmacy Research and Practice » Volume 11
Prescribing Pattern of Dermatological Compounding in Ethiopia: The Case of ALERT Hospital
Authors Selam MN , Ababu A , Bayisa R, Abdella M, Diriba E, Wale M , Alemu T , Marew T, Baye AM
Received 3 November 2021
Accepted for publication 21 December 2021
Published 6 January 2022 Volume 2022:11 Pages 1—8
DOI https://doi.org/10.2147/IPRP.S346395
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Muluken Nigatu Selam,1 Andualem Ababu,2 Regasa Bayisa,2 Mahdi Abdella,2 Edessa Diriba,2 Minychel Wale,3 Tadesse Alemu,4 Tesfa Marew,1 Assefa Mulu Baye5
1Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia; 2Pharmaceutical and Medical Equipment Directorate, Ministry of Health, Addis Ababa, Ethiopia; 3ALERT Hospital, Addis Ababa, Ethiopia; 4Universal Medical and Business College, Addis Ababa, Ethiopia; 5Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Muluken Nigatu Selam
Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia
Tel +251912159807
Email [email protected]
Background: Skin diseases are among the major contributors of disease burden in Ethiopia affecting individuals of all age. Extemporaneous compounding of topical medications serves as a necessary option to treat skin diseases when manufactured medications could not meet specific patient needs. Different classes of drugs are commonly used for the treatment of dermatologic diseases. Failure to periodically assess the prescribing pattern and patient needs may lead to inappropriate planning and implementation that ultimately compromise the service. Periodic prescription analysis for compounded medications helps to monitor the prescription pattern with respect to medication selection, disease condition, dosage form types and other relevant parameters. The current study was conducted to analyze the pattern of compounding prescriptions for dermatologicals in ALERT hospital.
Methods: A cross-sectional design was conducted by retrospectively evaluating compounding prescription records of January and July, 2021. A total of 460 prescriptions in the hospital community pharmacy were systematically selected. Data related to disease pattern, product selection and dosage form type were extracted and analyzed. Data analysis was done using software for the statistical package for social science version 25.0.
Results: A total of 441 prescriptions containing dermatological products for compounding were analyzed. Most patients were female (62.8%) and aged 30– 64 years (44.0%). Psoriasis (36.2%), acne vulgaris (15.3%), and rosacea (13.4%) were the top 3 skin diseases for which the compounding preparations were prescribed. Salicylic acid (38.0%) was the most frequently prescribed drug followed by betamethasone (20.2%); while white petrolatum (47.2%) was the most common diluting agent used for compounding.
Conclusion: Psoriasis was the major dermatologic disease for compounding prescriptions and salicylic acid was the most frequent product used in compounding for treatment of the prescribed skin diseases.
Keywords: compounding, dermatological preparations, prescription pattern, ALERT hospital
Introduction
Globally, skin problems were the 4th leading causes of nonfatal disease burden in 2010.1 Psoriasis and scabies are among the common skin diseases observed in developing countries.2 Dermatological disorders are among the leading causes of hospital visit in Ethiopia accounting for 25% of Outpatient Department (OPD) cases.3 Skin disorders have significant psychosocial impact on patients and also result in financial burden as most of the complications are chronic and require longer duration of treatment.4
Extemporaneous compounding plays a valuable role in providing access to medications for individuals during shortage of licensed drug supply and for patients with special needs that cannot be met with commercially available products.5–8 This time-honored practice is still ordered and remain a viable option by many practitioners.9,10 A typical study indicated that a dermatologist prescribes 2 to 5 compounded medications daily.11
Compounding can be defined as combining, admixing, diluting, reconstituting, or otherwise altering a drug or bulk drug substance to create a desired medication.12,13 Most commercially available medicines are formulated as solid dosage forms and may not be in the desired strength and dosage forms to meet specific patient needs particularly for dermatological complications.10,14 Buurma et al reported that dermatological dosage forms accounted a significant share (62.1%) among compounded medicines.15 Similar studies also revealed that dermatological preparations were among the most frequently prescribed therapeutic categories for extemporaneous compounding.16–19
In Ethiopia, extemporaneous compounding service for dermatological preparations is given only in 17 hospitals7 which may be associated with shortage of dermatologists, lack of standard compounding facilities and inadequate access to input ingredients. It is clearly indicated that the compounding service is an essential hospital pharmacy service in the country.20,21 Despite the fact that health-care facilities expansion and healthcare coverage have recorded significant improvement in the country over the last two decades, access to dermatological care and compounding services is still very limited. Lack of adequate facilities, shortage of input materials and trained personnel and weak planning and resource mobilization at all levels to respond to the dire demand for the service have been major challenges. In addition, the spectrum of the problem has not been adequately investigated; which would have been used by health sector policy formulation and planning. Moreover, there is lack of evidences showing the prescription pattern of dermatologic products that need compounding in Ethiopian hospitals. Absence of such evidences will have its own impact on the compounding services of common dermatologic preparations in terms of infrastructure, financing and professional training. Inconsistent availability of raw materials and commercial products that need dose or dosage form modification are among the problems encountered in health facilities.
Hence, periodic evaluation of the drug compounding practices and prescribing pattern is important for proactive planning and strategic intervention, to improve dermatological service delivery in health facilities. Prescribing pattern studies for dermatologic conditions helps to inform common skin diseases, types of prescribed products and dosage forms in which compounding is to be performed.19 The current study aimed at analyzing compounding prescription pattern of dermatological products at ALERT Hospital in Addis Ababa.
Methods
Study Setting
The study was conducted at ALERT hospital in Addis Ababa, Ethiopia. The hospital was established in 1934, and has been serving as the main referral center for skin-related cases in the country.
Study Design and Period
A cross-sectional design was conducted by retrospectively analyzing prescription records for dermatological compounding issued in January and July, 2021. The data collection period was selected to accommodate the seasonal variation of diseases.
Sample Size Determination and Sampling Technique
The sample size for this study was calculated using single population proportion formula considering assumptions of 95% confidence level, 0.05 margin of error and proportion of 50%.
Zα/2 = the value of the standard normal distribution at a given confidence level (at 95% CL = 1.96).
p = prescribing pattern of dermatological compounding (50.0%).
W = marginal error tolerated.
α = level of significance (5%).
Considering a 20% for incomplete prescription information, a final sample size of 460 patient prescriptions in the hospital’s community pharmacy was included in this study.
A systematic sampling technique was used to select the patients’ prescriptions. The total number of prescriptions for the data collection period (January and July) was 1934. Hence, by dividing the total prescriptions during the two months (1934) with the total sample size (460) (N/n), a sampling interval (K) of 4 was obtained. The first prescription was selected at random and consecutive prescriptions were selected every fourth prescription.
Inclusion and Exclusion Criteria
All extemporaneous compounding prescriptions in the community pharmacy of ALERT hospital were included for the study. Prescriptions without a drug item, ie, those that contain only bases, were excluded from the study.
Data Collection and Data Analysis
A data abstraction checklist was used to collect the data. A pre-test was conducted at St. Peter Specialized Hospital to ensure the consistency of the data abstraction format with study parameters prior to data collection. Two trained pharmacy professionals collected the data on prescriptions including sex, age, diagnosis, type and number of dermatologic products and diluents, and types of final dosage forms using data abstraction format. The data were entered, cleaned and analyzed using SPSS version 25 computer software. Results were reported with frequency, mean and percentage, and presented in figures and tables as found appropriate.
Ethical Approval and Consent to Participate
Prior to the data collection, ethical approval was obtained from Addis Ababa University, School of Pharmacy Ethical Review Board (ERB/SOP/215/11/2020). Above all, this study was carried out according to the Helsinki Declaration of ethical principles for research. In addition, permission from the hospital was obtained for data collection. Since the study was a retrospective review of patients’ prescription data, the ethical review board took into account the nature of the research and waived the need for individual informed consent from the participants. The patient’s personal identifiers, such as name, were not used in the research report or any other in order to maintain patient confidentiality.
Results
From 460 reviewed prescriptions, 441 (95.9%) were analyzed after excluding incomplete prescriptions where diagnosis, age or sex was not indicated. Majority, 277 (62.8%) were prescribed for female patients. The mean age of patients was found to be 30.2 (±14.5) years.
Twenty-three (5.2%) patients were affected by two skin disorders. Among the total 464 reported skin diseases, psoriasis accounted for 168 compounding prescriptions with nearly similar distribution in females (51.2%) and males (48.8%). Acne vulgaris was the second most common dermatological problem among females (67.6%) and affected mainly the age group of 18–29 years (67.6%). Melasma was found to dominantly affect female patients (85.7%) in the age group of 30–64 years (57.1%). Detailed diagnoses and patient information are presented in Table 1.
 |
Table 1 Distribution of the Top-Ten Dermatological Diseases by Age and Sex for Which Compounded Products Were Prescribed at ALERT Hospital, 2021 (N = 464) |
A total of 771 active substances were prescribed to compound dermatological preparations. Majority of the prescriptions contained two drugs (59.9%) and single drug was indicated in 35.4% of prescriptions (Table 2). Salicylic acid plus betamethasone (27%) was the most common combination used for compounding (Table 3).
 |
Table 2 Number of Drugs per Prescription of Dermatological Compounding Medicines at ALERT Hospital, 2021 |
 |
Table 3 Top-Five Combinations of Drugs for Dermatological Compounding at ALERT Hospital, 2021 |
Salicylic acid (38.0%) followed by betamethasone (20.2%) were the two most frequently prescribed extemporaneous preparations (Table 4).
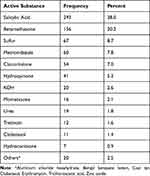 |
Table 4 Frequency of Dermatological Compounding Medicines at ALERT Hospital, 2021 |
Among the frequently utilized diluting agents for the compounding of dermatological preparations, white petrolatum, 221 (47.2%) followed by nivea cream®, 174 (37.2%) were the commonly used ones (Figure 1).
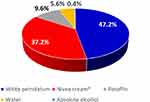 |
Figure 1 Diluents used for the compounding of dermatological preparations at ALERT hospital, 2021. |
From the total of 464 final products compounded, more than half (53%) of them were in the form of ointments followed by creams 186 (40.1%). Solutions (5.8%) and lotions (1.1%) were the other dosage forms.
Discussion
The study attempted to shed light on the prescription pattern of compounded medicines for skin disorders in ALERT hospital. It described common skin diseases and their prescribed drug preparation for compounding.
For the majority of skin problems, compounded dermatological products were meant for adults and old age patients (Table 1). This could be due to the need for combining commercial products or dosage modifications, which is reflected in the finding that majority (65%) of the prescriptions contained combined drugs. This result is in contrary with other studies in which children under 12 years of age received relatively more compounded medicines than other age groups,22–24 which could be due to the need for patient-tailored therapy for children.
Psoriasis (36.2%) being the major disease for compounding may reflect the need of patient tailored therapy for the problem (Table 1). The majority of patients with acne vulgaris (67.6%) were found in the age group range of 18 to 29 years, which is a reflection that this skin disease is common in adolescents and often continues into adulthood.25 While this disease usually begins at puberty, it can start even younger. The current study showed 18.3% of acne patients were less than 5 years of age. Though acne in this age group is very rare, it can occur because of a hormonal imbalance and certain medications.26
Majority (65%) of the compounded prescriptions contained two or more drugs. A compounding prescription pattern study conducted in Jogjakarta also reported similar findings in which the majority prescriptions (86.96%) contained more than one active substances.27 Such prescribing of combined products may be from the nature of certain skin conditions that could fail to respond to a single active substance or due to the absence of combined commercial products in the market. A survey of Australian general practitioners mentioned these as the major reasons for prescribing certain extemporaneous products.16
Among the prescribed drugs, salicylic acid (38.0%) and betamethasone (20.2%) were the two most commonly utilized ingredients in the hospital for compounding demonstrating the need for their sustainable availability to respond to patients’ customized need. Salicylic acid is available as powder form in the hospital whereas betamethasone used for the compounding purpose was obtained from market as cream.
In this study, ointments and creams took the larger share of dosage forms from the compounded preparations, which is in agreement with findings from similar studies.19,24,28,29 The presentations of dermatological preparations in the form of these dosage forms may be due to the nature of skin lesions (dry or oozy) diagnosed on patients and easier availability of diluting agents: white petrolatum and nivea cream®, as indicated from the result (Figure 1). Several semisolid formulations are available for topical applications intended to suit the type of skin lesion and its location. Ointments are suitable for dry, non-hairy skin due to their occlusive and greasy properties; and for wet or oozy skin conditions, creams are preferred. Additional reasons for the preference of these semisolid dosage forms by prescribers could be the simplicity of their formulation and the relatively longer residence time on the application site compared with liquid preparations which in turn enhances the therapeutic benefits. It is common to see semi-solid formulations for topical application in the market for the treatment of certain skin diseases.30
Equivalent to uninterrupted access to dermatological care services, ensuring the quality of compounded dermatological preparations is very essential. By the nature of most dermatological complication, preparations for skin disorders, particularly those to be applied on open and/or broken skin requires having the highest level of quality and safety. Safety issues and quality defects of compounded dermatological preparations have been causing a significant health risk.31–33 Cognizant of the risk level, regulatory authorities in many countries have been strengthening their regulatory systems. In Ethiopia, regulation of dermatological compounding practices has been mandated to the Ethiopian Food and Drug Authority (EFDA) and also strengthened under the revised proclamation (Proclamation No. 1112/2019).34 There are also directives and guidelines to regulate dermatological compounding and extemporaneous pharmaceutical manufacturing practices in the country.35,36 Compounding hospitals and pharmacies are often exempted from Good Manufacturing Practice (GMP) requirements where quality defects have been causing health risks.37,38
Even though the drug substances used in dermatological compounding pass through rigorous safety, efficacy, and quality evaluations, extemporaneously compounded preparations may not be adequately investigated for their safety and quality requirements. Compounding facilities, particularly those from developing countries (Ethiopia included) lack the requisite technology, facilities, and practices. It is recommended for a regulator to give due attention to enforcing quality standards and conduct regular inspections to prevent potential risks due to quality defects and improve dermatological health-care outcomes.39 In Ethiopia, it has been reported that the majority of public hospitals have started compounding services without passing through in-depth pre-licensing assessment;7 which may pose potential health risks to the public.
Regulation of compounding practices was officially started in Ethiopia following the establishment of the Drug Administration and Control Authority of Ethiopia (DACA);40 which is now restructured as EFDA. The official standard outlines requirements for premises, equipment, starting materials, personnel, packaging, and labeling, and documentation. However, a study in Addis Ababa reported a lack of awareness on the standards among pharmaceutical compounding facilities citing it as a major reason for poor compliance to regulatory requirements.28
EFDA is mandated to regulate products for their safety, efficacy, and quality; while compounding practices are regulated by regional regulatory bodies structured under regional health bureaus creating mandate and functional overlaps at times. The lack of periodic facility inspection could also compound the challenges in the regulation of compounded products and compounding practices. Extemporaneously prepared compounded products are categorized under low-risk products by the EFDA.41 However, because of the increasing health risks associated with poor quality extemporaneous products, EFDA and regional regulatory bodies need to undertake effective enforcement of established regulatory requirements related to premises, personnel qualification, compounding practices, sanitation and hygiene, quality control, and documentation.
The choice of ALERT hospital was based on the fact that it is the pre-eminent teaching/tertiary hospital in Ethiopia for skin disorders and extemporaneous preparations is old-rooted in the facility. The hospital remains a major referral center for the skin problems. Therefore, the impact of the practice at the study site as a determinant of practices in other hospitals of the country was a strong consideration. Hence, this study provides important baseline information that could serve as an input to hospitals and other stakeholders to take appropriate measures and improve dermatologic care services.
Limitation of the Study
Despite the findings of this study, we acknowledge that it has limitations. First, the study findings may not be extrapolated to other hospitals in the country as a non-probabilistic sampling method was employed. Additionally, products that were not available in the hospital and sent to the outside pharmacies for compounding were not included in the study, which may underestimate the existing dermatological problems and preparations in the hospital.
Conclusion
The use of extemporaneous products appeared to be an important source of medicines for psoriasis and acne vulgaris which were the major diseases for which compounding was performed. Salicylic acid accounted the most common product prescribed for compounding and white petrolatum was the major diluting agent used for compounding of preparations. Hence, inadequate access to these ingredients may affect the quality of pharmaceutical care in the hospital.
Recommendation
The hospital could use the findings from the study to select, quantify, and procure raw materials used for compounding of dermatological products. Respective government bodies should work to ensure the sustainable availability of raw materials used for compounding of dermatological products. Additionally, evaluation of the rational prescribing practice for compounding dermatological products needs to be considered in future studies; and also to expand the service to other facilities. The researchers also recommend the evaluation of prescribing pattern of compounded dermatological preparations in hospitals shall be carried out periodically as prescribing of these products for extemporaneous compounding in hospitals will continue to play an important role in patient therapy.
Data Sharing Statement
The datasets used for this publication including the data abstraction checklist can be obtained from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Ministry of Health- Ethiopia and staff of the ALERT hospital community pharmacy for their kind facilitation of this study. Our appreciation also goes to data collectors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534. doi:10.1038/jid.2013.446
2. Tegegne A. Prescribing pattern for skin diseases in dermatology OPD of in Boru Meda Hospital, North East, Ethiopia. J Basic Clin Pharma. 2018;9:31–33.
3. Ethiopia Public Health Training Initiative (EPHTI). Module: common skin diseases; 2005. Available from: https://www.cartercenter.org/resources/pdfs/health/ephti/library/modules/Degree/CommonSkinDiseasesDegree.pdf.
4. Joel JJ, Jose N, Shastry CS. Patterns of skin disease and prescribing trends in rural India. Sch Acad J Pharm. 2013;2(4):304–309.
5. Mohiuddin AK. Extemporaneous compounding: selective pharmacists with separate skill. Int J Pharm Chem Anal. 2018;5(4):165–178. doi:10.18231/2394-2797.2018.0027
6. Baum MZ. Pharmaceutical compounding: an essential piece of the health care reform puzzle; 2016. Available from: www.imprimisrx.com/assets/Imprimis-Drug-Pricing-Monograph.pdf.
7. Selam MN, Ababu A. Extemporaneous compounding practice for dermatologic preparations in Ethiopian public hospitals: regulatory requirements and quality concerns. Risk Manag Healthc Policy. 2021;14:1933–1938. doi:10.2147/RMHP.S300906
8. Hapsari I, Hadimartono M, Wiedyaningsih C, et al. Prescribing pattern of extemporaneous compounding in primary health care centers. Glob J Health Sci. 2018;10(12):104–115. doi:10.5539/gjhs.v10n12p104
9. Ling MR. Extemporaneous compounding. The end of the road? Dermatol Clin. 1998;16(2):321–327. doi:10.1016/S0733-8635(05)70014-0
10. Yusuff KB. Extent of extemporaneous compounding and pattern of prescribing and use of extemporaneous medicines in a developing setting. Pharm Health Serv Res. 2019;10(2):255–260. doi:10.1111/jphs.12297
11. Krochmal L, Patel B. Topical product design and extemporaneous compounding in dermatology. Adv Dermatol. 1992;7:231–252.
12. USP. (795) pharmaceutical compounding - non sterile preparations; 2019. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/gc795.pdf.
13. Lau ET, Jones AL, Kairuz T, et al. Compounding practices in Queensland: experiences and perceptions of pharmacists and pharmacy students. J Pharm Pract Res. 2013;43(1):19–24. doi:10.1002/j.2055-2335.2013.tb00209.x
14. Kristina SA, Wiedyaningsih C, Widyakusuma NN, et al. Extemporaneous compounding practice by pharmacists: a systematic review. Int J Pharm Pharma Sci. 2017;9(2):42–46. doi:10.22159/ijpps.2017v9i2.15681
15. Buurma H, de Smet PAGM, van den Hoff OP, et al. Frequency, nature and determinants of pharmacy compounded medicines in Dutch community pharmacies. Pharm World Sci. 2003;25(6):280–287. doi:10.1023/B:PHAR.0000006521.41736.db
16. Pappas A, MacPherson R, Stewart K. Extemporaneous prescribing: whatever happened to it? A survey of Australian general practitioners. J Pharm Pract Res. 2002;32(4):310–314. doi:10.1002/jppr2002324310
17. Lindblad AK, Isacson D, Eriksson C. Assessment of the appropriateness of extemporaneous preparations prescribed in Swedish primary care. Int J Pharm Pract. 1996;4:117–122. doi:10.1111/j.2042-7174.1996.tb00852.x
18. Karara AH, Hines R, Demir Z, et al. Evaluation of the most frequently prescribed extemporaneously compounded veterinary medications at a large independent community pharmacy. Int J Pharm Compd. 2016;20(6):461–467.
19. Giam JA, McLachlan AJ, Krass I. Specialized compounding—practices and opinions of Australian community pharmacists. J Pharm Pract Res. 2007;37(4):260–264. doi:10.1002/j.2055-2335.2007.tb00761.x
20. Ministry of Health. Ethiopian hospital services transformation guidelines; 2017. Available from: http://repository.iifphc.org›bitstream›handle.
21. Ministry of Health – Ethiopia. Health sector transformation plan II 2020/21–2024/25. 2021. Available from: https://e-library.moh.gov.et/library/index.php/knowledge-product/health-sector-transformation-plan-ii-hstp-ii-2020-21-2024-25/.
22. Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 2003;92(4):486–490. doi:10.1111/j.1651-2227.2003.tb00583.x
23. Richey RH, Shah UU, Peak M, et al. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013;13:81. doi:10.1186/1471-2431-13-81
24. Kairuz T, Myftiu J, Svirskis D, et al. Extemporaneous compounding in New Zealand hospitals. Int J Pharm Pract. 2007;15(2):129–131. doi:10.1211/ijpp.15.2.0008
25. Collier CN, Harper JC, Cantrell WC, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59. doi:10.1016/j.jaad.2007.06.045
26. Schnopp C, Mempel M. Acne vulgaris in children and adolescents. Minerva Pediatr. 2011;63:293–304.
27. Wiedyaningsih C, Widyaswari R, Hasani M, Dhani W. Compounding prescription patterns: factors influencing the physicians to prescribe compounded medicines for pediatric outpatients. Res Social Adm Pharm. 2012;8(6):e29. doi:10.1016/j.sapharm.2012.08.067
28. Seid S, Belete A, Gebre-Mariam T. Assessment of non-sterile pharmaceutical compounding practices in selected community and hospital pharmacies in Addis Ababa, Ethiopia. Ethiop Pharm J. 2016;32:2.
29. Masupye EM, Suleman F, Govender T. Investigating extemporaneous compounding practices in the Polokwane tertiary hospital pharmacies in South Africa - a pilot study. Afr J Pharm Pharmacol. 2015;9(48):1099–1105. doi:10.5897/AJPP2015.4282
30. Korting HC, Schöllmann C. Medical devices in dermatology: topical semi-solid formulations for the treatment of skin diseases. J Dtsch Dermatol Ges. 2012;10(2):103–109. doi:10.1111/j.1610-0387.2011.07764.x
31. Winckler SC. Extemporaneous compounding: a return to regulatory limbo? J Pain Palliat Care Pharmacother. 2002;16(4):71–78. doi:10.1080/j354v16n04_08
32. Shaughnessy AF. Meningitis outbreak shines light on compounding pharmacies. Br Med J. 2012;345(nov05 1):e7432. doi:10.1136/bmj.e7432
33. Quertermous J, Desai S, Harper J, et al. The practice of compounding, associated compounding regulations and the impact on dermatologists. J Drugs Dermatol. 2018;17(7 Suppl):s17–s22.
34. Ethiopian Food and Drug Authority (EFDA). The food and medicine administration proclamation (No.1112/2019); 2019. Available from: http://www.fmhaca.gov.et/wp-content/uploads/2020/06/Food-and-Medicine-Administration-Proclamation-1112.pdf.
35. Ministry of health-Ethiopia. National guideline for compounding of dermatological preparations; 2020. Available from: https://www.moh.gov.et/ejcc/sites/default/files/2020-07/Final_FMOH%20-%20National%20Guideline%20for%20Compounding%20of%20Dermatological%20Preparations.pdf.
36. Ethiopian Food and Drug Authority (EFDA). Cosmetic manufacturing directive; 2020. Available from: http://www.fmhaca.gov.et/wp-content/uploads/2020/06/Cosmetic-manufacturer-Directive-2020.pdf.
37. Gudeman J, Jozwiakowski M, Chollet J, et al. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1–8. doi:10.1007/s40268-013-0005-9
38. Myers CE. History of sterile compounding in U.S. hospitals: learning from the tragic lessons of the past. Am J Health Syst Pharm. 2013;70(16):1414–1427. doi:10.2146/ajhp130112
39. FDA. Evaluation of bulk drug substances nominated for use in compounding under section 503B of the federal food, drug, and cosmetic act. Guidance for industry; 2019. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluation-bulk-drug-substances-nominated-use-compounding-under-section-503b-federal-food-drug-and.
40. Drug Administration and Control Authority (DACA) of Ethiopia. Standards for the establishment and practice of pharmaceutical compounding laboratory; 2002. Available from: http://www.ethiopianreview.com/pdf/001/Labcomp.pdf.
41. Ethiopian Food and Drug Authority (EFDA). Guideline for registration of low-risk medicines; 2020. Available from: http://www.fmhaca.gov.et/publication/guideline-for-registration-of-low-risk-medicines-2/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.