Back to Journals » Journal of Inflammation Research » Volume 14
Predictive Value of the Platelet-to-Albumin Ratio (PAR) on the Risk of Death at Admission in Patients Suffering from Severe Fever with Thrombocytopenia Syndrome
Received 24 August 2021
Accepted for publication 11 October 2021
Published 29 October 2021 Volume 2021:14 Pages 5647—5652
DOI https://doi.org/10.2147/JIR.S335727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Yonghui Gui,1 Yuanhong Xu,2 Peng Yang1
1Department of Blood Transfusion, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China
Correspondence: Peng Yang
Department of Blood Transfusion, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China
Email [email protected]
Yuanhong Xu
Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, Anhui, People’s Republic of China
Email [email protected]
Objective: The purpose of this study was to evaluate the predictive value of the platelet-to-albumin ratio (PAR) on the risk of death in patients with severe fever with thrombocytopenia syndrome.
Methods: Between Jan 2019 and June 2021, 127 cases which were admitted to the First Affiliated Hospital of Anhui Medical University have been included in this study. The laboratory data were selected at the time of admission. To identify the potential independent risk factors for severe fever associated with thrombocytopenia syndrome, multivariate logistic regression analysis was performed. Receiver operating characteristic (ROC) curve analysis was used to evaluate the prediction accuracy of PAR in identifying patients exhibiting severe fever with thrombocytopenia syndrome.
Results: Multiple logistic regression analysis showed that PAR could potentially serve as an independent risk factor for the death in patients with SFTS (OR = 4.023, 95% CI 1.204– 13.436, P=0.024). The prediction of the risk of death in patients with SFTS was assessed using the AUC. The AUC for the PAR was 0.729 (95% CI, 0.637– 0.82, P < 0.001), whereas the optimal cut-off value of PAR was found to be 1.43, with 54.9% sensitivity and 86.1% specificity.
Conclusion: Our study demonstrated for the first time that PAR could act as an independent predictor for mortality in adult patients with SFTS.
Keywords: severe fever with thrombocytopenia syndrome, risk factors, platelet-to-albumin ratio
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is a lethal tick-borne infectious disease caused by a novel Banyang virus (SFTS) virus.1,2 SFTSV was first isolated from China, and the affected patients developed fever, thrombocytopenia, leukopenia, and multiple organ dysfunction.1 The cases of SFTSV have been reported in central and eastern China, South Korea, Japan and Vietnam.3 Patients suffering from SFTS showed the symptoms of fever, general fatigue, and gastrointestinal symptoms such as bloody diarrhea.4 SFTS has been established as a serious disease with mortality rates varying from 6.4 to 20.9%.5 The SFTSV genome consists of three different single-stranded negative RNA fragments, which are large (L), medium (M) and small (S) fragments. Moreover, S-fragment PCR amplification or SFTSV specific antibodies are often used to diagnose or confirm SFTSV infection.6
A number of previous studies have reported that the pathogenesis of SFTS is related to abnormal immune response.7,8 Moreover, additional recent findings have confirmed that platelet-albumin ratio (PAR) can be used as a potential marker of inflammation in patients, and can serve as a prognostic factor or progression indicator for a variety of diseases,9–12 For instance, preoperative PAR has been found to be a useful and potential prognostic biomarker in patients with non-small cell lung cancer undergoing primary resection.9 Preoperative PAR has also been described as a novel and effective independent prognostic indicator of survival after pancreatectomy.10 Thus, PAR as an emerging inflammation index, has attracted significant attention. Moreover, another report on the prognostic value of PAR has shown that preoperative PAR is closely associated with prognosis in patients with cholangiocarcinoma and liver cancer.12 However, there is currently no report that has evaluated the possible role of PAR in SFTS. For this reason, we aimed to analyze the potential role of PAR in predicting the mortality in adult patients with SFTS in this study.
Methods
Study Population
For this prospective study, we recruited SFTS patients from Jan 2019 to June 2021 from the First Affiliated Hospital of Anhui Medical University. The enrolled patients were found to be positive for SFTS viral RNA by real-time fluorescent polymerase chain reaction (RT-PCR) and/or SFTSV IgM was positive in the patient’s blood. However, patients with the following conditions were excluded from this study: (1) Those lacking the clinical and laboratory data presented in this study and (2) patients with other diseases, such as hematological diseases, cancers etc. The study protocol complied with the Declaration of Helsinki and was approved by the hospital’s ethics review board. All the procedures included in this study were undertaken as part of the routine clinical practice, and the data which could lead to identification of enrolled patients was removed. We confirmed that all the data was anonymized and maintained with confidentiality. Hence, the requirement for informed consent has been waived because of the retrospective nature of the current study.
Data Collection
We collected patient demographic data such as the gender and age, and the onset laboratory indicators in the blood of the patient during hospitalization such as white blood cell count (WBC), neutrophil count (NEU), platelet count (PLT), lymphocyte count (LYM), monocytes (MONO), hemoglobin (Hb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), activated partial thromboplastin time (APTT), lactic uric acid (UA), blood urea nitrogen (BNU), and PAR. The deaths were defined as patients who died during the course of the disease.
Statistical Analysis
The various quantitative were presented as the mean ± standard deviation (SD) and analyzed using independent Student’s t-tests, medians (interquartile range) and analyzed using Mann–Whitney U-test, depending on their distribution. The categorical variables were expressed as percentages (n, %) and were analyzed using Chi-square or Fisher’s exact tests, The correlation between the two continuous variables were examined using Pearson or Spearman correlation test. The multivariate logistic regression analysis was performed to evaluate if PAR could be established as an independent risk factor for the presence and severity of severe fever with thrombocytopenia syndrome. The prediction accuracy was evaluated using the area under the receiver operating characteristic (ROC) curves, The cut-off point showing the greatest accuracy was determined using Youden’s index (sensitivity + specificity – 1). All the data analysis was performed using SPSS 23.0 software (SPSS Inc., Chicago, Illinois, USA). A two-sided P value of <0.05 was considered as statistically significant.
Results
Basic Characteristics of the Patients
A total of 168 laboratory-confirmed SFTS patients were enrolled in our study between April 2019 and December 2021. After excluding cases based on the above-described exclusion criteria, 127 patients were finally included in our study. After grouping by year and quarter, it can be observed that the main infection time was primarily distributed is in the second and third quarters (Figure 1). Among the included cases, 91 survived whereas 36 died. There were 61 male patients and 66 female patients. The mean age of all patients was 62.3 years. Notably, the mean age and the occurrence of neurological symptoms in patients who died were significantly higher than in those who survived (67.14 vs 60.36; P=0.001). The demographic characteristics of the patients have been presented in Table 1. From our data, was found that the ALT, AST, UA, BUN, and APTT levels in the group that died were significantly higher than those in the group that survived (P<0.001). When the PAR is significantly lower than the survival group (p<0.001). We also found that the levels of LYM, MONO and PLT in the group that died were lower than those in the group that survived (p<0.05). There were no significant differences observed between the two groups in the number and content of WBC, NEU, HB and ALB.
 |
Table 1 The Basic Characteristics of the Patients |
 |
Figure 1 The number of confirmed cases was grouped by year and quarter. |
Risk Factors for Fatal Outcomes
Based on our assignment of the corresponding indicators (Table 2), we performed univariate and multivariable binary logistic regression analysis. However, univariable logistic regression analyses revealed that older age, reduction in PAR, PLT, LYM, and elevated levels of ALT, AST, BUN, UA and APTT were considered as important risk factors for fatal outcomes. After adjusting age, sex, AST, ALT, BUN and UA levels, PAR was found to be an independent risk factor for death in patients with SFTS (OR = 4.023, 95% CI 1.204–13.436, P=0.024). Meanwhile, our data also showed that age and BUN were independent risk factors for the fatal outcomes. The detailed results of the multivariable analysis have been shown in Table 3.
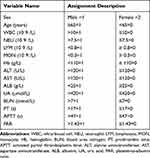 |
Table 2 The Variables and Assignments of SFTS Risk Factors Analysis |
 |
Table 3 Univariable and Multivariable Logistic Regression Analyses of the Various Features Associated with the Fatal Outcomes in Patients with SFTS |
Diagnostic Performance of the PAR on the Risk of Death in Patients with SFTS
The prediction of the risk of death in patients with SFTS was thereafter assessed using the AUC. As shown in Figure 2, the AUC for the PAR was 0.729 (95% CI, 0.637–0.82, P < 0.001). The optimal cut-off value of PAR was 1.43, with 54.9% sensitivity and 86.1% specificity respectively.
 |
Figure 2 The distribution of SFTS in high or low PAR groups. |
Discussion
Severe fever with thrombocytopenia syndrome (SFTS) is a novel viral disease with a high fatality rate, which is caused by tick-borne sandfly virus SFTSV.13 The onset of SFTS is sudden and can progress rapidly, causing several complications. The main clinical manifestations of SFTS are fever, leukopenia, thrombocytopenia, and hepatic and renal dysfunctions,14 So there is an unmet need to develop a novel model that can identify patients who are critically ill, as well as to diagnose patients who are at higher risk of death and treat them faster and earlier.
A number of previous studies have demonstrated that there exists a close correlation between ALB and inflammation.15,16 For instance, the platelet-albumin ratio (PAR) has recently been proposed as a novel indicator that can reliably reflect the systemic inflammation and immune nutritional status in several diseases.10 This is primarily because the possibility of being affected by the various physiological and/or disease conditions is less likely for the PAR than its PLTs and ALB components individually. Moreover, PAR has the ability to accurately reflect the status of immune system, systemic inflammation, and nutritional status of the patient simultaneously after considering the individual functions of PLTs and ALB.12 Additionally, past investigations on the prognostic worth of PAR showed that the preoperative PAR was significantly associated with the patients’ prognoses in pancreatic adenocarcinomas and non-small cell lung carcinomas.9,10
A few previous studies have reported all cases that occurred between April and October,14,17 This is consistent with our conclusions, that after grouping by year and quarter, it can be observed that the main infection time distribution was primarily found in the second and third quarters (Figure 1). The mean age of all patients was 62.3 years. Notably, the mean age in patients who died was significantly higher as compared to those who survived (67.14 vs 60.36; P=0.001), This is also consistent with the previous reports,18 and based on our data, we found that the ALT, AST, UA, BUN, and APTT in the group that died were significantly higher than those in the group that survived (P<0.001). When the PAR is significantly lower than the survival group (p<0.001). We also found that the numbers of LYM, MONO and PLT in the group that died were significantly lower than those in the group that survived (p<0.05). However, there was no significant difference between the two groups in the WBC, NEU, HB and ALB. Our univariate and multivariable binary logistic regression analysis revealed that older age, reduction in PAR, PLT, LYM, and elevated levels of ALT, AST, BUN, UA and APTT could be considered as potential risk factors for the fatal outcomes. After adjusting age, sex, AST, ALT, BUN and UA, PAR was proved to be an independent risk factor for the presence of SFTS (OR = 4.023, 95% CI 1.204–13.436, P=0.024). Meanwhile, our data also showed that age and BUN could serve as an independent risk factor for fatal outcomes, which was consistent with most of the previous studies.17,19
We further investigated the possible predictive effect of PAR as an independent predictor of mortality in adult patients with SFTS. The survival group displayed a significantly a higher PAR value than the death group. Thereafter, then the prediction of the risk of death in patients with SFTS was assessed using the AUC, the AUC for the PAR was 0.729 (95% CI, 0.637–0.82, P < 0.001). The optimal cut-off value of PAR was 1.43, with 54.9% sensitivity and 86.1% specificity. The ROC curve analysis indicated that the PAR had a well discriminatory power in predicting mortality in adult patients with SFTS. Moreover, PLT and ALB results were readily available at admission, allowing us to quickly identify patients at high risk of death and thus to intervene early to improve overall patient outcomes.
However, several limitations were also associated with our study design. First, it was a retrospective single-center study and we did not further track the future clinical outcomes in the present study. Second, due to the small number of deaths found in our cohort, there might be a possible statistical bias. Therefore, due to major these limitations of this research, further studies are needed in the future.
Conclusion
Our study clearly demonstrated that PAR could serve as an independent predictor for mortality in adult patients with SFTS.
Ethical Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved the Hospital Ethics Review Board of the First Affiliated Hospital of Anhui Medical University. We also confirm that all the data was anonymized and maintained with confidentiality; therefore, the requirement for an informed consent has been waived because of the retrospective nature of the current study.
Funding
The Natural Science Foundation of Anhui Province (grant numbers1808085MH273).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Takayama-Ito M, Saijo M. Antiviral drugs against severe fever with thrombocytopenia syndrome virus infection. Front Microbiol. 2020;11:150. doi:10.3389/fmicb.2020.00150
2. Chung JK, Kim CM, Kim DM, et al. Severe fever with thrombocytopenia syndrome associated with manual de-ticking of domestic dogs. Vector Borne Zoonotic Dis. 2020;20(4):285–294. doi:10.1089/vbz.2019.2463
3. Li J, Li S, Yang L, Cao P, Lu J. Severe fever with thrombocytopenia syndrome virus: a highly lethal bunyavirus. Crit Rev Microbiol. 2021;47(1):112–125. doi:10.1080/1040841X.2020.1847037
4. Saijo M. Pathophysiology of severe fever with thrombocytopenia syndrome and development of specific antiviral therapy. J Infect Chemother. 2018;24(10):773–781. doi:10.1016/j.jiac.2018.07.009
5. Tian B, Qu D, Sasaki A, Chen J, Deng B. Acute pancreatitis in patients with severe fever with thrombocytopenia syndrome virus infection. Pancreatology. 2020;20(8):1631–1636. doi:10.1016/j.pan.2020.09.024
6. Li XK, Dai K, Yang ZD, et al. Correlation between thrombocytopenia and host response in severe fever with thrombocytopenia syndrome. PLoS Negl Trop Dis. 2020;14(10):e0008801. doi:10.1371/journal.pntd.0008801
7. Li XK, Lu Q, Chen WW, et al. Arginine deficiency is involved in thrombocytopenia and immunosuppression in severe fever with thrombocytopenia syndrome. Sci Transl Med. 2018;10:eaat4162. doi:10.1126/scitranslmed.aat4162
8. Park SJ, Kim YI, Park A, et al. Ferret animal model of severe fever with thrombocytopenia syndrome phlebovirus for human lethal infection and pathogenesis. Nat Microbiol. 2019;4:438–446. doi:10.1038/s41564-018-0317-1
9. Guo M, Sun T, Zhao Z, Ming L. Preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann Thorac Cardiovasc Surg. 2021;27(2):84–90. doi:10.5761/atcs.oa.20-00090
10. Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–793. doi:10.21873/anticanres.11378
11. Huang C, Xia YQ, Xiao L, Huang J, Zhu ZM. Combining the platelet-to-albumin ratio with serum and pathologic variables to establish a risk assessment model for lymph node metastasis of gastric cancer. J Biol Regul Homeost Agents. 2021;35(2):811–817. doi:10.23812/20-626-L
12. Haksoyler V, Topkan E. High pretreatment platelet-to-albumin ratio predicts poor survival results in locally advanced nasopharyngeal cancers treated with chemoradiotherapy. Ther Clin Risk Manag. 2021;17:691–700. doi:10.2147/TCRM.S320145
13. Qi R, Qin XR, Wang L, et al. Severe fever with thrombocytopenia syndrome can masquerade as hemorrhagic fever with renal syndrome. PLoS Negl Trop Dis. 2019;13(3):e0007308. doi:10.1371/journal.pntd.0007308
14. Wang JY, Wu H, Tong ZD, Yan JB, Li KF, Tang A. The epidemiological research progress of fever with thrombocytopenia syndrome. Chin J Epidemiol. 2016;37(2):294–298. doi:10.3760/cma.j.issn.0254-6450.2016.02.029
15. Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi:10.1016/j.jhep.2014.04.012
16. Li T, Li X, Wei Y, et al. Predictive value of C-reactive protein-to-albumin ratio for neonatal sepsis. J Inflamm Res. 2021;14:3207–3215. doi:10.2147/JIR.S321074
17. Wang F, Wu Y, Jiao J, Wang J, Ge Z. Risk factors and clinical characteristics of severe fever with thrombocytopenia syndrome. Int J Gen Med. 2020;13:1661–1667. doi:10.2147/IJGM.S292735
18. Wang X, Lin L, Zhao Z, et al. The predictive effect of the platelet-to-lymphocyte ratio (PLR) and the neutrophil-to-lymphocyte ratio (NLR) on the risk of death in patients with severe fever with thrombocytopenia syndrome (SFTS): a multi-center study in China. Ann Transl Med. 2021;9(3):208. doi:10.21037/atm-20-4736
19. He Z, Wang B, Li Y, et al. Severe fever with thrombocytopenia syndrome: a systematic review and meta-analysis of epidemiology, clinical signs, routine laboratory diagnosis, risk factors, and outcomes. BMC Infect Dis. 2020;20(1):575. doi:10.1186/s12879-020-05303-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
