Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Predictors of Poor Plasma Glucose Maintenance in Type II Diabetic People with Ophthalmic Complication: The Case of Dessie Hospitals in Ethiopia
Authors Abdu Seid M , Dagnew B
Received 17 November 2020
Accepted for publication 27 April 2021
Published 24 May 2021 Volume 2021:14 Pages 2317—2324
DOI https://doi.org/10.2147/DMSO.S291674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Mohammed Abdu Seid,1 Baye Dagnew2
1Unit of Human Physiology, Department of Biomedical Sciences, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Human Physiology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Mohammed Abdu Seid Email [email protected]
Background: Diabetes mellitus, the commonest metabolic disorder, leads to cardiovascular diseases, neurological problems, kidney injury, and visual disturbances. Such complications can be prevented by maintaining plasma glucose level in the normal range, including ophthalmic complications. Hence, this study intended to pinpoint predictors of poor plasma glucose maintenance in type II DM people with ophthalmic complication.
Methods: We conducted a cross-sectional survey using simple random sampling approach to recruit participants. An interviewer-based questionnaire was used and ophthalmic complication was determined by visual acuity test. We used statistical package for social sciences version 23 to analyze the data and descriptive statistics were calculated. Predictors were ascertained using multivariable logistic regression at p≤ 0.05. Besides, AOR with 95% CI was also estimated to show extent of association.
Results: The prevalence of poor plasma glucose maintenance was 65.1% (56.6– 73.6). Poor plasma maintenance was predicted by the absence of formal education (OR: 0.67; 0.20– 2.23), DM history of family members (OR=4.29; 1.33– 13.83), longer duration of diabetes (OR: 3.02; 1.09– 8.63), insulin use (OR=10.05; 2.72– 52.35), and less physical exercise (OR=2.91; 1.47– 5.76).
Conclusion: Study subjects with no education, DM history of family members, prolonged DM, insulin medication, and inadequate exercise had higher rate of poor plasma glucose maintenance. Health professionals should educate patients on the importance of self-adherence to plasma glucose monitoring, and encourage them to practice recommended physical activity.
Keywords: maintenance, plasma glucose, predictors, ophthalmic complication, type II diabetes
Introduction
Diabetes mellitus (DM) is a serious chronic and pandemic illness characterized by hyperglycemia.1 Type II DM is one category of diabetes which is characterized by insulin resistance, compromised insulin secretion, and amplified blood glucose level.2 Diabetes pandemic is accelerated by the pooled effects of increasing rates of obesity, physical inactivity, and population ageing.3 In 2019, the number of people living with DM was estimated to be 463 million people, and expected to be 578 million in 2030 and 700 million by 2045. It affects more urban dwellers (10.8%) than rural dwellers (7.2%).4
Type II DM present at diagnosis with long-term complications1 and such common complications are cardiovascular diseases, neurological problems, kidney injury, and visual impairment.5,6 Ophthalmic complications are described as eye constraint due to lowered acuity, disturbed field, falsehood of sight, and others7 usually caused by errors of refraction, raised intraocular pressure, opacity of the lens, macular changes, and diabetes associated retinopathy.7–9 Nowadays, diabetic retinopathy is the major reason for blindness in adults living with diabetes.10 Global data showed that the number of visually impaired people increased sharply from 441.1 million11 to 2.2 billion.7 As a consequence, ophthalmic complications, particularly vision impairment, can lead to unemployment, loss of productivity, and hence economic crisis for the family and the country,3,7,12 and hindrance in terms of community engagement in different activities.13
Even though achieving good glycemic control is not easy due to patients’ non-compliance and adherence, it is still the major management protocol for type II DM patients to avert DM-associated impediments like diabetic retinopathy and visual impairment.2,14
In spite of the significance of better plasma glucose maintenance, scholars showed the existence of inadequate plasma glucose maintenance in Ethiopia.15,16
A large amount of literature disclosed that inadequate plasma glucose maintenance is associated with female sex,15,17,18 low educational level,18–20 age <65,17,18 longer duration of diabetes,17,18,20–24 insulin treatment,17,20–23 obesity,15,17,24 inadequate physical activity,24 and diet.19
The association between inadequate plasma glucose maintenance and living standards, medical parameters, and therapeutic options at local levels has not been well studied. Hence, this study envisioned to ascertain predicting factors of inadequate plasma glucose maintenance in type II DM people with ophthalmic complications.
Materials, Methods, and Setting
Study Location, Duration, and Design
This study was conducted at Dessie city, northeast Ethiopia from February-March 2020. Cross-sectional survey design was in place.
Participants
Type II DM patients who had medication follow-up at the selected hospitals in Dessie City with ophthalmic complications were eligible for the survey. Gravid women, those with severe illness, acquired immune deficiency virus, active trachoma, ocular injury, and neurological diseases like stroke were excluded.
Determination of Sample Size
Single population formula was used to compute the number of participants using proportion of 0.683 from another study in Ethiopia, 95% confidence bound, and 0.05 safety margins. After adding contingency of 5%, the number of subjects were 350.
Sampling Technique
Simple random sampling technique was applied to recruit samples and they were allocated proportionally for each selected institution.
Measurement of the Outcome Variable
The outcome variable was plasma glucose maintenance which was dichotomized as poor and good. A patient was considered as maintaining good plasma glucose if his/her fasting plasma glucose was <152mg%, otherwise it was poor.14 To diagnose ophthalmic complication (visual impairment), we implemented visual acuity test, a patient was defined as having ophthalmic complication if they had less than 20/40 to the absence of light perception in both or either eyes.25 The exposure variables included sex, age in years, residence, marital status, occupation, and the level of education, diabetes history of family members, DM duration since diagnosis, treatment, obesity, hypertension, physical activity, and adherence to diet.
Operational Definition
Visual acuity: measures the ability of the eye to distinguish shapes and the details of objects at a given distance that is intended to detect any changes in ocular vision.26
Obesity: body mass index of ≥30 kg/m2. It has three types namely type 1 obesity (BMI: 30–34.9 kg/m2), type 2 obesity (BMI: 35–39.9 kg/m2), and type 3 obesity (BMI: ≥40 kg/m2).2
Adherence to diet: when the study participants pursued a recommended diet for more than three days in the last seven days.
Physical activity: good if a person do physical aerobic exercise ≥150 minutes (3–5 days) per week otherwise it is poor.27
Questionnaire and the Survey Method
The trained data collectors (4 in number) collected the data using a questionnaire which comprised open ended and closed ended questions. The aforementioned variables, under outcome measurement, were included in the tool. All diabetic patients came to the next visit with FBS result and data collectors of this study used the new FBS result. History of hypertension for each participant was assessed and current blood pressure was measured with appropriate blood pressure device. We used a tape measure for height and a scale for weight measurement after appropriate calibration. The visual test was carried out in an isolated well-illuminated room using Snellen’s chart located at 6 meter distance. The investigator of this study gave orientation to the supervisor and data collectors regarding the measurement processes, ethical concerns, and the aim of the survey. The role of the supervisor was to control the data collection process and evaluate the data collection tools.
Validity
We employed content validity by distributing the tool to 17 individuals. Pre-test result was employed before entered to actual data collection of this study aimed to modify the tools. Few modifications were made after the pre-test.
Statistical Analysis and the Model
We used Epi-Data 3.1 for entering the data and SPSS-23 for descriptive statistics and regression. Due to the absence of normal distribution, we described continuous variables using interquartile range and median whereas number and percent were used for discrete variables. A p<0.25 was used as cut-off to select exposure variables for multivariable regression. In the last model, a variable with p≤0.05 was taken as statistical predictor of poor plasma glucose maintenance. Lemeshow-Hosmer test was executed to check model fitness at p>0.05 and multicollinearity was tested using variance inflation factor.
Results
Socio-Demographic Characteristics of Participants
A total of 339 people took part in the survey with 96.85% response rate. Among those type II DM participants, 129 (38.1%) had developed ophthalmic complications. The national median value for age was 58 years (50–65). Females represented 53.5% of the subjects and 87 (67.4%) were Muslims. Forty-four (34.1%) subjects reported no formal education. Married women constituted 124 (96.1%) and 87 (67.4%) resided in urban areas (Table 1).
 |
Table 1 Descriptive Profiles of Type II DM People With Ophthalmic Complication, Dessie City Hospitals, Ethiopia, 2020 (n=129) |
In the current study, 129 (38.1%) had developed ophthalmic complications. Among those diabetics with ophthalmic complications, 65.1% [95% CI: 56.6–73.6%] of them had poor plasma glucose maintenance (Figure 1).
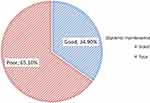 |
Figure 1 Prevalence of poor glycemic maintenance among type II DM people with ophthalmic complication at Dessie city Hospitals, Northeast Ethiopia, 2020 (n=129). |
Predictors of Poor Plasma Glucose Maintenance in Type II DM with Ophthalmic Complication
Among 129 visually impaired diabetic participants, eighty-two (63.6%) of them had no awareness of the fact that failing to take medication regularly rises plasma glucose level. Majority of study participants, 113 (87.6) and 96 (74.4%), had diabetic education and had no family history of diabetes respectively. Ninety-five (73.6%) patients had < 10 years DM duration and 1 (0.8%) was a new case. Fourteen (10.9%) and 50 (38.8%) patients had obesity and hypertension, respectively. The fasting plasma glucose level was 170mg% (IQR: 144–208mg/dl) (Table 2).
 |
Table 2 Clinical Characteristics of Type II DM Patients Who Had Ophthalmic Complication at Dessie City Hospitals (n=129) |
The simple logistic regression analysis showed that educational level, occupation, DM history of family member, diabetic education, DM duration, treatment option for DM, obesity, co-morbid hypertension, physical activity and diet were candidates for the final model. Subsequently, absence of formal education, DM history of family members, prolonged DM period, insulin injections for DM, and physical inactivity were statistically significant for poor plasma glucose maintenance.
The odds of poor plasma glucose maintenance were 4 times higher (AOR=4.29; 1.33–13.83) in type II DM people with DM history of family members compared to their counterparts. Poor plasma glucose maintenance was reduced by 33% (AOR=0.67; 0.20–2.23) among those with diploma relative to no formal education. Those with prolonged DM duration (≥ 10 years) had 3 times (AOR: 3.02; 1.09–8.63) better maintenance of plasma glucose compared to those with duration of less than 10 years. Inadequate physical exercise increases poor plasma glucose maintenance 2.91 times (AOR=2.91; 1.47, 5.76) compared to their counterparts. Poor plasma glucose maintenance was 10 times (AOR=10.05; 2.72–52.35) higher in DM patients with insulin medication compared to the references (Table 3).
 |
Table 3 Regression Model Illustrating Predictors of Poor Plasma Glucose Maintenance in Type II DM People Who Have Ophthalmic Complication at Dessie City Hospitals, Northeast Ethiopia, 2020 (n=129) |
Discussion
Plasma glucose maintenance plays a key role in preventing target organ damage as well as delaying diabetes-related impediments like retinal dysfunction, kidney injury, neuronal, and circulatory (cardiac and vascular) problems. Furthermore, good plasma glucose maintenance is also important to reduce morbidity and mortality of the disease.
In this study the overall glycemic control among diabetic patients so far was below the internationally recommended standards. DM patients with good plasma glucose maintenance are recommended to have hemoglobin A1C measurement two times annually and those with poor plasma glucose maintenance are required to have hemoglobin A1C check-up thrice per month. Unlike the way forward above, the findings of this study revealed that only fasting plasma glucose was used to observe plasma glucose maintenance that shows only a point in time of plasma glucose. In all visited hospitals, no patient received hemoglobin A1C test.
The prevalence of poor plasma glucose maintenance was 65.1% which is supported by studies in Gondar hospital (60.5%),28 Nekemte Referral Hospital,20 Debre Tabor referral hospital (71.4%),19 Ethiopian tertiary hospitals (68.3%),15 and Morocco (66.3%).21 However, this result was higher than in Ambo hospital (50%),29 and China (49.3%).30 On the other hand, this prevalence was lower compared to findings of Tikur Anbessa hospital (80%),22 Saudi Arabia (74.9%),24 Bangladesh (82%),18 and India (78.2%).17
A thirty-three33 percent reduction in poor plasma glucose maintenance was observed among those with a diploma compared to those with no education, which is in line with studies in Debre Tabor referral hospital,19 and Bangladesh.18 Possible justification for this might be that medication and practice of lifestyle and recommendations require knowledge or intellectual stimulation, which educated people have.
Diabetes history of family members was associated with poor plasma glucose maintenance and is supported by studies in India and Saudi Arabia.17,24 The reason might be the effect of heredity on disease brutality and prolongation.31
People living with type II DM with ≥ 10 year period since diagnosis had thrice higher probability to have poor plasma glucose maintenance compared to the references. Studies conducted in Ethiopian hospitals, ie, Debre Tabor, Tikur Anbessa and Nekemte and outside Ethiopia in Saudi Arabia and Morocco.19–22,24 Possible justification might be the fact that long duration of diabetes decreases insulin hormone production by the pancreas or results in target cell resistance.32
The association of insulin medication use with poor plasma glucose maintenance is consistent with studies done in India, Tikur Anbessa hospital (Ethiopia), and Morocco.17,20–22 Supposed causes might be due to the fact that continuous insulin injection leads to tissue resistance, and reduced patient adherence for insulin that in both cases result in increased plasma glucose level.33
Inadequate physical exercise was associated with poor plasma glucose maintenance which is similar to study done in Saudi Arabia.24 Possible reason for this may be the fact that inadequate exercise reduces tissue glucose uptake.34–36
Conclusion
In this study, poor plasma glucose maintenance was profound. The predictors of poor plasma glucose maintenance were no education, DM history of family members, prolonged DM period, insulin medication, and inadequate physical exercise. The health care providers should provide education about the need for self-monitoring of blood glucose, regular follow-ups, and encourage the patients to practice recommended physical activity. As a limitation, because of the snapshot nature of the design, we cannot infer cause and effect association. Some parts of the questionnaire relied on respondents’ memory, which may have resulted in recall bias. Hemoglobin A1C was not used as diabetic control standard due to clients’ financial issues.
Abbreviations
DM, diabetes mellitus; FPG, fasting plasma glucose; Type II DM, type 2 diabetes mellitus.
Data Sharing Statement
In addition to the data described in the manuscript, the dataset is available from the primary investigator upon request, email address: [email protected]
Corresponding author name: Mr. Mohammed Abdu Seid (MSc, BSc in Public Health).
Ethics and Consent Statement
We obtained ethical approval from the ethical review committee of the School of Medicine, University of Gondar (Ref: 1839/02/2020) and complied with the Declaration of Helsinki. The aim and objectives of the study were explained to the study respondents. Before commencing the interview and measurements, written consent was obtained from each study participant and would-be identifiers were disregarded to maintain confidentiality.
Acknowledgments
We are very grateful and forward our acknowledgment to the data collectors, supervisor, study subjects, hospitals, and University of Gondar, Ethiopia.
Author Contributions
Both authors contributed equally to data analysis, report write-up, drafting the manuscript, reviewing the manuscript, gave final approval for submission, and agree to be accountable for all aspects of the work.
Funding
The authors did not receive any specific financial support from any organization.
Disclosure
The authors declared that they have no competing interests.
References
1. Al PRW. IDF Diabetes Atlas. 9th edition. 12–18. Available from: https://www.idf.org/e-library/welcome/copyright-permission.html.
2. Fauci AS, Kasper DL, Longo DL, et al. Harrison’s: Principles of Internal Medicine.
3. Van Dieren S, Beulens JWJ, Van Der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(SUPPL. 1):3–8. doi:10.1097/01.hjr.0000368191.86614.5a
4. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates: results from the International Diabetes Federation Diabetes Atlas, 9 th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
5. Sayin N, Kara N, Pekel G, Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. doi:10.4239/wjd.v6.i1.92
6. Brownlee M. Biology of diabetic complications. Nature. 2001;414(13):813–820.
7. World Health Organization. World report on vision; 2019:5–29. Available from: http://apps.who.int/iris.
8. Cui Y, Zhang L, Zhang M, Yang X, Zhang L, Kuang J. Prevalence and causes of low vision and blindness in a Chinese population with type 2 diabetes: the Dongguan Eye Study. Sci Rep. 2017;7(8):1–9. doi:10.1038/s41598-017-11365-z
9. World Health Organization. Global initiative for the elimination of avoidable blindness. Vis 2020, the right to sight; 2007:2006–2011.
10. Vithian K. Microvascular complications: pathophysiology and management. Clin Med. 2010;10(5):505–509.
11. Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment. Lancet Glob Heal. 2015;(17):1–10.
12. Eckert KA, Carter MJ, Lansingh VC, et al. A simple method for estimating the economic cost of productivity loss due to blindness and moderate to severe visual impairment. Ophthalmic Epidemiol. 2015;22(5):349–355.
13. Kvam MH. Experiences of childhood sexual abuse among visually impaired adults in Norway: prevalenceand characteristics. J Vis Impair Blind. 2005;99(01):9–11.
14. Care M. Standards of medical care in diabetes — 2020. Clin Appl Res Educ. 2020;43(January):s66–74.
15. Demoz GT, Gebremariam A, Yifter H, et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow ‑ up care at a tertiary healthcare setting in Ethiopia. BMC Res Notes. 2019;12(207):1–7. doi:10.1186/s13104-019-4248-6
16. Alemayehu E, Id G, Kassie A, Id N. Glycemic control among diabetic patients in Ethiopia: a systematic review and meta- analysis. PLoS One. 2019;14(8):1–14.
17. Haghighatpanah M, Sasan A, Nejad M, Haghighatpanah M, Thunga G. Osong public health and research perspectives factors that correlate with poor glycemic control in Type 2 diabetes mellitus patients with complications. Osong Public Heal Res Perspect. 2018;9(4):167–174. doi:10.24171/j.phrp.2018.9.4.05
18. Afsana A, Liaquat A, Karim N, et al. Glycaemic Control for people with Type 2 diabetes mellitus in Bangladesh - An urgent need for optimization of management plan. Sci Rep. 2019;9(June):1–10. doi:10.1038/s41598-018-37186-2
19. Digssie A, Abebaw S, Achaw A, Getachew H, Achamyelew B. Level of glycemic control and its associated factors among type II diabetic patients in debre tabor general hospital, northwest Ethiopia. Metab Open. 2020;8:1–6. doi:10.1016/j.metop.2020.100056
20. Gebre M, Kebamo S, Mosisa B, Tadesse A. Predictors of poor glycemic control and level of glycemic control among diabetic patients in west Ethiopia. Ann Med Surg. 2020;55(January):238–243. doi:10.1016/j.amsu.2020.04.034
21. Chetoui A, Kaoutar K, Elmoussaoui S, Boutahar K, El A. Prevalence and determinants of poor glycaemic control: a cross-sectional study among Moroccan type 2 diabetes patients. Int Health. 2020;12(1):1–8. doi:10.1093/inthealth/ihz011
22. Ababa A, Tekalegn Y, Addissie A, Kebede T, Ayele W. Magnitude of glycemic control and its associated factors among patients with type 2 diabetes at Tikur Anbessa Specialized. PLoS One. 2018;13(3):5–16. doi:10.1371/journal.pone.0193442
23. Juarez DT, Sentell T, Tokumaru S, Goo R, Davis JW, Marjorie M. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006 – 2009. Prev Chronic Dis. 2013;9:1–10.
24. Alzaheb RA, Altemani AH. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab Syndr Obes. 2018;11:15–21.
25. Cui Y, Zhang L, Zhang M, Yang X, Zhang L, Kuang J. Prevalence and causes of low vision and blindness in a Chinese population with type 2 diabetes. Sci Rep. 2017;7(1):
26. Han X, Scheetz J, Keel S, et al. Development and validation of a smartphone-based visual acuity test. TVST. 2019;8(4):1–10. doi:10.1167/tvst.8.4.27
27. Dugan JA. Exercise recommendations for patients with type 2 diabetes. J Am Acad Physician Assist. 2016;29(1):13–18. doi:10.1097/01.JAA.0000475460.77476.f6
28. Fasil AB, Belete AM. Glycemic control and diabetes complications among diabetes mellitus patients attending at University of Gondar Hospital, Northwest Ethiopia. Diabetes Metab Syndr Obes. 2019;12:75–83. doi:10.2147/DMSO.S185614
29. Mellitus D, Woldu MA, Wami CD, et al. factors associated with poor glycemic control among patients with Type 2. EndocrinolMetab Syndr. 3(4):2–7.
30. Zhu H, Yu M, Hu H, He Q, Pan J, Hu R. Factors associated with glycemic control in community-dwelling elderly individuals with type 2 diabetes mellitus in Zhejiang, China: a cross-sectional study. BMC Endocr Disord. 2019;19(57):1–11. doi:10.1186/s12902-019-0384-1
31. Kao W, Gary-webb TL. Association between parental history of type 2 diabetes and glycemic control in urban African Americans. Diabetes Care. 2008;31(9):1773–1776. doi:10.2337/dc08-0618
32. Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi:10.1007/s00125-014-3369-7
33. Umpierrez GE, Hellman R, Korytkowski MT, et al. management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi:10.1210/jc.2011-2098
34. Asano RY, Sales MM, Alberto R, et al. Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes. 2014;5(5):659–665.
35. Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS One. 2013;8(11):1–8. doi:10.1371/journal.pone.0080436
36. Thomas D, Ej E, Ga N. Exercise for type 2 diabetes mellitus (Review). Cochrane Database Syst Rev. 2009;(1):10–11.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
