Back to Journals » Open Access Surgery » Volume 17
Peri-Operative Outcomes of Patients with Inflammatory Bowel Disease After the Introduction of an ERAS Protocol – A Retrospective Cohort Review
Authors Lendzion RJ, Sidhu A, D'Souza B
Received 13 October 2023
Accepted for publication 11 January 2024
Published 22 January 2024 Volume 2024:17 Pages 1—9
DOI https://doi.org/10.2147/OAS.S440460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Luigi Bonavina
Rebecca J Lendzion,1,2 Ankur Sidhu,1 Basil D’Souza1,3
1Department of Colorectal Surgery, St. Vincent’s Hospital, Fitzroy, Melbourne, VIC, 3065, Australia; 2Department of Colorectal Surgery, Macquarie University Hospital, Macquarie University, Sydney, NSW, Australia; 3Department of Colorectal Surgery, The Northern Hospital, Epping, Melbourne, VIC, 3076, Australia
Correspondence: Rebecca J Lendzion, Department of Colorectal Surgery, Macquarie University Hospital, 3 Technology Place, Macquarie Park, Sydney, NSW, 2109, Australia, Tel +61 2 9812 3880, Fax +61 2 9812 3886, Email [email protected]
Purpose: Enhanced recovery after surgery (ERAS) programs are evidence-based protocols designed to standardize medical care, improve outcomes, and lower costs. Research shows that ERAS in colorectal surgery is associated with reduced length of stay (LOS) and morbidity, faster recovery and comparable or reduced readmission rates versus traditional models. Very little evidence exists assessing ERAS outcomes in inflammatory bowel disease (IBD) surgery. We hypothesized that ERAS protocols following IBD surgery is associated with a reasonable LOS and morbidity. Secondary aims were to identify factors affecting patient selection for ERAS programs in IBD surgery.
Patients and methods: A retrospective review of 119 patients undergoing abdominal surgery in a high volume IBD tertiary referral centre with a well-established ERAS protocol.
Results: During the study period, 119 patients with IBD underwent surgery. Of these, 78 patients were allocated to an ERAS protocol; compliance was 72%. The ERAS cohort were more likely to have laparoscopic surgery (53.8%), compared to the non-ERAS cohort (N-ERAS) (46.3%). Median hospital stay was significantly shorter in the ERAS cohort compared to the N-ERAS cohort (7 vs 9 days; p < 0.05). Operative time was significantly longer in the N-ERAS cohort (233 ± 73.0 vs 266 ± 96; p = 0.040). Complication rates were higher in the N-ERAS cohort (48.8% vs 37.1%; p = 0.33).
Conclusion: Patients undergoing surgery with an ERAS protocol have improved outcomes compared with patients deemed not suitable for ERAS. Factors affecting suitability are longer operations, a requirement for stoma, malnourished patients, patients with a higher ASA and the commencement of a new fellow to the unit.
Keywords: Crohn’s disease, ulcerative colitis, enhanced recovery after surgery, perioperative care
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and Ulcerative Colitis (UC), is characterized by chronic relapsing, and remitting gastrointestinal inflammation. The availability of a large repertoire of immunosuppressive medications for the treatment of IBD has reduced hospital admission rates.1 Despite these improvements, however, 70–90% of CD patients will require surgery, largely due to disease-related complications or refractoriness to medical treatment.2 While 40–50% of these patients will require further surgery 10–15 years after their index operation.3,4 In UC, 40% of patients requiring inpatient admission eventually proceed to proctocolectomy.5
Australasia has seen a rise in the incidence and prevalence of IBD and has one of the highest rates in the world.6 A cross-sectional study of general practices within Australia found the estimated crude prevalence of IBD was 653 per 100,000 patients; CD: 306 per 100,0000 patients and UC: 334 per 100,000 patients.6
Enhanced recovery after surgery (ERAS) programs are evidence-based protocols designed to standardise medical care, improve outcomes, and lower health care costs. Protocols were developed in colorectal surgery to reduce physiological stress and postoperative organ dysfunction by optimizing perioperative care.7,8 An ERAS protocol typically involves 10–15 components, spanning over the peri-operative period to provide a multimodal pathway to recovery.
Data from observational studies and randomized trials show that ERAS protocols in colorectal surgery are associated with reduced hospital length of stay (LOS) and morbidity, faster recovery, comparable or reduced readmission rates, and cost savings when compared with traditional care.7–10 Despite these benefits, only a few studies have assessed outcomes of ERAS protocols in IBD surgery.11,12 This is likely due to concerns about placing complex, immunosuppressed patients on a more aggressive enhanced pathway. Particularly given that patients undergoing surgery nowadays typically have more severe disease, having tried (and failed) various medications, with resultant malnutrition, anemia, and debilitation due to chronic disease. It is estimated that up to 85% of CD patients awaiting surgery are malnourished.5
This is a single centre retrospective cohort study to assess the feasibility and short-term outcomes of an ERAS protocol in IBD patients undergoing surgery. We also looked at factors that surgeons considered important in excluding patients from an ERAS protocol.
Methods
Patient Population and Study Design
Between January 2013 and December 2019, in a high volume IBD practice, at A Hospital in Melbourne, Australia, 119 patients with IBD underwent abdominal surgery. This study was retrospectively performed and was reported in line with the STROCSS 2021 criteria.13 Eligible patients were identified for inclusion from a database that maintained a recorded of all emergency and elective surgery at the facility. Data was extracted from medical records by authors RL and AS and recorded in a database designed for research and teaching purposes. Ethics approval was obtained from the St Vincent's Hospital Human Research Ethics Committee (HREC) in accordance with the National Health and Medical Research Council (NHMRC) Guidelines on Ethical Considerations in Quality Assurance and Evaluation Activities (2014), and the National Statement on Ethical Conduct in Human Research (2007 updated 2018). Patient consent was not required as per the HREC as the study was performed under Quality Assurance and Evaluation; patient confidentiality was maintained in accordance with the Declaration of Helsinki. This study was registered with the Research Registry – the Unique Identifying Number (UIN) is as follows: researchregistry8745.
Due to the retrospective nature of this research, there was no patient or public involvement. Patients eligible for inclusion were adult patients (>18 years old) who were discussed at a multidisciplinary team meeting and had histologically proven CD or UC with small bowel or colorectal disease involvement requiring surgery (eg, stricturing or fistulising disease). Exclusion criteria was surgery for malignancy.
From January 2013 the Colorectal unit’s ERAS protocol was accepted as the standard of care post-operatively, however, at times, the operating surgeon would decide that a patient would be deemed not suitable for the ERAS protocol. This was recorded in the post-operative orders. Patients not allocated to ERAS (N-ERAS) received individualised post-operative care at the discretion of the treating surgeon.
In the ERAS group, preoperative carbohydrate loading consisted of four drinks the day prior to surgery, and two drinks two hours prior to surgery. Gastrointestinal tubes were not used, and postoperative mobilization and oral intake (high energy, high protein) commenced from four hours post-operatively. The urinary bladder catheter was removed routinely at midnight on day two. All patients were discharged if they complied with the following predefined discharge criteria: (1) pain controlled with oral analgesia; (2) tolerating diet and fluids; (3) passage of first stool or flatus; (4) afebrile and (5) safely mobilizing.
Data Collection and Outcomes
Peri-operative data was collected for each patient. Preoperative data included age, sex, body mass index (BMI), disease type, American Society of Anesthesiologists (ASA) score, preoperative medical therapy, smoking status and hemoglobin and albumin levels. Operative data included history of previous surgery, duration of surgery, intraoperative complications, and additional intraoperative details. Sub-group analysis of patients undergoing right hemicolectomy was planned.
Primary outcome was total post-operative hospital stay (days in hospital). This was defined as the number of days from surgery (day zero) until day of discharge; this did not include days admitted prior to surgery. Secondary outcomes were compliance with ERAS protocol, time to first flatus and stool, overall morbidity (according to the Clavien-Dindo classification), reoperation rate, in-hospital mortality, and 30-day re-admission rates. Compliance with major items of the ERAS protocol was assessed and variations from standard practice recorded.
Statistical Analysis
Descriptive analysis and data collection were performed using Excel (Microsoft, Redmond, WA). Values were expressed as medians with inter quartile range (IQR) for continuous variables. Chi-squared tests were used to compare categorical data. The Mann–Whitney U-test was used for continuous not normally distributed outcomes. For continuous normally distributed data, the independent sample t-test was used. A 2-sided P value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 28.0 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
During the study period, abdominal surgery was performed in 60 females and 59 males (Figure 1. Patient demographics are summarized in Table 1). Surgery was performed in 74 patients with CD and 45 patients with UC. Median age was 38 years (range: 18–79 years). The median BMI in CD was 24.9 kg/m2 (range: 14.9–44 kg/m2) and 25.2 kg/m2 (range: 18–41 kg/m2) in UC patients. Twenty-nine patients had a history of smoking; 79% had CD.
 |
Table 1 Patient Demographics |
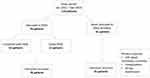 |
Figure 1 Patient cohort. |
Table 2 shows the surgical procedures performed in both groups. The majority of patients were prescribed either one (33; 28%) or two (44; 37%) immunosuppressive drugs. Overall, the mean Albumin level within the cohort was below the normal range (33.2 g/L; range 17–46 g/L). According to the World Health Organisation (WHO) guidelines for anemia,14 the mean hemoglobin level for women with UC was below the recommended guidelines of a hemoglobin level greater than 120 g/L (108.8 g/L; range 79–141 g/L).
 |
Table 2 Surgical Procedures |
ERAS Cohort
During the study period 78 patients were treated with the ERAS protocol; adherence was achieved in 56 (72%) patients. Reasons for failure are discussed below.
Immunosuppressive drugs were used in 68 patients (87.2%). The most commonly used immunosuppressive drugs were Azathioprine (31 patients; median dose 150 (IQR: 112.5–150) mg/day) and Prednisolone (18 patients; median dose 12.5 (IQR: 6.25–25) mg/day). Thirteen patients were receiving either regular or rescue therapy with Infliximab. More than half of patients (42; 53.9%) were prescribed two or more immunosuppressive drugs at the time of surgery.
The delivery of surgery included a combination of emergency (11; 14%) and elective (68; 86%) surgery. The most commonly used surgical approach was laparoscopic surgery (42; 53.8%). Median operating time was 210 (IQR: 185–282.5) minutes.
Median time to flatus was 2 (IQR 1–3) days and median time to first stool was 2 (IQR: 2–4) days. Median LOS was 7 (IQR: 5–9.75) days. There were 29 (37.1%) complications; the majority were grade two (23; 79.3%) and included eight infective complications. In patients with a complication, 24 (83%) were prescribed an immunosuppressive drug (Table 3). Use of an ERAS program was not associated with risk of complications (p = 0.222).
 |
Table 3 Post-Operative Complications and Use of Immunosuppressive Medications |
N-ERAS Cohort
Forty-one patients were excluded from the ERAS protocol (N-ERAS). The most common indications for exclusion were, extensive division of adhesions (DOA) (6;14.6%) and surgery for enterocutaneous fistula (ECF) (3; 7.3%). A reason was not provided in 17 (41.4%) cases; this was most frequently seen in the months following a new clinical fellow joining the unit (Table S1).
Immunosuppressive drugs were used in 33 (80.5%) patients. The most commonly used drugs were Azathioprine (19 patients; median dose 125 (IQR 100–150) mg/day) and Prednisolone (14 patients; median dose 11.25 (IQR 10–25) mg/day). Hydrocortisone was used in four patients. Seven patients were receiving either regular or rescue therapy with Infliximab. Similar to the ERAS cohort, just over half of patients (23; 56%) were prescribed two or more immunosuppressive drugs at the time of surgery.
The delivery of surgery included a combination of emergency (12; 29.3%) and elective (29; 70.7%) surgery. The most commonly used surgical approaches were laparoscopic (19; 46.3%) and open surgery (19; 46.3%). Median operating time was 240 (IQR 190–330) minutes.
Median time to flatus was 2 (IQR 1–3) days and median time to first stool was 2 (IQR 2–5) days. Median LOS was 9 (IQR 7–14) days. There were 20 (48.8%) complications; sixteen classified as grade two. In patients who had a complication, 14 (70%) received a regular immunosuppressive drug.
Primary and Secondary Outcomes
Median hospital stay was significantly shorter in the ERAS cohort compared to the N-ERAS cohort (7 vs 9 days; p < 0.05). Time to postoperative gastrointestinal recovery was similar in both groups (flatus: 2 vs 2 days; p = 0.882; first stool 2 vs 2 days; p = 0.93). Operative time was significantly longer in the N-ERAS cohort (233 ± 73.0 vs 266 ± 96; p = 0.040).
There was no statistical significance in complication rates (48.8% vs 37.1%; p = 0.333) and 30-day readmission rates (14.6% vs 10.2%; p = 0.481) in both groups respectively.
In patients who developed a post-operative complication, 57% were prescribed an immunosuppressive drug at the time of surgery. However, this was not significantly associated with the development of either overall post-operative complications (p = 0.215) or readmission rates (p = 0.254). Use of an anti-TNF drug (p = 0.511) or corticosteroid (CS) (p = 0.746) was not associated with increased incidence of post-operative complications.
Sub-Group Analysis
Ileo-Colic and Right Hemicolectomy – ERAS vs N-ERAS
A total of 35 patients in the entire cohort underwent ileocolic resection or right hemicolectomy. Twenty-six (74.3%) patients were allocated to the ERAS protocol. The remaining nine were excluded due to extensive DOA (2), ECF (1), four quadrant peritonitis (1), difficult anastomosis (1) and surgeon preference (1); a reason was not provided in three cases.
Median time to flatus was 3 days in both ERAS and N-ERAS patients. The median time to first stool was quicker in the N-ERAS group compared to the ERAS group, 3 days vs 4.5 days respectively. The median LOS for patients that were ERAS compliant was 6.55 (IQR 4–7) days, compared to 8 (IQR 7.5–9.5) days in the N-ERAS group; this was not statistically significant (p = 0.103).
Discussion
Since 2013, in our department, use of an ERAS protocol has become the standard of care in IBD patients undergoing abdominal surgery. The program itself was established with the co-ordinated efforts of the Anesthetic, Surgical and Nursing teams, with “ERAS leads” in each group. The obvious benefits of introducing a protocol have been a general understanding of what represents best practice in this complex group of patients along with a standardization of care. This study represents the largest cohort of published outcomes in IBD patients and ERAS in Australia and New Zealand. Our data supports that an ERAS protocol can be safely used in select IBD patients undergoing abdominal surgery.15,16
A prospective, non-randomized study of 29 patients with CD undergoing open ileocolic resection found an ERAS pathway, including epidural analgesia, early oral intake and early mobilization, was associated with reduced LOS without increased morbidity or readmission rates.15 While some authors have reported a prolonged post-operative stay following surgery for CD when an ERAS protocol was used, our results demonstrate that an ERAS pathway can be safely used in IBD patients with a reasonable time to discharge.17
Numerous studies have demonstrated the proven benefits of ERAS in patients undergoing colectomy for neoplasm, and as such this is where the benchmark for outcomes is derived. However, whether this data can be extrapolated and applied to IBD patients remains to be seen. IBD patients are a heterogenous group that undergo more extensive, variable, and often multiple surgical procedures on a background of poor nutrition, long-term immune modulating medications and chronic disease. In 2018, Ban et al18 published a retrospective registry-based cohort study assessing the impact of neoplasm, diverticulitis and IBD on the outcome of ERAS protocols. Patients undergoing colectomy for IBD had higher odds of adverse outcomes (OR, 1.62; 95% CI:1.13–2.32), prolonged length of stay (OR, 1.98; 95% CI: 1.46–2.69) and readmission (OR, 1.54; 95% CI: 1.15–2.08) compared to patients with neoplasm.18 Patients with IBD took longer to achieve adequate postoperative pain control and tolerate a diet.18 Additionally, IBD was identified as an independent predictor of adverse outcomes, including unplanned reoperation and postoperative ventilator dependency.18
Overall morbidity in our ERAS cohort was 37.1%, which is in keeping with the range observed in a recent meta-analysis where morbidity ranged from 5.7% up to 48%.12 Anastomotic leak was 2.6%, which is also consistent with the Vigorita meta-analysis (0−3.4%).12.
Median hospital stay observed in our ERAS cohort is greater than expected and more prolonged (1–2 days more) than that seen following scheduled surgery for colorectal cancer in our institution. This likely reflects the above findings by Ba et al18 as well as the heterogeneity of the operative procedures performed in this cohort. Additionally, IBD patients are often malnourished and immunosuppressed, which increases the risk of postoperative complications and prolongs LOS.11
Despite this however, our LOS is in-fact less than other fast track IBD reports from Enriquez-Navascues et al (8 days)17 and Dai et al (10.9 days).19 Given that the majority of currently available literature in this space has assessed outcomes following fast track surgery in ileocolic resection for CD, we performed a sub-group analysis of this cohort. We found that when only ERAS patients undergoing ileocolic or right hemicolectomy are examined, the median LOS is slightly lower at 6.55 days, which is consistent with other reports.19 So, while our LOS may appear prolonged (when compared to colectomy for neoplasm), it is on par with reported LOS following right sided colectomy in IBD where ERAS protocols are used.17,19 We acknowledge that our data may be limited by selection bias.
Corticosteroids have been shown to be associated with an increased rate of intra-abdominal sepsis following ileocolic resection in patients with CD.20,21 Two papers22,23 have recently demonstrated an increased risk of sepsis following ileocolic resection with either dual therapy with a steroid and a biologic or triple therapy with CS, a biologic, and an immunomodulator. The TREAT register showed steroids at any stage were associated with higher rates of infection.24 The lack of an association between infectious complications and history of CS in our cohort is likely secondary to the low numbers and as such is underpowered to demonstrate a difference. Given this, the authors still recommend a degree of caution in recommending ERAS protocols after operating on this sub-set of patients.
Owing to the heterogeneity of operations performed for CD and the varying use of proximal diversion, the literature with respect to anti-TNFs impact on anastomotic leak has been controversial. Most recently however, the PUCCINI study,25 a multi-centre prospective cohort study aimed to determine if exposure to anti-TNFs is an independent risk factor for post-operative infection following intra-abdominal surgery. In their assessment of 956 patients undergoing intra-abdominal surgery, the authors found that pre-operative use of anti-TNF drugs, as determined by history or drug levels, was not an independent risk factor for post-operative infections.25 In our ERAS cohort, thirteen patients were receiving either regular or rescue therapy with Infliximab, without a significant increase in post-operative complications, although once again, the authors acknowledge small case numbers.
In our cohort the factors associated with non-suitability for ERAS were longer operating time, presence of a stoma, more immunosuppressive medications (in particular CS and anti-TNF agents), low albumin and higher ASA grade – all suggestive of more challenging surgery and pathology in a more malnourished cohort. There were 21 patients deemed not suitable for ERAS who may have been excluded for the reasons above. It was also noted that exclusion from ERAS rates increased with the commencement of a new fellow in the unit. Fellows in this unit tend to operate independently of consultant surgeons and hence may initially take a more conservative approach to avoid perceived risks of ERAS in these patients.
A Polish questionnaire study26 assessing ERAS compliance in colorectal surgeons found high levels of acceptance in areas such as patient education, antibiotic, and venous thromboembolism prophylaxis. However, a number of evidence-based clinical practices were not followed, including no bowel preparation, use of laparoscopy, no drains, early oral intake, and early mobilisation. Similarly, a Spanish study of colorectal surgeons found only 3% were utilising pre-operative carbohydrate loading.27 Interestingly surgeons were more accepting of elements managed by Anesthesiologists.26
We acknowledge that our study is at risk of bias and is limited by its retrospective nature, heterogeneity, low patient numbers and lack of propensity matching. For patients in the N-ERAS cohort a reason was not provided in 41.4% of patients which the authors attributed to surgeon preference or in the context of new staff joining the unit. Due to the retrospective nature and multiple non-significant confounding factors unique to this cohort, the exact indications are challenging to establish and may have resulted in selection bias. We acknowledge that a large multi-centre trial would be of value in further assessing this uniquely challenging and diverse patient cohort.
Despite this, we have the largest single institutional study in Australia and New Zealand reporting safe use of an ERAS protocol in patients with both UC and CD. With acceptable results, our data, although limited, suggests that use of an ERAS program in IBD patients is safe. The authors accept that those rejected from the ERAS cohort, would likely be higher risk and therefore have worse outcomes and longer length of stay.
Our data demonstrates that an ERAS protocol can successfully be implemented in patients undergoing re-do abdominal surgery. Factors deemed important in excluding patients from ERAS are longer operations, a requirement for stoma, malnourished patients, patients with a higher ASA and the commencement of a new fellow to the unit.
Author Contributions
All authors contributed equally as per the IMCJE authorship guidelines. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There has been no financial support for this work that could have influenced its outcome.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterol. 2011;140(6):1729–1737. doi:10.1053/j.gastro.2011.02.012
2. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association institute technical review on the management of crohn’s disease after surgical resection. Gastroenterol. 2017;152(1):277–295. doi:10.1053/j.gastro.2016.10.039
3. Hasegawa H, Watanabe M, Nishibori H, et al. Laparoscopic surgery for recurrent crohn’s disease. Br J Surg. 2003;90:970–973. doi:10.1002/bjs.4136
4. Hindryckx P, Jairath V, D’Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016;13(11):654–664. doi:10.1038/nrgastro.2016.116
5. Stoner PL, Kamel A, Ayoub F, et al. Perioperative care of patients with inflammatory bowel disease: focus on nutritional support. Gastroenterol Res Pract. 2018;2018:1–13. doi:10.1155/2018/7890161
6. Elford AT, Leong RW, Halmos EP, et al. IBD barriers across the continents: a continent-specific analysis – Australasia. Therap Adv Gastroenterol. 2023;16. doi:10.1177/17562848231197509
7. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi:10.1001/jamasurg.2016.4952
8. Scott MJ, Baldini G, Fearon KC, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59(10):1212–1231. doi:10.1111/aas.12601
9. Muller S, Zalunardo MP, Hubner M, et al; Zurich Fast Track Study Group. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroentero. 2009;136(3):842–847. doi:10.1053/j.gastro.2008.10.030
10. Wind J, Polle SW, Fung Kon Jin PH, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–809. doi:10.1002/bjs.5384
11. Peng D, Cheng YX, Tao W, et al. Effect of enhanced recovery after surgery on inflammatory bowel disease surgery: a meta-analysis. World J Clin Cases. 2022;10(11):3426–3435.
12. Vigorita V, Cano-Valderrama O, Celentano V, et al. Inflammatory bowel diseases benefit from Enhanced Recovery After Surgery [ERAS] protocol: a systematic review with practical implications. J Crohns Colitis. 2022;16(5):845–851. doi:10.1093/ecco-jcc/jjab209
13. Mathew G, Agha R, Albrecht J; for the STROCSS Group. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int Surg J. 2021;96:106165. doi:10.1016/j.ijsu.2021.106165
14. Assessing the iron status of populations: report of a joint World Health Organization/ Centres for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, 2nd ed. Geneva:World Health Organization; 2007. Available from:http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107.pdf.
15. Andersen J, Kehlet H. Fast track open ileo-colic resections for crohn’s disease. Colorectal Dis. 2005;7:394–397. doi:10.1111/j.1463-1318.2005.00788.x
16. Xhu Y, Xiang J, Liu W, et al. Laparoscopy combined with enhanced recovery pathway in ileocecal resection for crohn’s disease: a randomized study. Gastroenterol Res Pract. 2018;11. PMID: 30534152; PMCID: PMC6252211. doi:10.1155/2018/9648674
17. Enriquez-Navascues JM, Elorza G, Placer C, et al. Fast track and intestinal surgery for Crohn’s disease: factors associated with prolonged hospital stay. Cir Esp. 2016;94:531–536. doi:10.1016/j.ciresp.2016.09.002
18. Ban K, Berian J, Liu J, et al. Effect of diagnosis on outcomes in the setting of enhanced recovery protocols. Dis Colon Rectum. 2018;61(7):847–853. doi:10.1097/DCR.0000000000001102
19. Dai X, Ge X, Yang Y, et al. Increased incidence of prolonged ileus after colectomy for inflammatory bowel diseases under ERAS protocol: a cohort analysis. J Surg Res. 2017;212:86–93. doi:10.1016/j.jss.2016.12.031
20. McKenna NP, Lightner AL. Preoperative considerations in inflammatory bowel disease. Surg Clin North Am. 2019;99(6):1083–1094. doi:10.1016/j.suc.2019.08.002
21. Alves A, Panis Y, Bouhnik Y, et al. Risk factors for intra-abdominal septic complications after a first ileocecal resection for crohn’s disease: a multivariate analysis in 161 consecutive patients. Dis Colon Rectum. 2007;50(3):331–336. doi:10.1007/s10350-006-0782-0
22. McKenna NP, Habermann EB, Glasgow AE, et al. Intra-abdominal sepsis after ileocolic resection in crohn’s disease: the role of combination immunosuppression. Dis Colon Rectum. 2018;61(12):1393–1402. doi:10.1097/DCR.0000000000001153
23. Serradori T, Germain A, Scherrer ML, et al. The effect of immune therapy on surgical site infection following crohn’s disease resection. Br J Surg. 2013;100:1089–1093. doi:10.1002/bjs.9152
24. Irving PM, Gearry RB, Sparrow MP, et al. Review article: appropriate use of corticosteroids in crohn’s disease. Aliment Pharmacol Ther. 2007;26(3):313–329. doi:10.1111/j.1365-2036.2007.03379.x
25. Cohen B, Fleshner P, Kane SV, et al. Anti-tumor necrosis factor therapy is not associated with post-operative infection: results from prospective cohort of ulcerative colitis and crohn’s disease patients undergoing surgery to identify risk factors for postoperative infection I (Puccini). Gastro. 2019;56:S–80.
26. Kisielewski M, Rubinkiewicz M, Pędziwiatr M, et al. Are we ready for the ERAS protocol in colorectal surgery? Wideochir Inne Tech Maloinwazyjne. 2017;12(1):7–12. doi:10.5114/wiitm.2017.66672
27. Roig JV, García-Fadrique A, Redondo C, et al. Perioperative care in colorectal surgery: current practice patterns and opinions. Colorectal Dis. 2009;11(9):976–983. doi:10.1111/j.1463-1318.2008.01699.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
