Back to Journals » Journal of Inflammation Research » Volume 17
Predictive and Prognostic Potentials of Lymphocyte-C-Reactive Protein Ratio Upon Hospitalization in Adult Patients with Acute Pancreatitis
Authors Xu XY, Gao Y , Yue CS, Tang YJ, Zhang ZJ, Xie FJ, Zhang H, Zhu YC, Zhang Y, Lai QQ, Wang XT, Xu JX, Zhang JN, Liu BW, Zhang JN, Kang K
Received 19 November 2023
Accepted for publication 7 March 2024
Published 13 March 2024 Volume 2024:17 Pages 1659—1669
DOI https://doi.org/10.2147/JIR.S450587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Xiao-Yu Xu,1,* Yang Gao,2,* Chuang-Shi Yue,3,* Yu-Jia Tang,4,* Zhao-Jin Zhang,5 Feng-Jie Xie,6 Hong Zhang,6 Yu-Cheng Zhu,7 Yan Zhang,7 Qi-Qi Lai,4 Xin-Tong Wang,4 Jia-Xi Xu,4 Jia-Ning Zhang,4 Bo-Wen Liu,4 Jian-Nan Zhang,4 Kai Kang4
1Department of Critical Care Medicine, The Second People’s Hospital of Beihai, Beihai, People’s Republic of China; 2Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 3Department of Critical Care Medicine, Henan Provincial People’s Hospital, Zhengzhou, People’s Republic of China; 4Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 5Department of Critical Care Medicine, The Yichun Central Hospital, Yichun, People’s Republic of China; 6Department of Critical Care Medicine, The Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, People’s Republic of China; 7Department of Critical Care Medicine, The Hongxinglong Hospital of Beidahuang Group, Shuangyashan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian-Nan Zhang; Kai Kang, Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Nangang District, Harbin, Heilongjiang, 150001, People’s Republic of China, Tel +86-13836119816 ; +86-13904618016, Email [email protected]; [email protected]
Purpose: In this study, our objective was to investigate the potential utility of lymphocyte-C-reactive protein ratio (LCR) as a predictor of disease progression and a screening tool for intensive care unit (ICU) admission in adult patients with acute pancreatitis (AP).
Methods: We included a total of 217 adult patients with AP who were admitted to the First Affiliated Hospital of Harbin Medical University between July 2019 and June 2022. These patients were categorized into three groups: mild AP (MAP), moderately severe AP (MSAP), and severe AP (SAP), based on the presence and duration of organ dysfunction. Various demographic and clinical data were collected and compared among different disease severity groups.
Results: Height, diabetes, lymphocyte count (LYMPH), lymphocyte percentage (LYM%), platelet count (PLT), D-Dimer, albumin (ALB), blood urea nitrogen (BUN), serum creatinine (SCr), glucose (GLU), calcium ion (Ca2+), C-reactive protein (CRP), procalcitonin (PCT), hospitalization duration, ICU admission, need for BP, LCR, sequential organ failure assessment (SOFA) score, bedside index for severity in AP (BISAP) score, and modified Marshall score showed significant differences across different disease severity groups upon hospitalization. Notably, there were significant differences in LCR between the MAP group and the MSAP and SAP combined group, and the MAP and MSAP combined group and the SAP group, and adult AP patients with ICU admission and those without ICU admission upon hospitalization.
Conclusion: In summary, LCR upon hospitalization can be utilized as a simple and reliable predictor of disease progression and a screening tool for ICU admission in adult patients with AP.
Keywords: lymphocyte-C-reactive protein ratio, acute pancreatitis, lymphocyte count, C-reaction protein, disease progression, ICU admission, screening tool
Introduction
Acute pancreatitis (AP) is characterized by an excessive and destructive inflammatory reaction, triggered by dysregulated activation of pancreatic zymogens and subsequent self-digestion of the pancreas, and usually associated with tissue injury, organ dysfunction, and local or systemic complications.1,2 Disease severity, risk stratification, and clinical prognosis of AP depend on the presence and progression of organ dysfunction and local or systemic complications.3–5 While most AP cases fall within the manageable range of mild to moderately severe, severe AP (SAP) is characterized by single or multiple organs involvement or even dysfunction, need for intensive care unit (ICU)-level care and organ support, and high mortality, thus posing a significant risk to human health.6,7 Current tailor-made management of AP and its complications has moved towards a multidisciplinary collaboration of the step-up approach and minimally invasive strategy based on blood purification (BP).1,8–11 Early identification of AP patients at high risk of developing SAP, single or multiple organs dysfunction, and the need for ICU-level care and organ support, as well as subsequent promptly proactive management are crucial for improved clinical prognosis and rational utilization of limited critical care resources.12 Therefore, there is an urgent need to develop clinical predictors for AP with the aims of early risk stratification, tailored interventions, and prognostic assessment.
Lymphocyte-C-reactive protein ratio (LCR) is a simple and easy-to-obtain clinical parameter compared to the complex and time-consuming existing scoring systems, which are not conducive to clinical application in AP patients. Furthermore, no single existing scoring system performs well across all forms of AP induced by different etiologies.13 Lymphocyte count (LYMPH) and C-reactive protein (CRP) reflect the status of protective immune activation and the extent of systemic inflammatory cascade reaction of the body, respectively.14 LCR, calculated as the ratio of LYMPH to CRP, has demonstrated superior predictive capabilities for disease severity and progression, early risk stratification, therapeutic responsiveness, short-term and long-term complications, and clinical prognosis compared to other established immune-inflammation biomarkers and existing scoring systems in patients with malignant tumors.15–17 As a result, LCR, as a non-specific predictor, has expanded its clinical application from initial malignant tumors to various other areas encompassing the aforementioned pathogenesis, particularly infectious diseases.14,18,19 The scope of its clinical application continues to widen with ongoing researches, increased understanding, and growing recognition of LCR.
However, there is a lack of related researches to explore the predictive and prognostic potentials of LCR in adult patients with AP to date. To address this gap, our study aimed to investigate whether LCR could serve as a simple and reliable predictor of disease progression and a screening tool for ICU admission in adult patients with AP. Our findings will have significant implications in terms of clinical value and practical significance.
Materials and Methods
Study Design
This single-center retrospective study included a total of 217 adult patients with AP who were admitted to the First Affiliated Hospital of Harbin Medical University from July 2019 to June 2022. Various demographic and clinical data were collected and compiled from the medical records of the enrolled patients during their hospitalization. Complete Blood Count (CBC) and C-reactive protein (CRP) assays were performed by detecting blood samples. Semiconductor laser flow cytometry and sheath flow direct current impedance method were applied for CBC, with reagents from Sysmex Corporation and equipment from XN1800 and XN9000. Scattering turbidity method was utilized for CRP assay with Pumen Super Sensitive CRP Assay Kit and PA-990/PA-990Pro Specific Protein Analyzer for further analysis. When calculating LCR, the blood volume used to measure CBC is 17 ul, while the concentration unit of CRP is mg/L. Body mass index (BMI), LCR, sequential organ failure assessment (SOFA) score, bedside index for severity in AP (BISAP) score, and modified Marshall score upon hospitalization were calculated based on the aforementioned data combined with other relevant information extracted from the medical records. All of these indicators were compared among different disease severity groups. The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University on March 14, 2023 (IRB number: 2023IIT025) and is valid for one year from the date of issuance. Written informed consent from participants was not required for this study, in compliance with national legislation and institutional requirements.
Study Population
The inclusion criteria for this study consisted of adult patients (aged 18 years or older) who were admitted to the First Affiliated Hospital of Harbin Medical University and diagnosed with AP between July 2019 and June 2022. The exclusion criteria included recurrent AP, chronic organ failure, leukemia, immunotherapy or organ transplant within 6 months, acquired immunodeficiency syndrome (AIDS), autoimmune disorders, uncontrolled malignant tumors with multiple metastases, pregnant or breastfeeding women, and incomplete medical records (Figure 1).
 |
Figure 1 Flowchart of patient enrollment. Abbreviations: AP, acute pancreatitis; AIDS, acquired immunodeficiency syndrome. |
Diagnosis and Classification of AP
According to the revised Atlanta classification (2012), the diagnosis of AP was established when at least two of the three diagnostic criteria, namely clinical presentation (typical epigastric abdominal pain), laboratory parameters (serum amylase/lipase levels at least three times higher than the upper limit of normal), and abdominal cross-sectional radiographic evidences (including abdominal ultrasound [US], computed tomography [CT], or magnetic resonance imaging [MRI]), were met.20,21 Subsequently, adult patients with AP were divided into three groups: mild AP (MAP), moderately severe AP (MSAP), and severe AP (SAP) based on the absence of organ dysfunction and the duration of organ dysfunction less than 48 hours and more than 48 hours.3 For adult AP patients with changes in disease severity, the most severe classification of AP during hospitalization was taken as the final one.
Calculation of LCR
LCR was calculated through multiplying lymphocyte count (LYMPH) by 10,000 and then dividing by CRP within 24 hours of admission.
Initiation and Implementation of BP
In this study, BP was initiated in adult patients with AP according to the Expert Consensus on Emergency Diagnosis and Treatment of Acute Pancreatitis.22 Vascular access was established by ultrasound-guided placement of a temporary double-lumen central venous catheter (11.5 F). Therapeutic mode, anti-coagulation method, ratio of pre- to post-dilution, blood flow rate, dehydration volume, and amount of substitute fluid were individually adjusted according to the different etiologies, conditions, and treatment needs of each adult AP patient receiving BP.
Data Collection
Various demographic and clinical data were collected and compiled from the medical records during hospitalization by dedicated personnel within our research team. The confidentiality of the enrolled patients was maintained.
Statistical Analyses
Statistical analyses were conducted using SPSS 24.0 (SPSS, Inc., Chicago, IL, USA). Continuous data with a normal distribution were presented as mean ± standard deviation (SD), while those without a normal distribution were expressed as median (P25, P75). Frequency was used to describe count data. Inter-group comparisons of continuous data with a normal distribution were performed using one-way analysis of variance (ANOVA). In case of a significant difference, least significant difference (LSD) method was employed for further pairwise comparisons with p < 0.05 as the statistically significant difference between groups. Kruskal–Wallis rank-sum test was used for inter-group comparisons of continuous data without a normal distribution. In case of a significant difference, pairwise multiple comparisons were conducted. Chi-square (χ2) test was adopted for three-group comparisons of categorical data, and Fisher exact probability method was applied when the conditions for χ2 test were not met. If a significant difference was found, pairwise inter-group comparisons were performed, and the significance level was adjusted using Bonferroni correction with p < 0.016 (0.05/3) as the statistically significant difference between groups. Spearman correlation analysis was used to assess the correlations between LCR and SOFA, BISAP, and modified Marshall scores. The receiver operating characteristic (ROC) curve of LCR was analyzed, and the area under the ROC curve, the cut-off value of LCR, and the corresponding sensitivity and specificity were calculated.
Results
Comparison of Demographic and Clinical Data Among MAP, MSAP, and SAP Groups Upon Hospitalization
A total of 217 adult patients with AP who were admitted to the First Affiliated Hospital of Harbin Medical University from July 2019 to June 2022 were included in this study. These patients were categorized into three groups: MAP group (N = 127), MSAP group (N = 48), and SAP group (N = 42). Upon hospitalization, several demographic and clinical data showed significant differences among different disease severity groups (Table 1).
 |
Table 1 Comparison of Demographic and Clinical Data Among MAP, MSAP, and SAP Groups Upon Hospitalization |
The Correlations Between LCR and SOFA, BISAP, and Modified Marshall Scores Upon Hospitalization
LCR exhibited significant negative correlations with SOFA, BISAP, and modified Marshall scores upon hospitalization, with correlation coefficients of −0.276, −0.414, and −0.385, respectively (Table 2).
 |
Table 2 The Correlations Between LCR and SOFA, BISAP, and Modified Marshall Scores Upon Hospitalization |
Comparison of LCR Between the MAP Group and the MSAP and SAP Combined Group Upon Hospitalization
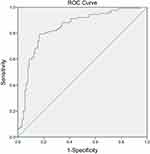
A significant difference was observed in the comparison of LCR between the MAP group and the MSAP and SAP combined group upon hospitalization (p = 0.000) (Table 3). The area under the ROC curve of LCR to classify adult patients with AP into the MAP group was 0.842 (Figure 2). The cut-off value of LCR, along with the sensitivity and specificity of the ROC curve, was determined as 88.63, 79.5%, and 83.3%, respectively.
 |
Table 3 Comparison of LCR Between the MAP Group and the MSAP and SAP Combined Group Upon Hospitalization |
Comparison of LCR Between the MAP and MSAP Combined Group and the SAP Group Upon Hospitalization
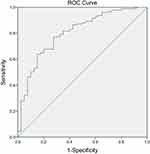
The comparison of LCR between the MAP and MSAP combined group and the SAP group upon hospitalization revealed a significant difference (p = 0.000) (Table 4). The area under the ROC curve of LCR to classify adult patients with AP into the SAP group was 0.845 (Figure 3). The cut-off value of LCR, along with the sensitivity and specificity of the ROC curve, were determined as 56.50, 77.1%, and 78.6%, respectively.
 |
Table 4 Comparison of LCR Between the MAP and MSAP Combined Group and the SAP Group Upon Hospitalization |
Comparison of LCR Between Adult AP Patients with ICU Admission and Those Without ICU Admission Upon Hospitalization
A significant difference was observed in the comparison of LCR between adult AP patients with ICU admission and those without ICU admission upon hospitalization (p = 0.000) (Table 5). The area under the ROC curve of LCR to classify adult AP patients with ICU admission was 0.802 (Figure 4). The cut-off value of LCR, along with the corresponding sensitivity and specificity of the ROC curve, was determined as 54.25, 76.8%, and 72.5%, respectively.
 |
Table 5 Comparison of LCR Between Adult AP Patients with ICU Admission and Those Without ICU Admission Upon Hospitalization |
Discussion
AP is a prevalent complex gastrointestinal disorder with an increasing incidence, presenting a huge challenge in prediction.23 A comprehensive approach involving the integration of available medical information from diverse sources, diagnostic invasive procedures, and even exploratory laparotomy should be carefully considered for diagnosis. Swift and accurate diagnosis forms the foundation for tailored interventions that can optimize patient outcomes. Following a definitive diagnosis, adult patients with AP exhibit significant variations in common etiologies, clinical manifestations, disease severity and course, and clinical prognosis, ranging from transient self-limited disease lasting a few days to temporary or permanent structural and functional damage to the pancreas, local or systemic inflammatory response, life-threatening single or multiple organs involvement or dysfunction, and even death.24–26 Early risk stratification is a prerequisite for optimal allocation of the limited critical care resources to the most needy adult patients with AP, thereby preventing overwhelming the healthcare system and ensuring timely implementation of tailor-made interventions. Among them, organ dysfunction and local or systemic complications are the most important determinant of early risk stratification, but they are also relatively lagging.3 An in-depth understanding and targeting of its precise pathogenesis will facilitate further research and pave the way for continuous advancements in clinical assessment and management, thereby holding great potential for improved clinical prognosis in adult patients with AP.
Currently, there is a lack of simple, effective, and practical biomarkers or simplified scoring systems in clinical practice, particularly routine peripheral blood parameters, for identifying high-risk cases that most require and benefit from close follow-up surveillance and intensive care, as well as predicting disease progression in adult patients with AP. Recent accumulating evidences have highlighted the significance of immune-inflammatory crosstalk in the pathogenesis of AP and its involvement in disease occurrence and development, thus presenting an entry point for clinical intervention. Among the numerous biomarkers, LYMPH and CRP are one of the most representative and commonly used markers that precisely reflect protective immunological responses and systemic inflammatory cascade reactions of the body in clinical settings, respectively.27–29 It is conceivable and achievable to employ a simple calculation using optimal biomarkers to stratify risk and predict disease progression and clinical prognosis in adult patients with AP. A decreased LCR may indicate compromised host immune status and/or enhanced systemic inflammatory responses, both of which are strongly associated with poor prognosis.15,30,31 Changes in the balance between LYMPH and CRP can effectively reflect the dynamics of immune-inflammation interactions. Importantly, a previous study from our research group demonstrated the predictive ability of LCR in adult patients with coronavirus disease 2019 (COVID-19) and its potential as an assistant screening tool for hospital and ICU admission.14 Based on these considerations along with the novel findings from our previous study, LCR, as a combination of LYMPH and CRP, is highly regarded as a feasible and promising predictor to facilitate early risk stratification for tailored interventions, taking into account both the immune and inflammatory perspective, in adult patients with AP. Similarly, other non-specific biomarkers from CBC such as LYMPH, neutrophil–lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) were also associated with disease severity and adverse outcomes in adult patients with AP.32–34 In addition, a 5-cytokine panel, including angiopoietin 2, hepatocyte growth factor, interleukin 8, resistin, and soluble tumor necrosis factor receptor 1A, can accurately predict persistent organ failure (POF) at the early stages of the disease process in patients with AP.35 Finally, novel digital tool and mechanistic model are also being attempted to forecast disease severity in patients with AP.36,37
To the best of our knowledge, our study represents the first attempt to investigate the predictive and prognostic potentials of LCR in adult patients with AP. Our results demonstrated that LCR upon hospitalization, along with existing scoring systems such as SOFA, BISAP, and modified Marshall scores, exhibited comparable effectiveness in predicting disease progression of AP. However, these existing scoring systems are intricate and time-consuming, whereas LCR stands out as one of the most readily available and convenient predictor for clinical application. Moreover, based on the cut-off values of different disease severity groups derived from this study, LCR upon hospitalization can be divided into three categories: greater than 88.63, between 56.60 and 88.63, and less than 56.60, which can stratify adult patients with AP into different disease severity groups, namely MAP, MSAP, and SAP, and accordingly guide tailored interventions of varying intensities. AP is a dynamic disease process, which means that disease severity in adult patients with AP may need to be reassessed as the disease progresses. It is self-evident that the earlier adult patients with AP at high risk of disease deterioration are identified, the more time there is for tailor-made interventions, and thus the more likely it is to improve clinical prognosis. Lastly, it is worth noting that the cut-off values of LCR to classify adult patients with AP into the SAP group and those with ICU admission are very close, indicating that SAP is a significant factor but not the sole determinant for ICU admission.
The present study has several potential limitations. Firstly, the retrospective nature of a single-center small-sample clinical study restricts the reliability and generalizability of our conclusion. Secondly, the sample size of this study is insufficient to support the training cohort and validation cohort, therefore, the novel findings from the training cohort need to be validated by future well-designed, multi-center, large-sample prospective randomized controlled clinical trials. Thirdly, it should be noted that LCR, as a simple and reliable predictor, is only applicable to adult patients with AP and cannot be directly expanded to other similar patient populations. Fourthly, the sensitivity and specificity of the ROC curve from our results still have room for improvement, suggesting the possibility of combining LCR with other biomarkers or simplified scoring systems in future studies. Fifthly, the predictive and prognostic potentials of dynamic monitoring of LCR were not explored in this study. Lastly, in order to elucidate the specific detailed mechanisms between AP and LCR, more high-quality basic studies will be necessary in the near future.
Conclusion
In conclusion, LCR holds great potential to serve as a simple and reliable predictor of disease progression and a screening tool for ICU admission in adult patients with AP. Its advantages of easy detection, cost-effectiveness, and high accuracy make it a promising candidate for broader application in clinical practice. Our study serves as the first pilot investigation to explore the predictive and prognostic potentials of LCR and provide a new strategy for risk stratification in adult patients with AP. Consequently, in the forthcoming years, further validation through well-designed, multi-center, large-sample prospective randomized controlled clinical trials is essential to ascertain its true benefits.
Data Sharing Statement
The authors will provide the raw data supporting the conclusion of this article, without any reservation.
Ethical Statement
This study received ethical approval (IRB number: 2023IIT025) from the Ethics Committee of the First Affiliated Hospital of Harbin Medical University on March 14, 2023, valid for one year. Written informed consent was not required due to the retrospective nature of the study and the absence of interventions or treatments. We strictly adhered to the Declaration of Helsinki’s ethical principles, safeguarding patient rights and privacy.
Acknowledgments
The authors extend their gratitude to all those who provided invaluable assistance, advice, and support for this article.
Funding
This work was supported by the National Natural Science Foundation of China (82372172), the Key research and development plan project of Heilongjiang Province (GA23C007), Heilongjiang Province Postdoctoral Start-up Fund (LBH-Q20037), Special Fund for Clinical Research of Wu Jie-ping Medical Foundation (320.6750.2022-02-16), the Research Project of Heilongjiang Provincial Health Commission (20231717010461), the Scientific Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (2021M08).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shi N, Sun GD, Ji YY, et al. Effects of acute kidney injury on acute pancreatitis patients’ survival rate in intensive care unit: a retrospective study. World J Gastroenterol. 2021;27(38):6453–6464. doi:10.3748/wjg.v27.i38.6453
2. Sundar V, Dutta A, Ramasamy S, et al. Sting pathway - A futuristic therapeutic target for acute pancreatitis? Gene. 2021;778:145469. doi:10.1016/j.gene.2021.145469
3. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
4. Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–734. doi:10.1016/S0140-6736(20)31310-6
5. Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–2023. doi:10.1053/j.gastro.2018.12.041
6. Waller A, Long B, Koyfman A, et al. Acute pancreatitis: updates for emergency clinicians. J Emerg Med. 2018;55(6):769–779. doi:10.1016/j.jemermed.2018.08.009
7. Cienfuegos JA, Valentí V, Rotellar F. Acute pancreatitis: an opportunity for gastroenterology hospitalists? Rev Esp Enferm Dig. 2022;114(2):73–75. doi:10.17235/reed.2022.8573/2022
8. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi:10.1136/bmj.l6227
9. Vishnupriya K, Chanmugam A. Acute pancreatitis: the increasing role of medical management of a traditionally surgically managed disease. Am J Med. 2022;135(2):167–172. doi:10.1016/j.amjmed.2021.08.021
10. Valverde-López F, Martínez-Cara JG, Redondo-Cerezo E. Acute pancreatitis. Med Clin. 2022;158(11):556–563. doi:10.1016/j.medcli.2021.12.012
11. Gliem N, Ammer-Herrmenau C, Ellenrieder V, et al. Management of severe acute pancreatitis: an update. Digestion. 2021;102(4):503–507. doi:10.1159/000506830
12. Zhou H, Mei X, He X, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: a retrospective study. Medicine. 2019;98(16):e15275. doi:10.1097/MD.0000000000015275
13. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390. doi:10.1001/jama.2020.20317
14. Zhang JN, Gao Y, Wang XT, et al. Lymphocyte-C-reactive protein ratio can differentiate disease severity of COVID-19 patients and serve as an assistant screening tool for hospital and ICU admission. Front Immunol. 2022;13:957407. doi:10.3389/fimmu.2022.957407
15. Lu LH, Zhong C, Wei W, et al. Lymphocyte-C-reactive protein ratio as a novel prognostic index in intrahepatic cholangiocarcinoma: a multicentre cohort study. Liver Int. 2021;41(2):378–387. doi:10.1111/liv.14567
16. Yildirim M, Koca B. Lymphocyte C-reactive protein ratio: a new biomarker to predict early complications after gastrointestinal oncologic surgery. Cancer Biomark. 2021;31(4):409–417. doi:10.3233/CBM-210251
17. Yugawa K, Maeda T, Kinjo N, et al. Prognostic impact of lymphocyte-c-reactive protein ratio in patients who underwent surgical resection for hepatocellular carcinoma. J Gastrointest Surg. 2022;26(1):104–112. doi:10.1007/s11605-021-05085-z
18. Erdogan A, Can FE, Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J Med Virol. 2021;93(9):5555–5559. doi:10.1002/jmv.27097
19. Wang J, Xie L, Lyu P, et al. Short-term prognostic efficacy of mGPS and LCS in patients with acute heart failure. Front Cardiovasc Med. 2022;9:944424. doi:10.3389/fcvm.2022.944424
20. Zhou W, Dong S, Chen Z, et al. New challenges for microRNAs in acute pancreatitis: progress and treatment. J Transl Med. 2022;20(1):192. doi:10.1186/s12967-022-03338-2
21. Beyer G, Hoffmeister A, Lorenz P, et al. Clinical practice guideline—acute and chronic pancreatitis. Dtsch Arztebl Int. 2022;119(29–30):495–501. doi:10.3238/arztebl.m2022.0223
22. Wang G, Xiao H, Ren E. Expert consensus on emergency diagnosis and treatment of acute pancreatitis. Clin Hepatol. 2021;37(5):1034–1041.
23. Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–496. doi:10.1038/s41575-019-0158-2
24. Ruan Q, Lu H, Zhu H, et al. A network-regulative pattern in the pathogenesis of kidney injury following severe acute pancreatitis. Biomed Pharmacother. 2020;125:109978. doi:10.1016/j.biopha.2020.109978
25. Ge P, Luo Y, Okoye CS, et al. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: a troublesome trio for acute pancreatitis. Biomed Pharmacother. 2020;132:110770. doi:10.1016/j.biopha.2020.110770
26. Matta B, Gougol A, Gao X, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18(7):1567–1575.e2. doi:10.1016/j.cgh.2019.11.017
27. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272(2):342–351. doi:10.1097/SLA.0000000000003239
28. Yildirim M, Dasiran F, Angin YS, et al. Lymphocyte-C-reactive protein ratio: a putative predictive factor for intestinal ischemia in strangulated abdominal wall hernias. Hernia. 2021;25(3):733–739. doi:10.1007/s10029-020-02174-x
29. Cheng CB, Zhang QX, Zhuang LP, et al. Prognostic value of lymphocyte-to-C-reactive protein ratio in patients with gastric cancer after surgery: a multicentre study. Jpn J Clin Oncol. 2020;50(10):1141–1149. doi:10.1093/jjco/hyaa099
30. Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734. doi:10.1002/jmv.25819
31. Ni HH, Lu Z, Huang X, et al. Combining pre- and postoperative lymphocyte-c-reactive protein ratios can better predict hepatocellular carcinoma prognosis after partial hepatectomy. J Inflamm Res. 2022;15:2229–2241. doi:10.2147/JIR.S359498
32. Akdur G, Bardakçı O, Das M, et al. Diagnostic utility of hematological indices in predicting adverse outcomes and severity of acute pancreatitis based on BISAP and modified Glasgow score. Ulus Travma Acil Cerrahi Derg. 2022;28(3):268–275. doi:10.14744/tjtes.2020.26348
33. Junare PR, Debnath P, Nair S, et al. Complete hemogram: simple and cost-effective in staging and predicting outcome in acute pancreatitis. Wien Klin Wochenschr. 2021;133(13–14):661–668. doi:10.1007/s00508-021-01821-2
34. Malheiro F, Nascimento ML, Carmo A, et al. Circulating blood lymphocytes and acute pancreatitis severity: a systematic review. Cureus. 2023;15(10):e47532. doi:10.7759/cureus.47532
35. Langmead C, Lee PJ, Paragomi P, et al. A novel 5-cytokine panel outperforms conventional predictive markers of persistent organ failure in acute pancreatitis. Clin Transl Gastroenterol. 2021;12(5):e00351. doi:10.14309/ctg.0000000000000351
36. Paragomi P, Spagnolo DM, Breze CR, et al. Introduction and validation of a novel acute pancreatitis digital tool: interrogating large pooled data from 2 prospectively ascertained cohorts. Pancreas. 2020;49(10):1276–1282. doi:10.1097/MPA.0000000000001686
37. Komara NL, Paragomi P, Greer PJ, et al. Severe acute pancreatitis: capillary permeability model linking systemic inflammation to multiorgan failure. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G573–G583. doi:10.1152/ajpgi.00285.2020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.