Back to Journals » Infection and Drug Resistance » Volume 12
Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds
Authors Li T , Ma Y, Wang M, Wang T, Wei J, Ren R, He M, Wang G, Boey J , Armstrong DG, Deng W , Chen B
Received 6 September 2018
Accepted for publication 27 December 2018
Published 29 January 2019 Volume 2019:12 Pages 297—309
DOI https://doi.org/10.2147/IDR.S186651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Tao Li,1 Yu Ma,2 Min Wang,1 Tao Wang,3 Jing Wei,4 Rui Ren,1 Min He,1 Guixue Wang,2 Johnson Boey,5 David G Armstrong,6 Wuquan Deng,2 Bing Chen1
1Department of Endocrinology, Southwest Hospital, Army Medical University, Chongqing, People’s Republic of China; 2Department of Endocrinology and Nephrology, Key Laboratory for Biorheological Science and Technology of Ministry of Education, Chongqing University, Affiliated Central Hospital of Chongqing University, Chongqing, People’s Republic of China; 3Institute of Combined Injury, State Key Laboratory of Trauma, Burn and Combined Injury, Army Medical University, Chongqing, People’s Republic of China; 4Department of Endocrinology, General Hospital of Xinjiang Military Region, The Chinese People’s Liberation Army, Urumqi, People’s Republic of China; 5Department of Podiatry, Singapore General Hospital, Singapore; 6Southwestern Academic Limb Salvage Alliance (SALSA), Department of Surgery, Keck School of Medicine of the University of Southern California, Los Angeles, CA, USA
Aim: This study was designed to examine the potential mechanism underlying these roles of platelet-rich plasma in treating diabetic foot ulcers (DFUs).
Methods: Staphylococcus aureus and HaCaT were co-cultured under high glucose conditions to serve as an in vitro model for infected cells in DFUs. Platelet-rich gel (PRG) or extract liquid of platelet-rich gel (EPG) were used to interfere with the model to observe the growth of HaCaT cells and S. aureus, and the effect of miR-21 changes in HaCaT cells on PDCD4, NF-κB activity and related inflammatory factors.
Results: Incubation of HaCaT cells with S. aureus promoted the decline of cell proliferation. Under this condition, the level of PDCD4 and the activity of NF-κB were increased in HaCaT cells with concomitant increased of IL-6, TNF-α and decreased IL-10, TGF-β1 in cultured supernatant. Both of PRG and EPG exhibited specific anti-S. aureus activity where they protect HaCaT cells from bacterial damage and promote cell proliferation. Meanwhile, EPG was observed to increase intracellular miRNA-21 while reduce PDCD4 expression and inhibit NF-κB activity to suppress the inflammation in HaCaT cells.
Conclusion: This in vitro model provides a valuable tool for study of wound healing in the treatment of DFUs. Our results suggest that miRNA-21 may regulate the expression of NF-κB through PDCD4 where it plays an anti-inflammatory role and promote proliferation in infected DFUs treated by PRP. These findings could provide novel therapeutic targets for refractory wounds.
Keywords: platelet-rich plasma, antibacterial, anti-inflammatory, cell proliferation-promoting, diabetic infected wound
Introduction
The global incidence of diabetes mellitus is increasing rapidly. The International Diabetes Federation estimated that there will be ~630 million of people diagnosed with diabetes by 2045.1 Among the complications associated with diabetes, diabetic foot ulcers (DFUs) severely debilitate the quality of life, and it was found that one in four individuals diagnosed with diabetes will develop DFU in their lifetime.2 Many pathophysiological mechanisms have been proposed to explain delayed wound healing in DFUs, one of which, wound infection plays an important role.3 Gram-positive bacterial infections are relatively common in DFUs, predominately caused by Staphylococcus aureus.4 Bacterial colonization in wounds causes direct cellular damage to the host cell, and an imbalance in the microenvironment. Once the bacterial bioburden reaches a critical level, the host body mounts an immune response, which is often deranged and yet insufficient to eradicate the offending bacteria. Persistent inflammation ensues, which further impedes diabetic wound healing.5,6 Antibiotics are often used to treat wound infections in DFUs. However, the frequent recurrence of DFUs for patients in DFU remission and emergence of multiple-drug resistance bacteria rendered antibiotics ineffective or poor efficacy, when used alone.2,7
In recent years, studies has revealed the wound healing potential of platelet-rich plasma (PRP) in the treatment of DFUs from their antimicrobial and regenerative properties.8–10 Platelet-rich gel (PRG) is produced from PRP which is activated by thrombin and/or calcium. These platelet derivatives have been shown to possess several important functions such as antibacterial and promotion of wound healing. For example, PRG has been proven to inhibit S. aureus, Staphylococcus epidermidis, Escherichia coli and Klebsiella pneumoniae, as well as methicillin-resistant staphylococcus aureus in a previous study.11 Moreover, PRG does not cause drug resistance and has a synergistic effect with antibiotics. The combination of these characteristics makes it more promising over traditional antibiotic prescriptions.12
Within activated platelets, platelet-derived antimicrobial peptides, chemokines, growth factors, and miRNAs have been observed to exert antibacterial effects and promote wound healing.13 There are more than 200 miRNAs found in platelets, in particularly, miRNA-21 and miRNA-223 are highly abundant.14 The level of miRNAs in DFU is directly correlated to wound healing.15 The expression of miR-21 is up-regulated during wound healing while the suppression of its expression is found in wounds of diabetic mice.16 Taken the above literature together, it has been speculated that miRNA-21 may play an important role in PRG treatment of DFUs. Furthermore, it has been reported that miRNA-21 can also play a role in inflammation by regulating nuclear factor-kappa (NF-κB),17 but the exact mechanism is not yet clear.
Programmed cell death factor 4 (PDCD4) is one of the currently validated miRNA-21 regulatory targets in which miRNA-21 inhibit PDCD4 expression at the post-transcriptional level.18,19 It has been reported that inhibition of miRNA-21 expression in mice may up-regulate PDCD4, induce the activation of NF-κB which eventually leads to increased synthesis of pro-inflammatory cytokines, and decreased synthesis of anti-inflammatory cytokines.20 Based on the above findings, we hypothesize that PRG may affect the activity of NF-κB, regulate wound inflammation, and promote wound healing through regulating PDCD4 mediated by miRNA-21.
To confirm this assumption, we firstly devised a diabetic wound infected in-vitro study model. The PRG was subsequently added into this model to observe the growth of HaCaT cells and S. aureus. In addition, to evaluate the potential ability of PRP in antibacterial and wound healing, the changes in miRNA-21, PDCD4 and NF-κB in HaCaT cells and related inflammatory factors were assessed.
Materials and methods
Preparation of platelet-poor plasma (PPP) and PRP
Whole blood samples were drawn from 21 patients (age 69±12) with DFUs but free of active infection and existing systemic coagulation disorders. PPP was isolated from the blood specimens with the first centrifugation in the department of blood transfusion. Platelets were sequestered by full automatic blood separator (CS-3000 plus; Baxter International, Inc., Deerfield, IL, USA), and condensed by the second centrifugation to produce PRP. The concentrations of platelets in PRP and PPP were determined by the automatic hematology analyzer (KX-21N; Sysmex Corporation, Kobe, Japan). The average platelet count was (1024±111)×109/L in PRP, while it was <10×109/L in PPP. The majority of the extracted PRP was used to treat the blood donor’s DFUs while the remaining PRP will be used in our study. The above processes were approved by the institutional review board of the first hospital affiliated to the Army Medical University. Written informed consent was obtained from all patients. The study was approved by the medical ethics committee of the first hospital affiliated to the Army Medical University (Chongqing, People’s Republic of China) and conducted in compliance with the Declaration of Helsinki.
Preparation of PRG
The PRG was harvested by platelet activation. The 10 mL PRP was activated by 1 mL calcium gluconate solution (100 g/L) and 1,000 IU thrombin.
Preparation of the extract liquid from PRG
The PRG was incubated at 37°C for 1 hour with constant thorough stirring with a sterile glass rod. Then, the mixture was centrifuged for 15 minutes with a centrifugation force of 3,000 × g at room temperature. The supernatant was collected as extract liquid of platelet-rich gel (EPG).
Establishment of an in vitro diabetic infected wound model
HaCaT cells and S. aureus (ATCC 25923) were bestowed gifts from the Institute of Combined Injury of the Army Medical University (Chongqing, People’s Republic of China) for research purposes. S. aureus was cultured in Tryptic Soy Broth (TSB) medium in a shaking 37°C incubator overnight. Then, the cultured bacteria were collected by centrifugation and re-suspended in high glucose DMEM culture medium (Hyclone). Bacterial density was adjusted to 108 CFU/mL and serial dilutions were prepared. Simultaneously, HaCaT cells were cultured in high glucose DMEM supplemented with 10% FBS at 37°C with 5% CO2 for 4 days. Then cells were harvested and seeded into 96-well plates at a density of 30,000 cells/cm2. After 48 hours, the culture medium was replaced by either fresh high glucose DMEM (HaCaT control) or S. aureus dilutions. To examine potential damage ability to HaCaT cells, four serial bacterial concentrations, from 10 to 104 CFU/mL, were chosen to set up the co-culture system. After pre-incubation of HaCaT cells with S. aureus for 1 hour, PRG or EPG accounted for different volume ratio was added to the co-culture system. In addition, in order to observe the direct effect on HaCaT cells, EPG was also added to the cell culture medium without bacteria. After 12, 24, 36 and 48 hours of intervention, cell proliferation was determined by Cell Counting Kit-8 (CCK-8) assay. Western blotting, quantitative reverse transcription (qRT-PCR), immunofluorescence and ELISA were used to determine the expression of miRNA, protein and cytokines, and the co-culture system was established in 6-well plates and divided into four groups: the H group, culture of HaCaT cells alone; the HP group, uninfected HaCaT cells interfered with EPG; the HS group, co-culture of HaCaT cells with S. aureus; and the HSP group, S. aureus-infected HaCaT cells interfered with EPG. The components of the co-culture system are shown in Table 1.
Evaluation of antibacterial activity
To compare the differential antibacterial ability of PRG and EPG, the bacterial concentration of 104 CFU/mL was chosen. Either PRG or EPG was prepared from equivalent PRP. After 12, 24, 48 and 72 hours of intervention, the bacteria were quantified by plate colony counting method. Furthermore, the antibacterial performance of EPG accounting for 10%, 20% and 30% volume were tested after 24 hours of intervention in the co-culture system with the initial bacterial concentration as 106 CFU/mL.
CCK-8 assay
Cell proliferation was measured according to the instructions of CCK-8. Briefly, 100 µL of DMED containing 10 µL of CCK-8 solution was added to each well and the plate was incubated for 2 hours at 37°C. Then the OD of wells was measured at 450 nm using a microtiter plate reader. Cell proliferation of experimental groups was expressed as a percentage of the HaCaT control group. There is a linear relationship between cell number and OD.
Plate colony counting
The bacterial suspension or cell lysate were collected and underwent serial dilution of up to seven times. Then 100 uL from each dilution was plated onto TSB agar plates. After incubation overnight at 37°C, the number of bacterial colonies was counted manually. The dilution times were adjusted to ensure the number of colonies in each plate was between 30 and 300.
Quantitative reverse transcription
Total RNA from HaCaT cells was extracted by Trizol (Thermo Fisher Scientific, Waltham, MA, USA). The purity of isolated RNA was determined by Nanodrop ND-1000 (Thermo Fisher Scientific). Reverse transcription was performed with PrimeScript RT reagent Kit (Takara Bio Inc., Kusatsu, Shiga, Japan). Quantitative PCR of miRNA-21, IL-6, IL-10, tumor-necrosis factor alpha (TNF-α) and TGF-β1 were performed using Mir-X™ miRNA SYBR® Kit, TB Green™ Premix Ex Taq™ II (Takara Bio Inc.) and iQ5 (Bio-Rad Laboratories Inc., Hercules, CA, USA) detection system supplied with analytical software. Primers were synthesized by RiboBio Co. Ltd (Guangzhou, People’s Republic of China). Forward (F) and reverse (R) primers are described in Table 2. The PCR results were normalized with U6 snRNAs for miR-21, and β-actin mRNAs for IL-6, IL-10, TNF-α and TGF-β1 as internal controls respectively. Results were expressed as relative expression compared with control samples.
  | Table 2 Primers used in the study Abbreviation: TNF-α, tumor-necrosis factor alpha. |
Western blotting
Total proteins from HaCaT cells were extracted by cell lysis buffer, containing supplementary protease and phosphatase inhibitor. Then, the proteins were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membrane. Subsequently, the membrane was blocked with 5% non-fat dry milk in tris buffered saline with Tween 20 buffer and incubated with primary antibodies at 4°C overnight, followed by incubation with secondary antibodies at 37°C for 1 hour. Protein blots were visualized with super-efficient chemiluminescence kit and detected by ChemiDoc XRS device (Bio-Rad Laboratories Inc.) supplied with Image Lab software. Antibodies purchased from Cell Signaling Technology (Danvers, MA, USA) were as follows: rabbit anti-PDCD4 antibody, rabbit anti-β-actin antibody, rabbit anti- phospho-NF-κB p65 antibodyand horseradish peroxidase-conjugated goat anti-rabbit IgG.
Immunofluorescence analysis
Sterile coverslips were pre-placed into a 6-well plate, then HaCaT cells were seeded on the coverslips and the co-culture system of each group was performed according to Table 1. After EPG intervention for 12, 24 and 48 hours, the cells were washed three times by PBS and fixed in 4% paraformaldehyde for 15 minutes. Subsequently the cells were treated with 0.5% Triton X-100 for 20 minutes, blocked with 10% goat serum at 37°C for 30 minutes and incubated with NF-κB p65 antibodies (1:200; Cell Signaling Technology) at 4°C overnight, followed by incubation with Cy3-conjugated secondary antibodies at 37°C for 30 minutes. Then the cell nucleus was stained with a DAPI for 5 minutes at room temperature. After rinsing with PBS, the coverslips were mounted on glass slides and observed under a fluorescence microscope.
ELISA
Cell culture supernatants in the four groups were collected for cytokine evaluation. The level of IL-6, IL-10, TNF-α, TGF-β1 were measured by enzyme immunoassay kit (Bosterbio, Wuhan, People’s Republic of China). The content of cytokines was calculated through a standard curve obtained by measuring the standards.
Statistical analysis
Data were expressed as mean ± SD. One-way ANOVA and LSD tests were used to analyze all experimental data. Analysis was carried out by SPSS 20.0 (IBM Corporation, Armonk, NY, USA). We used an alpha of 5% (*P<0.05; **P<0.01).
Results
Effect of S. aureus on the proliferation of HaCaT cells
Using the proposed co-culture system in-vitro model for infected cells in DFUs, we found that increasing initial concentrations of S. aureus results in a dose-dependent decline of HaCaT cells proliferation (Figure 1). For instance, there was significant reduction in cell proliferation after co-culture for 12 hours at a baseline bacterial concentration of 103 CFU/mL (P<0.05). Furthermore, independent to the initial concentration of bacteria, cell proliferation was severely impaired after 36 hours of co-culture (P<0.01) and almost complete destroyed by bacteria after 48 hours (P<0.01).
The antibacterial effect of PRG and EPG
Initially, the baseline bacterial concentration was set at 104 CFU/mL to evaluate the antibacterial activity of PRG and EPG (Figure 2A). Compared to the PPP group, both PRG and EPG had a significant reduction in bacterial count within 12 hours (P<0.01). In the next 36 hours, there were gradual increases in the bacterial counts but there were no statistical differences between the two groups (P<0.05). Moreover, in the previous 24 hours, the EPG group exhibited a higher bacterial growth rate than the PRG group (P<0.05). Secondly, the higher initial bacterial concentration (106 CFU/mL) was used to further assess the antibacterial activity of EPG. It was found that the bacterial count decreased considerably after EPG intervention for 24 hours (P<0.05) and this antibacterial effect was concentration dependent. For instance, 30% EPG suppressed the extracellular (Figure 2B) and intracellular (Figure 2C) bacteria concentration significantly (P<0.01).
EPG protects cells from bacterial damage and promotes cell proliferation
In Figure 3, EPG demonstrate that it could protect HaCaT cells from S. aureus with an initial bacterial concentration of 103 CFU/mL. Cell proliferation was restored in the first 24 hours with 20% EPG and promotion of cell proliferation occurred during the next 24 hours, while 10% EPG was unable to achieve this effect. Compared to the control group, cell proliferation was stimulated after 36 hours in a concentration-dependent manner when EPG was added to HaCaT cells in the absence of S. aureus infection.
EPG regulate miRNA-21, PDCD4 and NF-κB signaling pathway activity in HaCaT cells
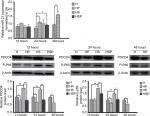
Before extraction of RNA from HaCaT cells, the four groups were photographed with an inverted microscope (Figure 4). In the HS group, there were hardly any viable cells while EPG displayed remarkable antibacterial effect in the HSP group. Increased cell proliferation was observed more in HaCaT cells in the HP group than in the H group at 48 hours after treatment with EPG. As Figure 5 illustrates, in the early stage at the 12th hour, application of 20% EPG did not cause significant changes to miRNA-21 in the experimental group. But at the 24th hour, increased miRNA-21 level was observed in the HSP group. Meanwhile, miRNA-21 level in the HP group was significantly increased after 24 and 48 hours of EPG intervention. At the 48th hour, due to the extensive damage caused by bacteria in the co-culture system, insufficient RNA was extracted from residual HaCaT cells in the HS group. Thus, no data were available for comparison of HS and HSP groups. Moreover, miRNA-21 level was found to be elevated in the HP group, and this elevation was more pronounced at the 48th than the 24th hour when compared to the H group. In the setting of bacterial infection, the expression of PDCD4 and p-NF-κB p65 protein was greatly stimulated in the HS group at the 12th and 24th hour. After intervention with EPG, PDCD4 and p-NF-κB p65 proteins were significantly reduced when the HS was compared to the HSP group. Results associated with bacterial infection at the 48th hour were not reported because of insufficient protein extracted from residual HaCaT cells. In comparison to the H group, PDCD4 and p-NF-κB p65 protein in the HP group were down-regulated by EPG at the 24th and 48th hour.
  | Figure 4 Images of the four groups under light microscope. Notes: Photos were taken before extraction of RNA from HaCaT cells at various time points. Scale bar: 100 µm. |
On the basis of detection in p-p65 protein expression, the translocations of p65 unit of NF-κB from cytosol to nucleus were determined in the four groups. As Figure 6 shows, antibody against NF-κB p65 produced red signal under fluorescence microscope, whereas the blue signal was produced by the DAPI staining of the cell nuclei. Areas where the red and the blue fluorescence overlapped appeared pink. In the H and HP groups, HaCaT cells exhibited red signal predominantly in the cytoplasm. Bacterial infection induced the pink staining in the HS group, indicating an increase in the nuclear translocation of NF-κB p65 protein. While after intervention with EPG, significantly reduced intensity of pink fluorescence was observed in the HSP group, suggesting EPG blocked the nuclear translocation of the NF-κB p65 protein.
  | Figure 6 Immunofluorescence microscopy analysis of the spatial localization of the NF-κB p65 protein. |
EPG promotes anti-inflammatory cytokines and inhibits pro-inflammatory cytokines
To examine the effect of EPG intervention on inflammation during bacterial infection, the mRNA expressions and concentrations of anti-inflammatory (IL-10 and TGF-β1) and pro-inflammatory cytokines (IL-6 and TNF-α) were measured in HaCaT cells and cultured supernatant respectively (Figures 7 and 8). Bacterial infection promoted the expression and secretion of IL-6 and TNF-α in the HS group. Whereas the intervention of EPG reduced the expression and release of these two pro-inflammatory cytokines in the HSP group, indicating an inhibition of inflammatory response. The same anti-inflammatory effect was also observed in the absence of bacterial infection in the HP group. In addition, a decrease in IL-10 expression and secretion were observed in the HS group but these were increased by EPG intervention in the HSP group. The expression and secretion of TGF-β1 were also increased by EPG intervention in the HSP group.
Discussion
Metabolic disorders and immune abnormalities in patients with diabetes increase the susceptibility of wounds to bacterial infections. Infection further aggravates these ulcers and impedes the healing process. S. aureus is a resident bacterium of human skin and colonizes in wounds after skin damage. It is one of the dominant sources of bacterial infection in DFUs.3 Bacteria causes cell necrosis and apoptosis by invading cells and releasing toxins. Bacteria also activates the body’s immune system, stimulating release of tissue-lysing enzymes and ROS from immune cells to cause tissue damage; causing sustained or excessive inflammatory reactions, stagnation of the wound healing process in the inflammatory response period and failure to enter the proliferative phase.21 Keratinocytes are the most important cell types in the epidermis and normally participate in the formation of barriers and protect the body from invading foreign bodies and pathogens. In the process of wound healing, keratinocytes proliferate, migrate and differentiate, and finally complete the re-epithelialization process.22 In addition, keratinocytes play various roles in the immune response of the skin, such as antigen presentation, release of immune mediators, and secretion of various cytokines.23 Thus, with reference to a previous study,24 we co-cultured human HaCaT keratinocytes with S. aureus under high-glucose conditions to establish an in-vitro high-glucose wound infection model. This model allows users to accurately set various conditions and avoid confounding factors. Compared to simple in-vitro antibacterial experiments, it is also possible to simultaneously observe the effects of bacteria and intervention on donor cells. The devised wound model is a good study tool for simulating the infected cells in DFUs and observing wound repair mechanism in vitro.
This study illustrates that HaCaT cells damaged by S. aureus appears to be concentration-dependent. The low concentration of bacteria causes cell proliferation to be severely impaired after 24 hours of co-culture, suggesting that HaCaT cells have immunomodulatory effects on their own, and have a certain resistance to low concentrations of S. aureus virulence in the early stages of cellular damage. High concentrations of bacteria exacerbate the rate of damage. PRG exhibits an antibacterial activity to S. aureus at various time-intervals within 72 hours, while PPP cannot exert this antibacterial effect, suggesting that activated platelets are the cornerstone of PRG function. These observations are consistent with previous reports.25,26 Platelets share many structural and functional properties with other native immune cells and thus play an important role in host immune defense.27 Platelets release alpha particles, antimicrobial peptides, chemokines, peroxides and other substances, all of which have antibacterial effect. These substances can directly inhibit or kill pathogens, and also exert indirect antibacterial effects through chemotaxis and activation of immune cells.28 Platelet concentration in PRG is three to five times that of normal human blood, and large amounts of active substances are released after platelet activation, which forms the basis of PRG to exhibit modest antibacterial effect.
In view of the fact that the jelly-like nature of PRG was difficult to pipette accurately, we extracted EPG from PRG. Our study showed that both of them had equivalent antibacterial capacity within 48 hours, but PRG had a more lasting antibacterial effect than EPG. This phenomenon, on the one hand, may suggest that our extraction method could not completely obtain the platelets and their released substances in PRG. On the other hand, it may also be related to the three-dimensional scaffold structure formed by fibrin polymerization in PRG, adhering to active substances released by platelets and slowly releasing these active substances.29 The sustained-release effect that leads to a more long-lasting therapeutic effect also necessitates the need for the replacement of PRG after covering the wound for 5 days in clinical treatment. However, EPG is considered a more suitable candidate for micro-manipulation, owing to its convenience and accuracy in a liquid form. Combined with the comparative results of antibacterial activity, we believe that EPG was an effective substitute for PRG in experiments within 48 hours, and this finding has not yet been reported before. EPG is found to play a role in our concentration-dependent model. An amount of 20% of EPG can significantly inhibit intracellular and extracellular S. aureus, protect HaCaT cells against S. aureus infection, and promote cell proliferation. In the absence of S. aureus infection, EPG promotes cell proliferation more strongly.
miRNAs are a class of small, non-coding, single-stranded RNAs that specifically recognize and bind to a target messenger RNA (mRNA), causing degradation of the mRNA or hindering its translation, thereby allowing post-transcriptional regulation of the gene.30 Among the miRNA family, miRNA-21 is considered an important member which is involved in the proliferation, differentiation, and apoptosis of regulated cells and associated with the occurrence and prognosis of a variety of tumors.31 Studies have also suggested that miRNA-21 may stimulate re-epithelization of the wound surface.16,32 PDCD4 is a tumor suppressor regulated by miRNA-21 and inhibits cell proliferation by blocking protein translation in eukaryotic cells. The absence or low expression of PDCD4 is associated with tumorigenesis.33 It has been reported that up-regulation of miRNA-21 in lipopolysaccharide-stimulated peripheral blood mononuclear cells can reduce PDCD4, inhibit NF-κB activity and increase IL-10 expression.34 It has also been reported that increased PDCD4 expression in coronary microembolized porcine cardiomyocytes will increase NF-κB activity which result in an increase of TNF-α.35 In either way, PDCD4 can activate NF-κB signaling pathway to influence the regulation of inflammation. NF-κB is an important transcriptional regulator that specifically binds to promoters and enhancers of various genes and plays an important role in the body’s immune response through its regulation of the expression of cytokines, adhesion molecules, chemokines, and immune receptors.36 The most common form of NF-κB is a dimeric form consisting of two family members of the p50 and p65 and is inactivated via the binding with IκB. Upon activation, IκB is degraded, p65 is phosphorylated, and NF-κB is transported from the nucleus into the cytoplasm and functions as a transcription regulator.37
This study found that S. aureus infection caused a simultaneous increase in the levels of PDCD4 and phosphorylated p65 (p-p65) in HaCaT cells. The increase in PDCD4 partially explained the impaired cell proliferation induced by bacterial infection, while the increase in p-p65 and its translocation from cytosol to nucleus reflected the activation of the NF-κB signaling pathway. After 12 hours of EPG intervention, we found that both PDCD4 and p-p65 were down-regulated and the translocation of p65 was significantly reduced, but up-regulation of miRNA-21 was not yet statistically significant at this time point. We speculated that EPG-mediated up-regulation of miRNA-21 was a relatively slow process. The down-regulation of PDCD4 and p-p65 in the early stage was mainly attributed to the bacteriostatic effect of EPG. After 24 hours of EPG intervention, we found that up-regulation of miRNA-21 in HaCaT cells were coordinated by the down-regulation of PDCD4 and p-p65, as well as the reduced p65 translocation to the nucleus. We speculated that this phenomenon was partially related to the inhibition on bacteria by EPG. In addition, it may also be attributed to the up-regulation of miRNA-21 by EPG and subsequent down-regulation of PDCD4 and inhibition of NF-κB activity. In the absence of bacterial infection, we used EPG to directly intervene in the cellular activity, in which the up-regulation of miRNA-21 appeared at the 24th hour but the degree of up-regulation was more pronounced at the 48th hour. This phenomenon also further supported our hypothesis. Synchronous reduction of PDCD4 and p-p65 observed with increased miRNA-21 in sterile conditions confirmed that EPG could regulate PDCD4 through miRNA-21 and thus affected the NF-κB signaling pathway, regardless of its antibacterial effect.
The pro-inflammatory cytokines, IL-6 and TNF-α accumulate in large amounts at the DFUs’ wound surface, making it difficult for inflammation to subside and aggravating tissue damage.5,38 Anti-inflammatory cytokine IL-10 can promote tissue repair by inhibiting these inflammatory responses.5 TGF-β1 can promote the proliferation, migration and differentiation of fibroblasts, accelerate epithelial-mesenchymal transition, coordinate the action of other growth factors, and facilitate wound healing.39 In the present study, it was found that the up-regulation of IL-6 and TNF-α caused by S. aureus infection was consistent with the activation of the NF-κB signaling pathway, and the down-regulation of IL-10 and TGF-β1 also reflects the perverse healing effect caused by the antibacterial infection. After intervention with EPG, the changes of the above-mentioned cytokines can be reversed; the anti-inflammatory cytokines are increased whereas the pro-inflammatory factors are reduced. In the absence of bacterial infection, direct action of EPG on cells validates this anti-inflammatory effect. Combined with the detection of relevant signal molecules, we consider miRNA-21/PDCD4/NF-κB signaling pathway as a direct mechanism for EPG to exert its anti-inflammatory effects, and that EPG also indirectly produces an anti-inflammatory effect by inhibiting bacterial growth and reducing harmful stimuli.
miRNA-21 may play an important role in PRG mediating anti-inflammation and promoting wound repair, and provide further theoretical support for PRG to effectively treat DFUs in clinical practice. Yet our studies have presented some deficiencies. At present, we have not been able to elucidate the mechanism of miRNA-21 changes in HaCaT cells. It has been reported that platelet-released exosomes are rich in miRNAs, and these exosomes can be taken up by other cells,40 suggesting that this change may be due to the direct release of miRNA-21 from platelets. It has also been reported that platelet-released growth factors, such as TGF-β can up-regulate miRNA-21,41 suggesting that this change may also be the result of the action of other platelet-derived substances. Further study is required to elaborate these changes. In addition, the elevated mRNA expression of TGF-β is contradicted by its reduced content in supernatants. This phenomenon may be involved in mRNA translation, protein modification, and protein secretion. The uncertain regulatory mechanism in these processes deserves our further study.
Conclusion
In conclusion, the high-glucose in vitro infection wound model established may be a valuable tool for future similar research. miRNA-21 appears to regulate NF-κB through PDCD4 and play a triple role of antibacterial, anti-inflammatory while stimulating cell proliferation in the diabetic wound model. Therefore, we speculate that miRNA-21 is a promising intervention target and may provide new strategies for the treatment of refractory wounds such as DFUs.
Acknowledgment
This work was supported by the National Nature Science Foundation of China (Grant No. 81500596).
The abstract of this paper was presented at the “54th EASD Annual Meeting of the European Association for the Study of Diabetes” as an oral presentation.
Disclosure
The authors report no conflicts of interest in this work.
References
Ogurtsova K, da Rocha Fernandes J D, Huang Y, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. | ||
Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. | ||
Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112–126. | ||
Spichler A, Hurwitz BL, Armstrong DG, Lipsky BA. Microbiology of diabetic foot infections: from Louis Pasteur to ‘crime scene investigation’. BMC Med. 2015;13:2. | ||
Acosta JB, del Barco DG, Vera DC, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530–539. | ||
Salazar JJ, Ennis WJ, Koh TJ. Diabetes medications: impact on inflammation and wound healing. J Diabetes Complications. 2016;30(4):746–752. | ||
Lipsky BA. Diabetic foot infections: current treatment and delaying the ‘post-antibiotic era’. Diabetes Metab Res Rev. 2016;32(Suppl 1):246–253. | ||
Deng W, Boey J, Chen B, et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care. 2016;25(7):393–397. | ||
Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8(3):307–312. | ||
Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23(5):638–643. | ||
Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion. 2013;53(1):138–146. | ||
Mercier RC, Dietz RM, Mazzola JL, Bayer AS, Yeaman MR. Beneficial influence of platelets on antibiotic efficacy in an in vitro model of Staphylococcus aureus-induced endocarditis. Antimicrob Agents Chemother. 2004;48(7):2551–2557. | ||
Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29(6):556–568. | ||
Nagalla S, Shaw C, Kong X, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117(19):5189–5197. | ||
Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics. 2011;43(10):543–556. | ||
Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J. 2012;9(4):355–361. | ||
Roy S, Sen CK. miRNA in innate immune responses: novel players in wound inflammation. Physiol Genomics. 2011;43(10):557–565. | ||
Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. | ||
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283(2):1026–1033. | ||
Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 2011;3(3):159–166. | ||
Bertesteanu S, Triaridis S, Stankovic M, et al. Polymicrobial wound infections: pathophysiology and current therapeutic approaches. Int J Pharm. 2014;463(2):119–126. | ||
Hosoya A, Lee JM, Cho SW, et al. Morphological evidence of basal keratinocyte migration during the re-epithelialization process. Histochem Cell Biol. 2008;130(6):1165–1175. | ||
Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–691. | ||
Wiegand C, Abel M, Ruth P, Hipler UC. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009;17(5):730–738. | ||
Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–437. | ||
Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25(18):4489–4502. | ||
Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections – complex interactions with bacteria. Front Immunol. 2015;6:82. | ||
Deppermann C, Kubes P. Platelets and infection. Semin Immunol. 2016;28(6):536–545. | ||
Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (Prf): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–e50. | ||
Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113(6):673–676. | ||
Wang W, Li J, Zhu W, et al. MicroRNA-21 and the clinical outcomes of various carcinomas: a systematic review and meta-analysis. BMC Cancer. 2014;14:819. | ||
Wang T, Feng Y, Sun H, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181(6):1911–1920. | ||
Yang HS, Jansen AP, Komar AA, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23(1):26–37. | ||
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11(2):141–147. | ||
Su Q, Li L, Zhao J, Sun Y, Yang H. Effects of trimetazidine on PDCD4/NF-κB/TNF-α pathway in coronary microembolization. Cell Physiol Biochem. 2017;42(2):753–760. | ||
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. | ||
Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. | ||
Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937–2947. | ||
Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2(1):46–53. | ||
Stakos DA, Gatsiou A, Stamatelopoulos K, Tselepis AD, Stellos K. Platelet microRNAs: from platelet biology to possible disease biomarkers and therapeutic targets. Platelets. 2013;24(8):579–589. | ||
Wang T, Zhang L, Shi C, et al. TGF-β-induced miR-21 negatively regulates the antiproliferative activity but has no effect on EMT of TGF-β in HaCaT cells. Int J Biochem Cell Biol. 2012;44(2):366–376. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.