Back to Journals » Patient Preference and Adherence » Volume 18
Practices, Efficacy, and Reported Side Effects Associated with Isotretinoin Treatment in Palestine
Authors Abukhalil AD , Yousef M, Ammar M , Jaghama W, Al-Shami N , Naseef HA , Rabba AK
Received 28 September 2023
Accepted for publication 13 February 2024
Published 23 February 2024 Volume 2024:18 Pages 487—501
DOI https://doi.org/10.2147/PPA.S442436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Abdallah Damin Abukhalil, Mai Yousef,* Marwa Ammar,* Weam Jaghama,* Ni’meh Al-Shami, Hani A Naseef, Abdullah K Rabba
Department of Pharmacy, Faculty of Pharmacy, Nursing, and Health Professions, Birzeit University, West Bank, State of Palestine
*These authors contributed equally to this work
Correspondence: Abdallah Damin Abukhalil; Ni’meh Al-Shami, Pharmacy Department, Faculty of Pharmacy, Nursing and Health Professions, Birzeit University, West Bank, State of Palestine, Email [email protected]; [email protected]
Background: Isotretinoin is a commonly prescribed medication for the treatment of acne. It is associated with serious side effects that require monitoring and adherence by patients and healthcare providers. No studies have been conducted in Palestine to explore isotretinoin prescribing and utilization.
Objective: This study aims to evaluate the current clinical practices, adherence to clinical guidelines, efficacy, and reported side effects associated with Isotretinoin treatment in Palestine.
Methods: A descriptive cross-sectional online questionnaire-based study using social media platforms (eg, Facebook and Telegram) was conducted among Birzeit University students in April 2023. This study included participants aged ≥ 18 years with a history of isotretinoin treatment; subjects with incomplete data were excluded. Statistical significance was set at P < 0.05. SPSS version 27 was used for data analysis.
Results: A total of 548 participants were included in the study, the majority of most of whom were female (96%). The most predominant side effects were cracked, dry lips and xeroderma (96.2%). Moreover, 12% of participants had depression. Most respondents were educated about medication side effects and only 39.1% were counseled about blood donation. Of the 59 sexually active women, only 4 (6.8%) were asked for a recent pregnancy test. A total of 60.2% of dermatologists adhered to the American Academy of Dermatology (AAD) guidelines, and 48.7% ordered the required laboratory tests before initiating isotretinoin treatment. Only 1.7% of pharmacists followed the FDA-suggested protocols for dispensing isotretinoin to childbearing females.
Conclusion: Adherence to isotretinoin safety prescribing protocols to provide patient education, monitoring, and ordering of laboratories to ensure patient safety can be improved by adapting policies and protocols in pharmacy and medical practice in Palestine to monitor and enforce adherence when prescribing, dispensing, or taking high-risk medications.
Keywords: isotretinoin, acne, guidelines, adherence, side effects
Introduction
Acne is a common skin disorder in adolescents. It can negatively affect patients’ quality of life, causing anxiety, depression, and low self-esteem due to lesions or scars, which have cosmetic effects on appearance and self-image.1 Therefore, treatment with safe and effective medications is the primary goal of both patients and healthcare providers. Isotretinoin is a medication chemically related to retinoic acid and has been used to treat acne for almost 40 years. Isotretinoin has been very effective in patients with severe acne by decreasing the size of the sebaceous glands, reducing sebum production, and decreasing skin scarring and lesions.2 Oral isotretinoin has shown efficacy in treating moderate and preventing psychosocial effects, prolonging remission, and offering a cure.2
Isotretinoin is indicated for treatment-refractory and moderate to severe nodulocystic acne, a type of severe inflammatory acne that presents as firm, painful lumps under the skin and red bumps on the skin’s surface. Isotretinoin has a promising off-label use in managing seborrhea, seborrheic dermatitis, severe rosacea, chemoprevention of non-melanoma skin cancer in at-risk patients, and advanced photoaging with multiple actinic keratoses.3
According to the American Academy of Dermatology (AAD) and the European Dermatology Forum, physicians should prescribe isotretinoin for acne treatment for 4–6 months, with a cumulative dose greater than 120 mg/kg, which can be reached by either 0.5–1 mg/kg/day. However, isotretinoin therapy guidelines and duration vary from case to case, according to the severity of acne. Some patients require even higher doses, especially truncal, severe acne, males, and younger patients.4 These guidelines appended some key points that patients must be enlightened with for safety reassurance during treatment, improved results, and increased patient satisfaction.
Isotretinoin has demonstrated superiority over other acne treatments, including antibiotics and other topical products, in improving patient outcomes and satisfaction in reducing inflammation and resolving acne symptoms.5 However, serious side effects have been associated with isotretinoin treatment. The main side effect is teratogenicity, which requires intense monitoring. Other side effects include elevated liver enzyme levels, kidney problems, and benign intracranial hypertension, which have been reported during treatment with oral retinoids and tetracyclines. It may also frequently cause increased cholesterol, severe skin rashes and dryness, dry eyes (intolerance to additional contact lenses), conjunctivitis, cracked lips, back, and muscles, joint pain, easy bruising, bleeding, and anemia. Some side effects are dose-related, and patients may not experience them. These side effects are usually reversible after changing the dose or stopping the treatment. However, some cases are severe, and the patient must consult a doctor immediately.6
Clinical Guidelines recommend monthly monitoring of laboratory parameters, such as low-density lipoprotein (LDL), high-density lipoprotein (HDL), Triglycerides, total cholesterol, liver function tests, and complete blood counts.2 However, the American Academy of Dermatology (AAD) guidelines recommend monitoring only lipid and liver profiles at baseline and at least once throughout treatment. However, while commonly ordered by physicians, complete blood count (CBC) testing is not required in AAD guidelines.7 Additionally, all female patients of childbearing age should undergo pregnancy tests before initiating therapy and periodically thereafter. Depression is another critical side effect that needs to be monitored and discussed with patients, as 1 in 1000 patients experience depression, suicidal ideation, and other related mental health issues.8 Although depression is reported throughout the treatment, it is controversial whether it is drug-related, considering how acne can alter self-esteem and cause anxiety and depression. Patients’ overall health and well-being are crucial and worth monitoring using depression scales. Isotretinoin is associated with a history of dry eye syndrome, contact lens intolerance, photosensitivity, and reduced night vision, necessitating regular eye exams during treatment.9
Many countries have initiated mandatory registry programs to increase awareness of the side effects of isotretinoin and ensure its safety. The most common is the iPLEDGE program in the USA, designed to ensure appropriate prescribing, dispensing, and patient education.1 In Palestine, the use of isotretinoin is widespread and prescribed by both dermatologists and non-dermatologists.9 Unfortunately, Palestine lacks monitoring programs for isotretinoin treatment. Therefore, this cross-sectional questionnaire-based study explored the current practices, efficacy, and reported side effects associated with the isotretinoin treatment in Palestine. This may have important implications for guiding Palestinian prescribing practices in developing new strategies to improve adherence and compliance with isotretinoin treatment. Furthermore, to implement programs that optimize awareness and understanding of the potential risks and benefits among isotretinoin users.
Methodology
Study Design and Sample
A descriptive cross-sectional online questionnaire-based study was conducted among Birzeit University students in April 2023. This study included participants aged ≥ 18 who were formerly or presently undergoing isotretinoin treatment. The questionnaire was distributed through the university and students’ social media platforms, Facebook, Inc. and Instagram, and was made available for three weeks, starting at the beginning of April. A total of 548 participants were included in the study; 50 were excluded because they did not meet the inclusion criteria, had missing information, or did not use isotretinoin for acne treatment.
The research project had a sample size of 548 participants with a 5% allowable deviation range and a 95% confidence level to ensure the statistical validity and robustness of the findings.
The Questionnaire
The questionnaire was designed to be user-friendly, easy to complete, and translated into Arabic, the official language of the participants, to facilitate their participation and comprehension. The questionnaire was developed after reviewing the American Academy of Dermatology (AAD) and European Academy of Dermatology and Venereology (EADV) guidelines and previous studies on the use of isotretinoin in acne treatment.10–12
A pilot study was conducted with 20 participants who met the inclusion criteria and were asked to complete the questionnaire and provide feedback to confirm its consistency, validity, and clarity. It is important to note that the questionnaire responses from the pilot study were not included, and the final questionnaire was modified accordingly.
The questionnaire consisted of 34 structured and standardized questions divided into seven sections. In the first section, respondents were asked to provide demographic information, including age, gender, marital status, and geographic location. In the second section of the questionnaire, information about the respondents’ experience with isotretinoin therapy was collected, including previous usage, reasons for use, age at the time of treatment, duration of treatment, daily dosage, adherence to instructions, and any previous treatment with isotretinoin. Subsequently, the respondents were evaluated to assess the adherence of prescribers and pharmacists to globally recognized guidelines for isotretinoin usage (eg AAD and EADV). The fourth section consisted of a single question on the side effects experienced during the treatment period. In the fifth section, the respondent’s psychological state regarding the development of depression during treatment was evaluated using the Hospital Anxiety and Depression Scale (HADS), which included seven questions. The questionnaire addressed treatment efficacy, including satisfaction with the results, changes in acne scars, post-treatment routine skincare, occurrence and severity of acne. Finally, the seventh section provides an optional question for respondents to report any health problems they may have experienced after using isotretinoin or side effects that persist even after the end of treatment. Furthermore, the participants were required to provide informed consent at the beginning of the questionnaire, granting voluntary agreement to participate. Only those who provided consent could proceed to the subsequent questionnaire sections, ensuring compliance with the ethical guidelines and participant autonomy.
Statistical Analysis
Data were analyzed using descriptive analysis, with categorical variables such as indication for use and dose presented as frequencies and percentages, whereas continuous variables, such as age and treatment duration, were presented as mean or median (± standard deviation).
This was followed by a chi-square test to evaluate the relationship between patient characteristics and the risk of relapse and the association between regular monitoring and the occurrence of side effects. Statistical significance was set at a threshold of P < 0.05. Data analysis, comparison of results, and definition of the disparities between isotretinoin prescription practice and guideline recommendations were performed using the Statistical Package of Social Sciences (SPSS) version 27.
The Ethics Committee of Birzeit University approved this study. Written informed consent was obtained from each patient in the first section of the questionnaire. This study adhered to the ethical guidelines of the Declaration of Helsinki.
Results
A total of 548 people responded to the questionnaire, with the majority of participants being female (96%), single (88.7%), and middle areas, with a mean age of 22.56 years and a standard deviation of 4.22. Demographic characteristics are shown in Table 1.
 |
Table 1 Patient’s Demographics N= 548 |
As shown in Table 2, the mean age of patients at treatment initiation was 20.08 years. Of all participants, 81.9% had previously used isotretinoin, and 18.1% were currently undergoing treatment. The majority (94.7%) had used prescriptions to obtain the medication. The average expected treatment duration was 6.2 months (± 2.5), while the average completed duration was 5.4 months (± 2.7). It is noticeable that 5.3% of participants used isotretinoin without a prescription.
 |
Table 2 Treatment Information N=548 |
Of the 104 respondents who discontinued isotretinoin, 9.7% stated that side effects were the reason for discontinuation. The most prescribed dose was 40 mg (60.9%), followed by 20 mg (11.1%) and other daily or weekly doses, as shown in Table 2.
As shown in Figure 1, cleansers (51.8%) were the most commonly used products prior to isotretinoin initiation, followed by topical or oral antibiotics (34.1%) and topical retinoids (31.6%).
 |
Figure 1 Medications and OTC Products Used Prior to Treatment N=548. |
Table 3 explores health provider adherence to patient counseling and monitoring during therapy. The majority of respondents were educated about medication adherence, side effects, appropriate administration, and lab monitoring. However, only 39.1% of participants were counseled about blood donation. Furthermore, of the 59 sexually active women, only 4 (6.8%) were asked for a recent pregnancy test, 15 (25.4%) were asked for a prescription no older than 7 days, and 13 (22%) were given a 30-day refill only.
 |
Table 3 Medication Dispensing and Patient Counseling by Health Care Provider N=548 |
The data revealed that the most frequently performed tests were liver function tests (ALT/AST) (91.3%) and complete blood count (74.8%). The lipid panel was less frequently ordered (51.4%), although it was recommended by guidelines.
Figure 2 illustrates the most frequently encountered side effects. Most patients treated with isotretinoin experienced side effects, with only 1.3% reporting no side effects. The most predominant side effects were cracked, dry lips and xeroderma, experienced by 96.2% of participants. The least prevalent side effects were anemia (5.5%), an increased risk of infection (4%), and bone fractures (2.7%).
 |
Figure 2 Side effects Associated with Isotretinoin Use N=548* (weight loss and delayed period). |
Table 4 presents the reported stages of mood changes and depressive thoughts during isotretinoin treatment, and the patients were categorized based on the Hospital Anxiety and Depression scale. 45.4% of patients could still enjoy things as they used to before isotretinoin treatment, and 4.1% hardly at all. Most of the patients were still able to laugh and see the funny side of things, as always (62.9%). 35.1% of patients were able to feel cheerful most of the time, 17.5% of patients felt slowed down nearly all the time, and none of the patients included in this study completely lost interest in appearance.
 |
Table 4 Depression Assessment Among Patients Currently on Treatment N=97 |
Figure 3 represents the total score of the hospital anxiety and depression scale employed on the patients that were included in this section of the study. The vast majority of the patients had no depressive symptoms (73%) according to the scale; 15% were borderline, 12% were abnormal, and out of the usual had depressive thoughts.
 |
Figure 3 Psychiatric Evaluation Results for Current Isotretinoin Users. N=97. |
The results presented in Table 5 provide insights into patient satisfaction, acne recurrence, the effect on acne scars, and daily routine post-treatment. Of the 548 participants, 47.4% expressed satisfaction with the treatment. Regarding acne recurrence, 35.6% of participants experienced recurrence. Moreover, most patients use a cleanser and SPF sunscreen as a daily routine post-treatment.
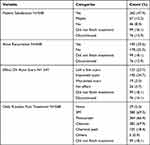 |
Table 5 Patient Satisfaction, Acne Recurrence, Effect on Acne Scars, and Daily Routine Post Treatment |
Table 6 presents the characteristics of the patients and their corresponding relapse status. A statistically significant difference was observed based on age, with participants aged ≤ 20 exhibiting a higher proportion of relapses than those aged ≥ 21 years. Similarly, a significant association was found between sex and relapse rates, with females showing a higher likelihood of relapse than males. On the other hand, no significant associations were observed between relapse rates and marital status, medication doses, or completion of the medication course.
 |
Table 6 Characteristics and Relapse Status of Patients |
Table 7 shows providers’ adherence to the treatment guidelines. Physician adherence was 100% when prescribing isotretinoin only for patients aged ≥12 years. 75% of physicians adhered to the recommended AAD treatment duration, which is a minimum of 3 months up to a maximum of 6 months. This study revealed a notable percentage (5.3%) of patients taking isotretinoin without prescription. 39.8% of the physicians were nonadherent to the treatment guidelines by prescribing isotretinoin for indications unsupported by the established protocols. 18.4% of patients were concurrently taking at least one contraindicated medication while on isotretinoin therapy. Most physicians and pharmacists (71%) did not adhere to patient counseling. The vast majority of pharmacists (98.3%) were nonadherent to the established protocols by the FDA concerning married women or women of childbearing age by repeating the pregnancy test with every new prescription. Most physicians (51.3%) neglected to order the required tests (pregnancy test, lipid profile, and liver enzymes) before initiating the isotretinoin therapy. 53.2% of physicians ignored the necessity of requesting to repeat the required tests monthly according to the guidelines.
 |
Table 7 Isotretinoin Treatment and Health Care Provider Guideline Adherence |
Discussion
This study focuses on significant elements of isotretinoin treatment in Palestine, including indications, prescribing, monitoring, adherence, and side effects reported by patients in Palestine.
Isotretinoin is indicated for patients with severe recalcitrant nodular acne, which is characterized by large, inflamed, and painful nodules beneath the skin’s surface that have not responded adequately to conventional treatment methods, such as topical or systemic antibiotics, topical retinoids, miscellaneous agents, or other therapies.13 In this study, most participants (60%) were on isotretinoin treatment appropriately and according to the protocol published by the American Academy of Dermatology and in accordance with the product labeling.2 A higher rate of appropriate prescribing was reported in a similar study conducted in Quebec City; 79% of the participants had the correct indication for isotretinoin treatment.10 A Complete adherence with all steps of the isotretinoin treatment guideline was reported among physicians in Singapore (97.8%), which may be due to patients’ inclination and their assertiveness in expressing their preference to the physicians about their chosen medication, influenced by social media, coupled with individuals’ insistent desire to attain a flawless appearance, and the fact that it is the quickest way to treat acne may also potentially contribute to this phenomenon.1,10,11
The duration of treatment with isotretinoin, as recommended by the AAD, is minimally 3 months, up to the maximum duration of 6 months. In this study, 25% of participants had used isotretinoin for an improper duration of treatment, which is lower than the findings of other studies; for example, the improper duration reported was 35% in a study conducted in Quebec City study,10 68% of a Saudi female college students’ students’ study,14 and 44.7% in the Jordan University of Science and Technology study,15 all had undergone a duration of therapy longer than 6 months.1,12,13 Adherence to the treatment duration is essential for the efficacy and prevention of long-term damage and complications with longer treatment durations, such as permanent loss of eyesight, erectile dysfunction, and loss of libido.16
The majority of isotretinoin prescriptions (94.7%) in the study were recommended and prescribed by a dermatologist, indicating patients’ adherence to prescription laws since isotretinoin is a prescription-only drug preferred to be prescribed by a specialist and a trained healthcare provider. This finding is much higher than that of other studies; for example, only 52% of prescriptions in Quebec City were prescribed by dermatologists.10 Even though specialists prescribed isotretinoin, many parameters were not followed during the patient care process while taking isotretinoin.1
According to the ADD guidelines, it is essential for physicians and pharmacists to provide counseling and patient education on several key aspects. Initially, they should emphasize the significance of maintaining adequate hydration to avoid liver-related complications and minimize skin dryness.17 Furthermore, they should highlight the necessity of using sunscreen to minimize hyperpigmentation.17 They should advise patients to refrain from sharing the medication with anyone else. Additionally, it is crucial to inform patients that blood donation is not permitted during the course of isotretinoin treatment due to the risk of the recipient being a pregnant woman in her first trimester, as the blood may contain high levels of isotretinoin.13 The data showed that only 29% of healthcare professionals were adherent in educating the patients about the required key points to follow during the treatment, which is significantly lower than that in other studies performed in other countries.2 For example, in a Jordanian study, 98.4% of patients were informed about the contraindication of blood donation, and 75.4% were informed about the importance of hydration and sunblock, respectively.15 Also, in a study performed in the western region of Saudi Arabia, 84.6% of patients were informed about isotretinoin side effects by physicians and pharmacists.18 The low adherence in clinical practice regarding patient counseling and educations is due to the lack of pharmacy practice laws that enforce patient counseling as not only ethical responsibility for practitioners but also a legal one that is required by laws. Moreover, 18.4% of the patients concurrently took at least one contraindicated medication, which may be due to insufficient patient education by physicians and pharmacists on potential side effects that may occur due to drug-drug interactions. Pharmacists in Palestine are not required by law to maintain patient profiles in community pharmacies which aids in conducting medication reviews and identifying drug-related problems when dispensing prescriptions. For example, Intracranial hypertension is a consequence of the concurrent use of tetracyclines with Isotretinoin.19
Laboratory tests, such as pregnancy, lipid profile, and liver enzyme tests, are important safety monitoring parameters that prescribers should order when initiating and during isotretinoin therapy. In this study, prescribers (51.3%) were nonadherent to the recommendation of clinical guidelines in ordering essential laboratory tests, which can compromise patient safety and expose patients to harm and toxicity. A similar finding was reported in a study in Singapore.11 This noncompliance may be due to the lack of a program to ensure appropriate prescribing and monitoring of isotretinoin, such as the IPLEDGE program. In contrast, a study conducted in California demonstrated a high compliance rate to the IPLEDGE program (approximately 90%).10 Which led to a 29% reduction in the number of patients treated with isotretinoin and limited isotretinoin prescription only for the cases that were suggested by the FDA.20
The IPLEDGE program is a system of protocols developed by the FDA that healthcare professionals should follow to prevent fetal exposure and minimize the risk of congenital disabilities associated with isotretinoin. The program consists of several protocols, such as refusal to dispense if the prescription was more than 30 days of supply or if the prescription was written more than 7 days before dispensing, emphasizing the importance of a pregnancy test at each refill and checking the prescription’s validity. Our data indicated that only 1.7% of pharmacists knew these essential guidelines. In comparison, as much as 98.3% did not provide counseling, compared with other countries, in a study in the Netherlands (94.4% of dermatologists and 95.9% of pharmacists were educated)21 and France (87% of pharmacists were also aware).22 This could be attributed to the neglect of pharmacists in providing essential information or their lack of education regarding this program. Therefore, adopting policies in pharmacy practice laws, such as continual education requirements for healthcare providers and adopting protocols for certain medications, such as isotretinoin, are essential for improving patient care and ensuring patient safety and adherence to clinical guidelines.
Commonly reported side effects associated with isotretinoin were observed in this study. The most common side effects were cracked, dry lips and extreme dryness of the skin, also known as xeroderma. These results are consistent with previous studies on the side effects of isotretinoin. For example, a study involving 50 participants observed a 98% rate of occurrence for cheilitis or dry lips.23 The frequency of this side effect is connected to isotretinoin’s suggested mechanism of action, as reducing sebaceous glands’ size and sebum production results in a change in the skin’s lipid composition and can cause dryness of the skin.24
Hair loss is another reported side effect associated with isotretinoin. Although the exact mechanism underlying hair loss in response to isotretinoin is unknown, several studies have highlighted the relationship between isotretinoin and hair growth. A meta-analysis of 22 studies showed that 3.2% (n = 18/565) of patients on <0.5 mg/kg/d of isotretinoin reported hair loss at a lower rate compared with those on ≥0.5 mg/kg/d, who experienced hair loss at a rate of 5.7% (n = 192/3375).25 When compared to these findings, this study has a much greater rate of hair loss, as 38.5% struggled with hair loss while on isotretinoin. Hair loss can be attributed to other factors, such as seasonal shedding in healthy individuals or stress-induced hair loss, which is common in college students, such as telogen effluvium, a scalp condition marked by excessive hair shedding that may be caused by trauma, drugs, etc. that may also affect the result.26 Nevertheless, it is important to counsel the patient on this possible side effect that psychologically impacts patient quality of life.
The effect of isotretinoin treatment on depression has been a subject of growing interest. Theoretically, the isotretinoin molecule (synthetic vitamin A derivative) is a fat-soluble compound that can cross the blood-brain barrier, the protective barrier surrounding the brain.27 This ability facilitates the interaction between isotretinoin and retinoid receptors and causes slight induction and alteration of the receptor.28 Retinoid receptors are found predominantly in the limbic brain regions, including the hippocampus. This region is involved in regulating emotions and may be linked to depression.29 Research done to investigate the association between isotretinoin and depression has produced contradictory findings.27–30 It includes various studies, including case reports, database studies, and retrospective studies, with the majority suggesting a possible link between isotretinoin and depression.29 However, reviews of prospective studies have shown no association, and some have even shown improvements in depressive symptoms.30
This study assessed 97 patients who currently received isotretinoin therapy for depression using the Hospital Anxiety and Depression Scale (HADS). Upon analyzing the data, most patients were regular, while a small percentage were borderline or abnormal, as shown in Figure 3. This study found a higher percentage of patients with depression (12%) at the end of treatment compared to a study conducted in Zaragoza, Spain, where only 3.5% of patients exhibited depression based on HADS measures.31 Conversely, a study in Venezuela concluded that isotretinoin was safe for regular dermatological patients regarding psychological side effects, with no significant differences observed in psychological well-being using the Zung scale.32 In Sweden, a distinct finding emerged, showing an increased risk of attempted suicide up to six months after the completion of isotretinoin treatment.29,30 This finding was noteworthy, as suicide risk was also high both before and after treatment cessation. These varying outcomes underscore the complexity of the relationship between isotretinoin and psychological effects, warranting further investigation into the nuanced factors influencing its impact on mental health.33 Nevertheless, there are several limitations to consider.31 The study’s reliance on a single self-report measure to evaluate depression, the HADS, introduces the possibility of subjective bias and the limited assessment of other important confounding factors that could contribute to patients’ experiences. Moreover, the absence of a control group in this study design limits the ability to establish a direct causal relationship between isotretinoin treatment and the observed depression symptoms.
Another significant side effect of isotretinoin treatment is liver enzyme (AST/ALT) elevation, which was reported by 43 (7.8%) of participants; a lower rate than reported by other studies was up to 15% of patients may experience liver test abnormalities.34 Furthermore, a very small number of patients (34) experienced hyperlipidemia while on isotretinoin treatment, which is much lower compared to other studies.35 These low findings are attributed to most of our sample being young females (96%), and females generally are at lower risk of developing hypercholesterolemia.36 Moreover, as shown in Table 7, the lack of regular monitoring might have affected this finding.32–34
Acne relapse, defined as the recurrence of acne symptoms after initial treatment, is a common and frustrating issue faced by patients and healthcare providers. Despite advancements in acne treatment options, relapse remains a persistent challenge, often leading to dissatisfaction and hindering long-term management of the condition. The occurrence of acne relapse varied significantly across studies conducted globally. According to a Spanish study published in the ADEV journal, the relapse rate ranges from 10% to 60%.37 These variations can be attributed to the specific treatment approaches used and the duration of the follow-up period. These variations can be attributed to the specific treatment approaches utilized and the duration of the follow-up period. Similarly, a cross-sectional study conducted in Saudi Arabia demonstrated that 45.12% of participants who used isotretinoin showed relapse.38 On the other hand, a long-term follow-up Suisse study demonstrated that only 14.6% of patients relapsed.39 In this study, 35.6% (195 out of 373) of participants experienced a relapse after completing a course of isotretinoin, which aligns with the range of relapse rates reported in other studies.40 Several factors can potentially influence relapse rate including, age, sex, side effects, and medication adherence. Younger age was associated with a higher probability of relapse (p = 0.019). In contrast, males demonstrated a lower likelihood of relapse (p = 0.026). The higher likelihood of relapse in females may be attributed to specific skin practices commonly seen in this population, including using comedogenic products such as makeup. Products like these can clog pores, increase sebum production, and eventually contribute to acne relapse.1 Additionally, hormonal changes that occur during the menstrual cycle can also play a role in increased sebum production and inflammation. Fluctuations in hormone levels during these periods can make the skin more vulnerable to the recurrence of acne.41 In a study conducted over one year, relapse occurred in 14 out of 20 individuals under the age of 12, 21 out of 47 individuals aged between 12 and 14, and 23 out of 66 individuals aged between 14 and 16.42 Some studies suggest that men have a higher relapse rate.28,40 However conclusive evidence supports the claim that acne relapse is higher in males or females, specifically post isotretinoin therapy.35
Moreover, the analysis did not find a significant association between medication dose, treatment course completion, marital status, and acne recurrence, and it is important to interpret these findings cautiously due to potential limitations. A possible explanation for this lack of significance could be the study’s sample size, which may have limited the statistical power to detect more minor, more negligible effects. Additionally, other unmeasured factors, such as genetic predisposition, lifestyle habits, or environmental influences, could have influenced the recurrence of acne independently of the variables examined. Future studies with larger, more extensive, and more diverse populations across Palestine must further explore these factors and their potential impacts on acne recurrence.
Strengths and Limitations
This cross-sectional study provides a snapshot of our sample and helps us determine the prevalence of the characteristic we seek, isotretinoin guideline adherence. This study sample size allowed for increased precision, generalizability, and statistical power. In addition, cross-sectional studies have another advantage, which is less imposing upon patients.
Despite efforts to ensure comparability with previous studies, several limitations may interrupt our findings. Although the study included 548 participants, the sample size was still a limitation for some data, such as married or male patients and current users of isotretinoin. Furthermore, this study has a cross-sectional design; we cannot generate statistical relations between variables, which is difficult to control, and recall, selection, and self-reporting biases may occur. In addition, prescribing physicians and dispensing pharmacists were not surveyed in this study, which could also be a limitation in drawing a conclusion on current physician and pharmacist awareness of isotretinoin and their current practices, which calls for further studies among healthcare providers.
Conclusions
This study explored many facts regarding prescribing and utilization of Isotretinoin in Palestine. There were varying degrees of adherence to isotretinoin guideline counseling regarding blood donation, and adherence to dispensing guidelines, particularly for sexually active women, was severely lacking. Adherence to isotretinoin safety prescribing protocols for providing patient education, monitoring, and ordering laboratory tests could be improved by adapting protocols and policies to pharmacy and medical practice laws to monitor and enforce adherence when prescribing or dispensing high-risk medications to ensure optimal therapeutic outcomes and patient safety.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi:10.1016/j.jaad.2015.12.037
2. Bagatin E, Costa CS. The use of Isotretinoin for acne - an update on optimal dosing, surveillance, and adverse effects. Expert Rev Clin Pharmacol. 2020;13(8):885–897. doi:10.1080/17512433.2020.1796637
3. Nickle SB, Peterson N, Peterson M. Updated physician’s guide to the off-label uses of oral isotretinoin. J Clin Aesthet Dermatol. 2014;7(4):22–34.
4. Leyden JJ, Rosso JQ D, Baum EW, Mcguigan KA. Faculty the use of Isotretinoin in the treatment of acne vulgaris clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2 Suppl):S3.
5. Chernyshov PV, Tomas-Aragones L, Manolache L, et al. Which acne treatment has the best influence on health-related quality of life? Literature review by the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J Eur Acad Dermatol Venereol. 2018;32(9):1410–1419. doi:10.1111/jdv.15048
6. Karaosmanoğlu N, Mülkoğlu C. Analysis of musculoskeletal side effects of oral Isotretinoin treatment: a cross-sectional study. BMC Musculoskelet Disord. 2020;21(1):631. doi:10.1186/s12891-020-03656-w
7. Acne clinical guideline. Available from: https://www.aad.org/member/clinical-quality/guidelines/acne.
8. British Association of Dermatologists. Isotretinoin patient guide. Available from: https://www.bad.org.uk/pils/isotretinoin/.
9. Caffery BE, Josephson JE. Ocular side effects of isotretinoin therapy. J Am Optom Assoc. 1988;59(3):221–224.
10. Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006;62(8):667–674. doi:10.1007/S00228-006-0151-X/METRICS
11. Tang MBY, Tan EST, Tian EAL, Loo SC, Chua SH. Electronic e-isotretinoin prescription chart: improving physicians’ adherence to isotretinoin prescription guidelines. Australas J Dermatol. 2009;50(2):107–112. doi:10.1111/j.1440-0960.2009.00516.x
12. Lelubre M, Hamdani J, Senterre C, et al. Evaluation of compliance with isotretinoin PPP recommendations and exploration of reasons for non-compliance: survey among French-speaking health care professionals and patients in Belgium. Pharmacoepidemiol Drug Saf. 2018;27(6):668–673. doi:10.1002/pds.4441
13. Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76(6):1068–1076.e9. doi:10.1016/j.jaad.2016.12.028
14. Albadr T, Alruhaimi D, Cahusac PB, Rohra D. Knowledge and use of isotretinoin in Saudi female college students: cross -sectional study. J Dermatol Dermatologic Surg. 2019;23(2):76. doi:10.4103/jdds.jdds_2_19
15. Jarab AS, Al-Azzam S, Almutairi S, Mukattash TL. Patients’ knowledge and information needs about isotretinoin therapy use in Jordan. Int J Clin Pract. 2022;2022:1–6. doi:10.1155/2022/9443884
16. Isotretinoin: the truth about side effects. Available from: https://www.aad.org/public/diseases/acne/derm-treat/isotretinoin/side-effects.
17. Younis NS, Al-Harbi NY. Public understanding and awareness of isotretinoin use and safety in Al Ahsa, Eastern Saudi Arabia. Ther Innov Regul Sci. 2019;53(5):618–622. doi:10.1177/2168479018807677
18. Bakheet KMA, Alghanemi RG, Alsiyoufi AM, Abduljabbar M, Hariri J. Females’ knowledge and use of isotretinoin (Roaccutane) in the Western Region of Saudi Arabia. Cureus. 2020. doi:10.7759/cureus.12148
19. Reserva J, Adams W, Perlman D, et al. Coprescription of Isotretinoin and tetracyclines for acne is rare: an analysis of the National Ambulatory Medical Care Survey. J Clin Aesthet Dermatol. 2019;12(10):45–48.
20. Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65(6):1117–1125. doi:10.1016/j.jaad.2010.09.017
21. Crijns HJMJ, van Rein N, Gispen-de Wied CC, Straus SM, de Jong-van den Berg LTW. Prescriptive contraceptive use among isotretinoin users in the Netherlands in comparison with non-users: a drug utilisation study. Pharmacoepidemiol Drug Saf. 2012;21(10):1060–1066. doi:10.1002/pds.3200
22. Autret-Leca E, Kreft-Jais C, Elefant E, et al. Isotretinoin exposure during pregnancy. Drug Saf. 2010;33(8):659–665. doi:10.2165/11536250-000000000-00000
23. Bhat R, Nandakishore B, Dandakeri S, Martis J, Kamath G, Rao P. Safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris. Indian J Dermatol. 2014;59(3):316. doi:10.4103/0019-5154.131455
24. Ward A, Brogden RN, Heel RC, Speight TM, Avery GS. Isotretinoin. Drugs. 1984;28(1):6–37. doi:10.2165/00003495-198428010-00002
25. Lytvyn Y, McDonald K, Mufti A, Beecker J. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125–142. doi:10.1016/j.jdin.2022.01.002
26. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen Effluvium: a Review of the Literature. Cureus. 2020. doi:10.7759/cureus.8320
27. Crandall J, Sakai Y, Zhang J, et al. 13- cis -retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci. 2004;101(14):5111–5116. doi:10.1073/pnas.0306336101
28. Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1(3):162–169. doi:10.4161/derm.1.3.9364
29. O’Reilly K, Bailey SJ, Lane MA. Retinoid-mediated regulation of mood: possible cellular mechanisms. Exper Biol Med. 2008;233(3):251–258. doi:10.3181/0706-MR-158
30. Oliveira JM, Sobreira G, Velosa J, Telles Correia D, Filipe P. Association of isotretinoin with depression and suicide: a review of current literature. J Cutan Med Surg. 2018;22(1):58–64. doi:10.1177/1203475417719052
31. Marron S, Tomas-Aragones L, Boira SA. Depression, quality of life and patient satisfaction in acne patients treated with oral isotretinoin. Acta Dermatol Venereologica. 2013;93(6):701–706. doi:10.2340/00015555-1638
32. Suarez B, Serrano A, Cova Y, Baptista T. Isotretinoin was not associated with depression or anxiety: a twelve-week study. World J Psychiatry. 2016;6(1):136. doi:10.5498/wjp.v6.i1.136
33. Sundstrom A, Alfredsson L, Sjolin-Forsberg G, Gerden B, Bergman U, Jokinen J. Association of suicide attempts with acne and treatment with isotretinoin: retrospective Swedish cohort study. BMJ. 2010;341(1):c5812. doi:10.1136/bmj.c5812
34. Kapała J, Lewandowska J, Placek W, Owczarczyk-Saczonek A. Adverse Events in Isotretinoin Therapy: A Single-Arm Meta-Analysis. Int J Environ Res Public Health. 2022;19(11):6463.
35. Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7(1):171. doi:10.4103/jfmpc.jfmpc_104_16
36. Gao Z, Chen Z, Sun A, Deng X. Gender differences in cardiovascular disease. Med Nov Technol Devices. 2019;4:100025. doi:10.1016/j.medntd.2019.100025
37. Morales-Cardona CA, Sánchez-Vanegas G. Acne relapse rate and predictors of relapse following treatment with oral isotretinoin. Actas Dermo-Sifiliográficas. 2013;104(1):61–66. doi:10.1016/j.adengl.2012.05.021
38. Sa A, Y A, Am A, Sa A, L P, Na A. Prevalence and associated risk factors of acne relapse among Saudi acne vulgaris patients using isotretinoin. Saudi Pharm J. 2020;28(3):374–379. doi:10.1016/j.jsps.2020.01.019
39. Harms M, Masouyé I, Radeff B. The relapses of cystic acne after isotretinoin treatment are age-related: a long-term follow-up study. Dermatologica. 1986;172(3):148–153. doi:10.1159/000249320
40. Azoulay L, Oraichi D, Bérard A. Isotretinoin therapy and the incidence of acne relapse: a nested case–control study. Br J Dermatol. 2007;157(6):1240–1248. doi:10.1111/j.1365-2133.2007.08250.x
41. Elsaie ML. Hormonal treatment of acne vulgaris: an update. Clin Cosmet Invest Dermatol. 2016;9:241–248. doi:10.2147/CCID.S114830
42. Leyden JJ. Oral Isotretinoin. Dermatology. 1997;195(1):29–33. doi:10.1159/000246017
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
