Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
PDL1-Based Nomogram May Be of Potential Clinical Utility for Predicting Survival Outcome in Stage III Breast Cancer
Received 18 August 2023
Accepted for publication 19 October 2023
Published 25 October 2023 Volume 2023:15 Pages 731—746
DOI https://doi.org/10.2147/BCTT.S435980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Xi Zhang, Ruzhe Li, Guonian Wang
Department of Anesthesiology, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China
Correspondence: Guonian Wang, Department of Anesthesiology, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, 150081, People’s Republic of China, Email [email protected]
Purpose: Programmed cell death ligand 1 (PDL1) has the predictive and prognostic value in a great deal of cancers. This study aims to explore the expression of PDL1 in stage III breast cancer (BC) and its correlation with clinical outcome.
Methods: The protein expression of PDL1 in tumor tissues was determined by immunohistochemistry (IHC). The correlations between PDL1 and clinicopathological variables were performed by χ²-tests or Fisher’s exact tests. The Cox proportional hazards model was used for univariate and multivariate analysis of the potential prognostic factors. Survival curves were estimated based on Kaplan–Meier analyses, and Log Rank test was used to contrast factors influencing the survival outcome.
Results: On the basis of the semiquantitative scoring method for PDL1 expression, the patients were divided into low PDL1 expression group (109 cases) and high PDL1 expression group (107 cases). PDL1 expression was correlated with positive lymph nodes, positive axillary lymph nodes, postoperative radiotherapy, and CK5/6 expression (P < 0.05). The PDL1 expression in tumor tissues was discovered to be a potential prognostic risk factor with the disease-free survival (DFS) and overall survival (OS) for stage III BC. Moreover, patients with high PDL1 expression showed longer lifetime (DFS and OS) compared to those with low PDL1 expression in total patient population (P < 0.05). Moreover, the nomogram showed that the prediction line is in good agreement with the reference line for postoperative 1-, 3-, and 5-year lifetime. The DCA curve showed that the 3- and 5-year lifetime by nomogram had so much better divination of the clinical application than only by PDL1.
Conclusion: PDL1 is a latent prognostic factor in stage III BC and is closely related to some clinicopathological features. PDL1 expression in tumor tissues is significantly associated with better lifetime rate in stage III BC.
Keywords: breast cancer, programmed death ligand-1, improved individual outcomes, patient stratification, tumor cell
Introduction
Breast Cancer (BC) Incidence Trends
On the basis of the current statistics, breast cancer (BC) represents the most common malignant tumor with the highest mortality in female worldwide, as the main cause of global cancer incidence, seriously endangers women’s health on the face of the earth.1–3 Currently, the incidence of breast cancer is showing an upward trend with each passing year, and onset age becomes younger in average age.4 Compared with patients aged ≥65 years, young patients diagnosed with metastatic cancer under the age of 35 have a significantly lower 5-year disease-free survival rate and a significantly higher incidence of distant metastasis.5 Compared with the data in 2012, the global cancer statistics in 2020 indicated that the mortality of BC in China had significantly increased.6
BC is a Highly Heterogeneous Patient Cohort
The common breast cancer subtype includes Luminal A, Luminal B, HER2-enriched, and triple negative breast cancer (TNBC). Research has pointed out that over 50% of affected individuals in TNBC die within the first 6 months of metastatic disease.7 According to the progress of BC, the disease is divided into four types: stage I BC, stage II BC, stage III BC and stage IV BC. Stage III BC is between stage II and stage IV BC and is located between the distal metastasis and non-metastasis of the focus. Stages I and II breast cancers are typically treated with breast-conserving surgery and radiation therapy. At present, the main curative treatments for stage III BC include surgery, and are supplemented by chemotherapy, radiotherapy, targeted and endocrine therapy.8,9 The stage III BC cannot solve the problem of recurrence and metastasis by expanding the scope of surgery and adding therapy, and the survival period cannot be prolonged, and the quality of life is poor.10 Therefore, it is necessary to look for the effective indicators to predict the prognosis of stage III BC.
Predictive Approach is Essential to Improve Individual Outcomes
The complex action of the immune system in the growth, elimination and metastasis of BC has been the object of increasing attention.11 Recent evidence highlights the essential role of immunotherapeutic targets in BC, especially in advanced breast cancer (ABC).12,13 In recent years, immune checkpoints are supposed to be important therapeutic targets, and immune checkpoint inhibitors (ICIs) have made rapid progress under medical treatment of several tumors, such as lung cancer, melanoma, and lymphoma.14–16 The biomarkers of ICIs can predict the response of immunotherapy, evaluate the efficacy of immunotherapy, and even be related to the prognosis of the disease after treatment. At the moment, programmed death ligand 1 (PDL1) has become the research hotspot of many tumors and prompting immune resistance or immune evasion to endogenous antitumor activity.17,18 Although considerable research has been devoted to PDL1 expression level in BC, less attention has been paid to PDL1 predicted value in stage III BC.
Working Hypothesis
Accurate prognostic models are of major significance in stage III BC. However, there is no prognostic model based on PDL1-based nomogram. Hence, it is essential to investigate the effect of PDL1 on stage III BC and the prognosis of clinical outcome. We hypothesized that PDL1 is related to the prognosis of stage III BC patients, and high expression of PDL1 is related to better tumor prognosis. It is a potential indicator that can be used for prognosis prediction of breast cancer patients. To further improve the clinical utility of PDL1, we will establish a predictive model for PDL1-based nomogram and its correlation with clinical outcome.
Materials and Methods
Patient Selection
The medical records of 216 patients diagnosed as BC in Harbin Medical University Cancer Hospital were retrospectively reviewed from June 2012 to November 2015. The patients were diagnosed with stage III BC following pathology testing and received surgical treatment. All procedures involving human participants were approved by the ethical committee of Harbin Medical University Cancer Hospital (No. 82172192) and had been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All enrolled participants gave their informed consent in writing prior to inclusion in the study.
The inclusion criteria were as follows: (1) diagnosed with stage III breast cancer by histopathology; (2) received operation, included mastectomy/breast-conserving surgery; (3) medical records of patient data were completely available. The exclusion criteria were as follows: (1) received hormone therapy, chemotherapy, and radiotherapy before surgery; (2) received anti-PD1/PDL1 therapy before treatment; (3) with any form of established metastatic disease (stage IV), or other tumors.
Tumor Tissue Samples
The specimens were taken from cancer patients after operation and were using archival, formalin-fixed, and paraffin-embedded (FFPE). A series of 4-μm-thick sections from each specimen were used to determine the histopathological features.
PDL1 Immunohistochemistry (IHC) Staining Assay
Primary antibody against PDL1 (1:1000 dilution, pH 6.0, GB11339A, Servicebio, Wuhan, China) was used. The goat anti-rabbit IgG H&L (1:5000 dilution, AS014, ABclonal, Wuhan, China) was conducted as the secondary antibody. All enrolled samples were the formalin-fixed paraffin-embedded (FFPE) tissue specimens in Harbin Medical University Cancer Hospital. The manual IHC staining was followed according to the standard protocols. 1) Baked slices at 60 °C for 1 hour. 2) Dewaxed in xylene, dehydrated in gradient alcohol (100%, 95%, 90%, 85%, 80%, 75%, 60%, 50%, 30%). 3) Cover the tissue with 3% H2O2 at room temperature for 30 minutes. 4) Microwave repair with citrate buffer. 5) With sheep serum working solution for 30 minutes. 6) Incubate with primary antibodies overnight. 7) Polymer helper for 30 minutes. 8) Incubate with secondary antibodies for 30 minutes. 9) DAB for color development and hematoxylin staining. 10) Gradient dehydration (30%, 50%, 60%, 75%, 80%, 85%, 90%, 95%, 100%, and xylene). 11) Neutral gum sealing piece. 12) The slices were scanned by the Aperio Image Scope system.
Determination of PDL1 Result
PDL1 determination criteria were as follows: (1) determined by semiquantitative scoring method; (2) histological analysis included the density and intensity of stained cells; (3) scoring was done for tumor tissues only; (4) examined independently by investigators blinded to the clinical information of the patients. The density of positively stained cells was as below: (1) 0 for less than 1% stained; (2) 1 for 1%–10%; (3) 2 for 11%–50%; (4) 3 for 51%–75%; (5) 4 for 76%–100%. The staining intensity was as follows: (1) 0 means no staining; (2) 1 indicates light yellow staining; (3) 2 for brown yellow dyeing; (4) 3 for yellowish brown dyeing. The immunoreactivity of PDL1 expression was assessed according to the density and intensity of stained cells. These patients were divided into: (1) negative group, 0–1 score; (2) weakly positive group; 2–4 scores; (3) median-positive, 5–8 scores; (4) strongly positive, 9–12 scores. In this study, up to 4 scores was taken into account low PDL1 expression (negative and weakly positive) and more than 4 was taken into account high PDL1 expression (median-positive and strongly positive).
Survival Time
Disease-free survival (DFS) was defined as the length of time from the date of tumor diagnosis to the date of the first evidence of disease local, distant recurrence at any site, to the date of death from any reason, or to time of the last visit. Overall survival (OS) was defined as the length of time from the date of tumor diagnosis to the date of the first evidence of death from any reason, or to time of the last visit.
Statistical Analysis
IBM SPSS advanced statistics (Statistical Package for Social Sciences), version 22.0, was used to analyze the data, and R (version 3.6.0; Vienna, Austria. URL: http:\\www.R-project.org\). The correlations between PDL1 and clinical and pathologic characteristics variables were performed by χ²-tests for trends or Fisher’s exact tests. The Cox proportional hazards model was used for univariate and multivariate analysis of the potential prognostic factors. Survival curves were estimated based on Kaplan–Meier analyses, and the Log Rank test was used to contrast factors influencing the survival outcome. P value less than 0.05 indicated were considered statistically significant.
Results
Patient Characteristics
From June 2012 to November 2015, 216 patients with stage III BC were enrolled for the current research. The clinical and pathologic data in the patients with stage III BC are described in Table 1. Ninety-two cases were postmenopausal patients with breast cancer, with the mean menopausal age 48.8 ± 4.5 years and ranged from 33 to 56 years. Twenty-four cases of breast cancer with tumor genetic history, included lung cancer, liver cancer, gastric cancer, ovarian cancer, esophageal cancer, bladder cancer, renal cell carcinoma, and cholangiocarcinoma. And 19 cases of breast cancer with basic diseases included hypertension, coronary atherosclerotic heart disease, and diabetes mellitus. Five cases (2.3%) were grade I, 162 (75.0%) cases were grade II, and 49 (22.7%) cases were grade III. Molecular subtypes consisted of 39 (18.1%) cases Luminal A, 34 (15.7%) cases Luminal B HER2 positive, 52 (24.1%) cases Luminal B HER2 negative, 40 (18.5%) cases HER2 enriched, and 51 (23.6%) cases triple negative breast cancer.
 |
Table 1 Patients’ Characteristics |
Expression of PDL1 by Immunohistochemistry and Its Expression Association with the Patients’ Clinical Characteristics
In this study, 216 human BC specimens, 109 patient samples (50.5%, 109/216) were observed to negative or weakly positive, and 107 patient samples (49.5%, 107/216) were observed to median-positive or strongly positive. Figure 1 shows the representative figures of different expressions of PDL1. According to the PDL1 expression, PDL1 was related to positive lymph nodes (P = 0.042), positive axillary lymph nodes (P = 0.030), and postoperative radiotherapy (P = 0.040) (Table 2).
 |
Table 2 PDL1 Protein Expression Association with the Patients’ Clinical Characteristics |
 |
Figure 1 The representative figures of different expressions of PDL1. |
Association PDL1 Expression with the Patients’ Pathology Parameters
Estrogen receptor (ER), progesterone receptor (PR) and Ki-67 were classified into four molecular subgroups as follows: 0–25%, 26–50%, 51–75%, and 76–100%. Human epidermal growth factor receptor 2 (HER-2) classified into two molecular subgroups as follows: negative (immunohistochemical score in the range of 0± to 1+, or 2+ but with a verified negative fluorescence in-situ hybridization test result) and positive (immunohistochemical score in the range of 3+ or 2+ but with a positive fluorescence in-situ hybridization test result). The detailed patients’ pathology parameters of the patients can be found in Table 3. According to the PDL1 expression, PDL1 was associated with CK5/6 expression (P = 0.020).
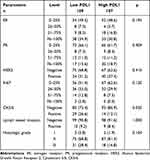 |
Table 3 Association PDL1 Expression with the Patients’ Pathology Parameters |
Association PDL1 Expression with the Nutritional and Blood Parameters
The median carbohydrate antigen153 (CA153), carcinoembryonic antigen (CEA), D-Dimer (D–D), fibrinogen (FBG), hemoglobin (Hb), neutrophils (Neu), and monocyte (Mono) were 12.05 ng/mL, 1.83 ng/mL, 0.16 mg/L, 2.79 g/L, 136g/L, 3.76×109/L, 1.95×109/L, and 0.44×109/L, respectively. Compared with the two groups, there were no significant difference in these nutritional and blood parameters except CA153 (P = 0.029). The detailed patients’ nutritional and blood parameters of the patients could be found in Table 4.
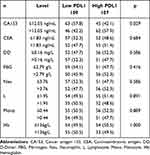 |
Table 4 Association PDL1 Expression with the Nutritional and Blood Parameters |
The Cox Proportional Hazards Model Was Used for Univariate and Multivariate Analysis of the Potential Prognostic Factors
Through the proportional hazards model for DFS and OS, the univariate analysis indicated that menarche age, D–D, Hb, total lymph nodes, PDL1 were associated with the prognosis of stage III BC, and the multivariate analysis showed that menarche age, D–D, Hb, total lymph nodes, annd PDL1 were the potential prognostic factors (Table 5 and Figure 2).
 |
Table 5 Univariate and Multivariate Analyses for Disease Free Survival (DFS) and Overall Survival (OS) |
 |
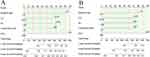
Figure 2 Forest plot of multivariate analyses. (A) Multivariate analyse for DFS, (B) multivariate analyse for OS. |
Association Between PDL1 Expression and Survival Outcomes
In total patient population, patients with high PDL1 expression showed longer DFS and OS compared to those with low PDL1 expression (χ2 = 6.244, p = 0.012 and χ2 = 6.499, p = 0.011). The 1-, 3-, and 5-year survival rates for DFS and OS in the low PDL1 group were 75.7% (95% CI: 0.679–0.843), 62.1% (95% CI: 0.535–0.721), 59.1% (95% CI: 0.504–0.693); and 83.3% (95% CI: 0.766–0.907); 73.0% (95% CI: 0.651–0.819), 64.6% (95% CI: 0.562–0.743), respectively. The 1-, 3-, and 5-year survival rates for DFS and OS in the high PDL1 group were 91.4% (95% CI: 0.862–0.969); 77.5% (95% CI: 0.696–0.863), 73.0% (95% CI: 0.646–0.825), and 97.1% (95% CI: 0.940–1.000); 91.0% (95% CI: 0.855–0.968), 77.4% (95% CI: 0.695–0.862), respectively (Figure 3).
 |
Figure 3 Kaplan–Meier curves for disease free survival (DFS) and overall survival (OS) by PDL1 protein expression in tumor cells. (A) Kaplan–Meier curves for DFS, (B) Kaplan–Meier curves for OS. |
Nomogram Conducted
According to the multivariate analysis, the menarche age, D–D, Hb, total lymph nodes, PDL1 were considered as potential prognostic factors affecting DFS and OS. And the nomogram was used to predict 1-, 3-, and 5-year DFS and OS probability (Figure 4). Moreover, the probability of 1-, 3-, and 5-year DFS and OS was predicted with a C-index of 0.828 (95% CI: 0.774–0.881). Furthermore, the calibration curve has shown that the prediction line was in good agreement with the reference line for postoperative 1-, 3-, and 5-year DFS and OS, and was performed well for the current nomogram (Figure 5).
Predictive Accuracy by Decision Curve Analysis (DCA)
The DCA was used to compare the clinical applicability and effectiveness of the 3- and 5-year DFS and OS nomogram (including menarche age, D–D, Hb, total lymph nodes, PDL1) with that of the PDL1. The DCA curve has shown that the 3- and 5-year DFS and OS by nomogram had so much better divination of the predictive clinical application than by PDL1 (Figure 6).
Discussion
The incidence rate of breast cancer continues to rise, and effective prevention of breast cancer is essential to reduce the overall impact of this disease.19 Identifying high-risk populations through risk prediction and early intervention can help reduce the incidence rate of patients and reduce the burden of social pressure.20 Though the current curative treatment has led to notable improvement in treatment efficiency and survival time of breast cancer, the ABC (stage III) and metastatic breast cancer (MBC) are still short of effective treatments.21,22
The PDL1 Has Potential as a Prognostic Indicator in Cancer
PDL1 is thought to express an inhibitory signal that prohibits the antitumor killing activity of T cells in the process of cancer development and has indicated relationship with survival outcomes in many of cancers.23,24 The blocking-up PDL1 furnishes a new manoeuvre for tumor immunotherapy.25 In Deng M and their colleges’ study, PDL1 expression in tumor tissues was related to the poor OS in intrahepatic cholangiocarcinoma (ICC) specimens, and the CD8+ T-cells are associated with the poor DFS and OS.26 Other study has shown that the biological behavior of NSCLC was more aggressive, and the survival expectancy time was shorter in NSCLC with high PDL1 expression.27 Another study indicated that the PDL1 expression in breast and their matched distant metastases might gain better survival.28 In Zong L’s study, the results performed that the PDL1 expression in tumor cells (TCs) and immune cells (ICs) was different, and the individual assessment of PDL1 in these different cells seems to be more relevant to selecting patients eligible in endometrial cancer patient’s immunotherapy.29 Despite the trend that PDL1 expression in tumor cell seems to predict prognosis in various cancer, the relationship between stage III BC and PDL1 remains not known.26–29 Hence, the expression of PDL1 in stage III BC tissues and its significance was analyzed in the current study.
The Role of PDL1 Expression and Its Correlation with Clinical Outcomes
As one of the immune regulatory marker, PDL1 represents the tumor microenvironment and is thought to reflect the immune function change during cancer development and progression.30,31 Thus far, the role of PDL1 expression and its correlation with clinical outcomes remain considerable controversy. There were reports about the role of increased PDL1 expression related to aggressive clinical behavior or poor prognosis in malignant tumors or breast cancer.32–34 For instance, HHLA2/PDL1 co-expression had an unfavorable impact on the prognoses of patients with ccRCC.32 In soft-tissue sarcomas (STS), the expression of PDL1 refined the prediction of metastatic relapse, and the PDL1 blockade holds the potential to improve patient survival.33 Another study also indicated that the positive expression of PDL1 by IHC was related to worse OS in MPM patients.34 However, some reports indicated that increased PDL1 expression is associated with favorable outcomes in solid cancers and shows no difference with our analysis.35–37 For example, PDL1 expression could improve the prognosis of adrenocortical carcinomas (ACCs), and high PDL1 expression was related to longer DFS.35 One study showed that PDL1 expression in cancer cells was an independent factor of favorable outcome in esophagogastric junction (AEG) and could improve DFS and OS.36 In one study of HER2-positive invasive breast cancer, PDL1 expression might predict a better outcome in this subtype breast cancer managed with HER2-blocking therapy and conventional chemotherapy.37 The divergence may stem from the heterogeneous components of various solid tumors in different studies, and the different definitions of PDL1 condition in every study may also have affected the contradictory results of previous reports. In addition, the different molecular characteristics and carcinogenic mechanisms between BC subtypes or other types of cancers may affect the role of PDL1, and the PDL1 may have relatively diverse roles among many cancers and different BC subtypes.37,38 Furthermore, PDL1 can be expressed on different cell types, and the presence of PDL1 in tumor microenvironment looks like to state clearly an immune resistance to antineoplastic activity.39,40
To our knowledge, the PDL1 expression in stage III BC tissues and its association with different BC subtypes was investigated rarely.41 In the current study, low PDL1 expression was related to poor survival outcomes in stage III BC. This is attributed to the fact that PDL1 expression in tumor cells. In Chen’s study, they performed that low PD-L1 protein expression was associated with significantly worse prognoses and shorter DFS and OS in breast cancer patients, and the protein expression of PDL1 was found to be a significant prognostic factor for patients who received neoadjuvant chemotherapy.42 The results of this study were basically consistent with ours. Their enrolled patients were received neoadjuvant chemotherapy; however, our enrolled patients were stage III breast cancer without undergoing adjuvant therapy. We also observed that the expression of PDL1 varies among different molecular subtypes. In median-positive or strongly positive expression of PDL1, the PDL1 expression was highest in TNBC (26.6%), followed by Luminal B HER2 (-) subtype (25.7%), Luminal B HER2 (+) subtype (16.5%), Luminal A subtype (16.5%), and then HER2 enriched subtype (14.7%). While in median-positive or strongly positive expression of PDL1, the PDL1 expression was highest in HER2 enriched subtype (22.4%), Luminal B HER2 negative subtype (22.4%), followed by triple negative breast cancer (20.6%), Luminal A subtype (19.6%), and then Luminal B HER2 positive subtype (15.0%). Unluckily, this discrepancy among these subtypes was not statistically significant. In Gupta A’ study, they also found that no significant association was observed between PDL1 and molecular subtypes of breast carcinoma.43
In our study, the univariate and multivariable analysis indicated that PDL1 was an independent prognostic factor for stage III BC prognosis. According to the multivariable analysis, we constructed the nomogram based on the independent prognostic factors, including menarche age, D–D, Hb, total lymph nodes, and PDL1, to determine the lifetime in stage III BC. The calibration plots for postoperative 1-, 3-, and 5-year DFS and OS indicated that PDL1-based nomogram predictions were basically accorded with actual observations. Furthermore, the DCA curves showed that PDL1 score-based nomogram offered prognostic assessment of 3- and 5-year DFS and OS had so much better divination of the clinical application. Therefore, PDL1 might be considered a good biomarker to indicate the prognosis of stage III BC. The constructed nomogram might be used to evaluate and predict the survival of stage III BC.
Limitations
There were some limitations which should be considered and counted during the interpretation of our results. Firstly, the utmost limitation of this study is the small sample size with the retrospective nature of the study, and the enrolled samples determined are the primary resection specimens. Despite this limitation, this study relies on a single PDL1 antibody clone, the PDL1 expressing cells and the cutoff values are different via using these antibodies. Additionally, the patients with stage III BC are enrolled and may affect the influence of PDL1 expression on prognosis. Finally, this study does not provide information to determine the predictive value of the efficacy of anti-PDL1 immunotherapy.
Clinically Relevant Conclusions
In conclusion, we demonstrated the comprehensive analysis of the role of PDL1 in stage III BC. We found that PDL1 was a potential prognostic factor in stage III BC. The expression of PDL1 was strongly correlated with the prognosis of stage III BC patients. PDL1 expression in tumor tissues is significantly related to a better DFS and OS rate in stage III BC. The PDL1-based nomogram model we established correctly evaluated the prognosis of stage III BC patients with C-index of 0.828. These findings provided new insights into the relationship between PDL1 and stage III BC and also provided a novel model to predict the survival rate of stage III BC. For the further application of PDL1-based nomogram model in cancer management, we recommend the following:
Predictive Diagnostics
Patients with high expression of PDL1 showed longer DFS and OS compared to those with low expression of PDL1, and PDL1 high expression is dramatically related to better prognosis. It is suggested that PDL1 can be used for predictive diagnosis of breast cancer.
Targeted Prevention
The abnormal expression of PDL1 affects the response rate of immunotherapy. The PDL1 expression in breast cancer may become a basis for tumor immunotherapy. Hence, the PDL1 expression may help us to identify the high-risk populations for targeted prevention and further provide immunotherapy strategies by interfering with the function of PDL1.
Data Sharing Statement
The data used to support the results of this study can be obtained from the corresponding authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (No.82172192).
Disclosure
The authors declare that there are no competing financial interests.
References
1. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi:10.1097/CM9.0000000000002108.
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
3. Mazurakova A, Koklesova L, Samec M, et al. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 2022;13(2):315–334. doi:10.1007/s13167-022-00277-2.
4. Du XL, Song L. Breast cancer incidence trends in Asian women aged 20 or older as compared to other ethnic women in the United States from 2000 to 2018 by time period, age and tumor stage. Cancer Epidemiol. 2022;76:102076. doi:10.1016/j.canep.
5. Wang J, Wang J, Li Q, et al. Young breast cancer patients who develop distant metastasis after surgery have better survival outcomes compared with elderly counterparts. Oncotarget. 2017;8(27):44851–44859. doi:10.18632/oncotarget.15268
6. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
7. Vaz-Luis I, Lin NU, Keating NL, et al. Factors Associated with Early Mortality Among Patients with De Novo Metastatic Breast Cancer: a Population-Based Study. Oncologist. 2017;22(4):386–393. doi:10.1634/thencologist
8. Ben-Dror J, Shalamov M, Sonnenblick A. The History of Early Breast Cancer Treatment. Genes. 2022;13(6):960. doi:10.3390/genes13060960.
9. Loibl S, Poortmans P, Morrow M, et al. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi:10.1016/S0140-6736(20)32381-3.
10. Mangieri CW, Ruffo J, Chiba A, et al. Treatment and Outcomes of Women With Large Locally Advanced Breast Cancer. Am Surg. 2021;87(5):812–817. doi:10.1177/0003134820956335.
11. Badiu DC, Zgura A, Gales L, et al. Modulation of Immune System – strategy in the Treatment of Breast Cancer. In Vivo (Brooklyn). 2021;35(5):2889–2894. doi:10.21873/invivo.12578.
12. Sivaganesh V, Promi N, Maher S, et al. Emerging Immunotherapies against Novel Molecular Targets in Breast Cancer. Int J Mol Sci. 2021;22(5):2433. doi:10.3390/ijms22052433.
13. Esteva FJ, Hubbard-Lucey VM, Tang J, et al. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175–e186. doi:10.1016/S1470-2045(19)30026-9.
14. Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40(6):586–597. doi:10.1200/JCO.21.01497.
15. Curti BD, Faries MB. Recent Advances in the Treatment of Melanoma. N Engl J Med. 2021;384(23):2229–2240. doi:10.1056/NEJMra2034861.
16. Kline J, Godfrey J, Ansell SM. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood. 2020;135(8):523–533. doi:10.1182/blood.2019000847.
17. Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer. 2022;22(3):174–189. doi:10.1038/s41568-021-00431-4.
18. Pezeshki PS, Mahdavi Sharif P, Rezaei N. Resistance mechanisms to programmed cell death protein 1 and programmed death ligand 1 inhibitors. Expert Opin Biol Ther. 2021;21(12):1575–1590. doi:10.1080/14712598.2021.1929919.
19. Kapinova A, Mazurakova A, Halasova E, et al. Underexplored reciprocity between genome-wide methylation status and long non-coding RNA expression reflected in breast cancer research: potential impacts for the disease management in the framework of 3P medicine. EPMA J. 2023;14(2):249–273. doi:10.1007/s13167-023-00323-7.
20. Liskova A, Samec M, Koklesova L, et al. Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021;12(2):155–176. doi:10.1007/s13167-021-00242-5.
21. Lima EOL, Silva MMD. Quality of life of women with locally advanced or metastatic breast cancer. Rev Gaucha Enferm. 2020;41:e20190292. doi:10.1590/1983-1447.
22. Verret B, Sourisseau T, Stefanovska B, et al. The Influence of Cancer Molecular Subtypes and Treatment on the Mutation Spectrum in Metastatic Breast Cancers. Cancer Res. 2020;80(15):3062–3069. doi:10.1158/0008-5472.CAN-19-3260.
23. Gou Q, Dong C, Xu H, et al. PDL1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020;11(11):955. doi:10.1038/s41419-020-03140-2.
24. Liu J, Chen Z, Li Y, et al. PD-1/PDL1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021;12:731798. doi:10.3389/fphar.2021.731798.
25. Emens LA. Breast Cancer Immunotherapy: facts and Hopes. Clin Cancer Res. 2018;24(3):511–520. doi:10.1158/1078-0432.CCR-16-3001.
26. Deng M, Li SH, Fu X, et al. Relationship between PDL1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021;21(1):371. doi:10.1186/s12935-021-02081-w.
27. Arak H, Aytekin A, Canoz O, et al. Prognostic and Predictive Significance of PDL1 Expression in Non-Small Cell Lung Cancer Patients: a Single-Center Experience. Turk Patoloji Derg. 2021;37(3):239–248. doi:10.5146/tjpath.2021.01545.
28. Manson QF, Schrijver WAME, Ter Hoeve ND, et al. Frequent discordance in PD-1 and PDL1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis. 2019;36(1):29–37. doi:10.1007/s10585-018-9950-6.
29. Zong L, Sun Z, Mo S, et al. PDL1 expression in tumor cells is associated with a favorable prognosis in patients with high-risk endometrial cancer. Gynecol Oncol. 2021;162(3):631–637. doi:10.1016/j.ygyno.2021.07.009.
30. Chen S, Crabill GA, Pritchard TS, et al. Mechanisms regulating PDL1 expression on tumor and immune cells. J Immunother Cancer. 2019;7(1):305. doi:10.1186/s40425-019-0770-2.
31. Tashireva LA, Muravyova DT, Popova NO, et al. Parameters of Tumor Microenvironment Determine Effectiveness of Anti-PD-1/PDL1 Therapy. Biochemistry (Mosc). 2021;86(11):1461–1468. doi:10.1134/S0006297921110092.
32. Zhou QH, Li KW, Chen X, et al. HHLA2 and PDL1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000157. doi:10.1136/jitc-2019-000157.
33. Bertucci F, Finetti P, Perrot D, et al. PDL1 expression is a poor-prognosis factor in soft-tissue sarcomas. Oncoimmunology. 2017;6(3):e1278100. doi:10.1080/2162402X.2016.1278100.
34. Rrapaj E, Giacometti L, Spina P, et al. Programmed cell death 1 ligand 1 (PDL1) expression is associated with poor prognosis of malignant pleural mesothelioma patients with good performance status. Pathology. 2021;53(4):462–469. doi:10.1016/j.pathol.2020.09.018.
35. Billon E, Finetti P, Bertucci A, et al. PDL1 expression is associated with longer postoperative, survival in adrenocortical carcinoma. Oncoimmunology. 2019;8(11):e1655362. doi:10.1080/2162402X.2019.1655362.
36. Kollmann D, Ignatova D, Jedamzik J, et al. PDL1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology. 2018;7(6):e1435226.
37. Hou Y, Nitta H, Wei L, et al. PDL1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J. 2018;24(6):911–919. doi:10.1111/tbj.13112.
38. Parvathareddy SK, Siraj AK, Ahmed SO, et al. PDL1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells. 2021;10(2):229. doi:10.3390/cells10020229.
39. Hou J, Zhao R, Xia W, et al. PDL1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z.
40. Sobral-Leite M, Van de Vijver K, Michaut M, et al. Assessment of PDL1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology. 2018;7(12):e1509820. doi:10.1080/2162402X.2018.1509820.
41. Chao X, Liu L, Sun P, et al. Immune parameters associated with survival in metaplastic breast cancer. Breast Cancer Res. 2020;22(1):92. doi:10.1186/s13058-020-01330-6.
42. Chen L, Huang S, Liu Q, et al. PD-L1 Protein Expression Is Associated With Good Clinical Outcomes and Nomogram for Prediction of Disease Free Survival and Overall Survival in Breast Cancer Patients Received Neoadjuvant Chemotherapy. Front Immunol. 2022;13:849468.
43. Gupta A, Chandra S, Chauhan N, et al. Study of PD-L1 Expression with Association of Pathological Factors and Molecular Subtypes in Breast Carcinoma. J Lab Physicians. 2022;14(4):491–496. doi:10.1055/s-0042-1757232.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.