Back to Journals » OncoTargets and Therapy » Volume 16
Optimizing the Continuum of Care in Gastric Cancer
Authors Riccò B, Martinelli G, Bardasi C, Dominici M , Spallanzani A , Salati M
Received 18 May 2023
Accepted for publication 15 November 2023
Published 23 November 2023 Volume 2023:16 Pages 995—1012
DOI https://doi.org/10.2147/OTT.S365505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Daniel Neureiter
Beatrice Riccò,* Giulio Martinelli,* Camilla Bardasi, Massimo Dominici, Andrea Spallanzani, Massimiliano Salati
Department of Oncology and Hematology, University Hospital of Modena, Modena, Italy
*These authors contributed equally to this work
Correspondence: Andrea Spallanzani, Department of Oncology and Hematology, University Hospital of Modena, Via del Pozzo, 71, Modena, MO, 41125, Italy, Tel +390594223310, Fax +390594223289, Email [email protected]
Abstract: Gastric cancer (GC) still ranks as the fifth most common malignancy and the fourth leading cause of cancer-related death worldwide. Despite the recent progress in the therapeutic algorithm of the advanced disease with the advent of immune checkpoint inhibitors (ICIs) and next-generation HER2-directed therapies, survival rates remain poor, with a median survival hardly exceeding 12 months. Furthermore, only 40% of patients remain eligible for second- and later-line treatments due to the aggressiveness of the disease and the rapid deterioration of performance status (PS). Thus, current research is focusing either on the identification of novel treatment options or the development of personalized strategies to optimize the continuum of care and ultimately improve patients’ outcome. In this article, we provide an overview of the current treatment landscape for advanced GC with a particular emphasis on later-line treatments and outline novel perspectives on the horizon.
Keywords: gastric cancer, gastroesophageal cancer, continuum of care, target therapies
Introduction
Over the Twentieth Century, a significant decrease in GC incidence and mortality has been observed thanks to better food preservation,1 Helicobacter pylori eradication,2 screening strategies in endemic areas3,4 and the spread of the Mediterranean diet.5,6 Nevertheless, GC still ranks as the fifth most common malignancy7 and the fourth leading cause of cancer-related death worldwide,8 mainly affecting men older than 60.9 According to the ICBP SURVMARK-2 population-based study in 7 Western countries, more than half of patients with GC have a regional or distant disease at diagnosis, with proportions ranging between 56% and 90% across countries.10 Furthermore, 40–50% of patients systemically relapse after radical surgery despite optimal multimodality management.10
Advanced gastric cancer (AGC) is one of the most aggressive cancer types, with a median overall survival (OS) of only 3 months in untreated patients.11 Nowadays, chemotherapy represents the mainstay of palliative treatment for patients with AGC with the aim of prolonging OS, delaying disease progression and improving cancer-related symptoms.12 Historical outcomes have been meager in this setting, apart from the minority subset of HER2-positive disease in which median OS (mOS) of 16 months has been reported in clinical trials with trastuzumab-based combinations.13
However, after decades of failures, the phase III CheckMate 649 is the first trial to overcome the barrier of 1 year in mOS for the vast proportion of HER2-negative AGC thanks to the addition of the anti-programmed cell death-1 (PD-1) nivolumab to platinum/fluoropyrimidine doublet.14 Interestingly, other novel targets such as fibroblast growth factor receptor 2 (FGFR2b) and Claudin18.2 have been recently validated in first-line randomized phase III trials, thus further exploiting the emerging molecular segmentation of AGC.15,16
In parallel, the last few years have witnessed the advent of an expanding number of evidence-based options in the late-line setting, including the antiangiogenic agent ramucirumab, the new cytotoxics trifluridine/tipiracil and checkpoint inhibitors. Although only moderately active, these newly available compounds have significantly shown to prolong survival in second- and third-line trials depicting a continuum of care for AGC patients. Crucial to this concept are also a close tumour assessment for a prompt recognition of disease progression and the implementation of simultaneous care including the nutritional support early along patient’s journey. As a result, a significantly higher proportion of patients go on to receive multiple lines of therapy and this treatment sequencing is favorably impacting the outcome leading to long-term survival in selected cases.
Notably, unprecedented results have been reported for the HER2-directed antibody-drug conjugated (ADC) trastuzumab deruxtecan (T-Dxd) in refractory patients with confirmatory randomized phase III trials data eagerly awaited.17
Here, we provide an overview of the current treatment landscape for AGC highlighting the most up-to-date evidence that is making the continuum of care a reality in this hard-to-treat cancer.
First-Line Setting
Combination chemotherapy has long been recognized as the standard-of-care for AGC with a platinum/fluoropyrimidine doublet being endorsed by international guidelines as the preferred first-line regimen for unresectable patients.18 In 2008, the REAL-2 phase III trial proved that capecitabine-fluorouracil and oxaliplatin-cisplatin are equally effective in previously untreated esophagogastric cancer (HR 0.86, 95%CI 0.80–0.99 and HR 0.92, 95%CI 0.80–1.10, respectively).19 Besides, the phase III GO2 study suggested that dose-reduced oxaliplatin-based chemotherapy (60% of standard doses of oxaliplatin 130 mg per square meter as per REAL-2 trial) is also feasible for elderly and/or frail patients, showing lesser toxicity and equivalent survival outcomes compared to conventional therapy.20 Nonetheless, the current treatment armamentarium still provides a mOS ranging from 9 to 11 months and a 5-year OS of less than 10%. Furthermore, over the last two decades, several trials failed to identify viable targets in unselected AGC patients’ populations,21,22 with HER2-directed therapies being the only exception. We herein present the state of the art regarding first-line treatment. Table 1 will guide the reader through the major randomized phase III trials in this setting.
 |
Table 1 Major Randomized Phase III Trials in the First-Line Setting |
HER2-Negative Patients
Since the advent of checkpoint inhibitors has revolutionized the oncology arena, various chemo-immunotherapy combinations have been explored in AGC. The KEYNOTE-062 was the first global, randomized, phase III trial evaluating a ICI as a single agent or in combination with chemotherapy in HER2-negative AGC in the first-line setting.30 In this study, pembrolizumab alone, chemotherapy alone (cisplatin plus fluoropyrimidine), or combined therapy was randomly assigned to 763 patients with previously untreated AGC or esophagogastric junction (EGJ) adenocarcinoma with a programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥1 (281 with a CPS ≥10). OS outcomes showed that, when compared to chemotherapy alone, pembrolizumab monotherapy was noninferior in the CPS ≥1 population (10.6 versus 11.1 months, HR 0.91, 99% CI 0.69–1.18) and was nonsuperior in combination with chemotherapy in both CPS ≥1 (12.5 versus 11.1 months, HR 0.85, 95% CI 0.70–1.03, p = 0.05) and ≥10 (12.3 versus 10.8 months, HR 0.85, 95% CI 0.62–1.17, p = 0 0.16) cohorts. Of note, it significantly improved mOS in the CPS ≥10 subgroup analysis (17.4 vs 10.8 months, HR 0.69, 95% CI 0.49–0.97), although this difference was not statistically tested.30
In contrast to the disappointing KEYNOTE-062, the CheckMate 649 trial recently reported practice-changing results for the front-line chemo-immunotherapy combination. In this multicenter phase III trial, 1581 patients with previously untreated, HER2-negative, AGC, EGJ, or esophageal adenocarcinoma (955 with CPS ≥ 5) were randomly assigned to receive nivolumab plus chemotherapy or chemotherapy alone (FOLFOX/XELOX).14 Combined therapy was associated with a significantly better median progression-free survival (mPFS) and OS in all enrolled patients (mPFS 13.8 versus 11.6 months, HR 0.79, 95% CI 0.71–0.88, two-year survival 28 versus 19%). The primary endpoint of the study was met: patients with CPS ≥ 5, had a substantial gain in survival with the combinatory approach (mPFS 14.4 versus 11.1 months, HR 0.70, 95% CI 0.61–0.81, two-year OS 31% versus 19%). Nevertheless, in patients with CPS < 1 (mOS 13.1 versus 12.5 months, unstratified HR 0.95, 95% CI 0.73–1.24), <5 (mOS 12.4 versus 12.3 months, unstratified HR 0.94, 95% CI 0.79–1.11), or <10 (mOS 12.4 versus 12.5 months, HR 0.91, 95% CI 0.78–1.06) there was no survival advantage for nivolumab with chemotherapy compared to chemotherapy alone.14 Based on these data, the Food and Drug Administration (FDA) approved nivolumab in combination with chemotherapy for metastatic gastric and EGJ cancer and esophageal adenocarcinoma irrespective of PD-L1 overexpression; whereas, the European Medicines Agency (EMA) restricted the approval only to patients with PD-L1 CPS ≥ 5.
Reasons of these disappointing data could be found in several factor.
First, the chemotherapy backbone was different in these two studies: in the KEYNOTE-062 a cisplatin-based backbone was used while in the CheckMate 649 was used as oxaliplatin backbone.
From some preclinical Oxaliplatin showed an induced immunogenic cell death by releasing tumor antigens, which led to the secretion of the danger-related molecules.35
On the other hand, cisplatin could down-regulate the activity of different immune cell subsets and immune phenotypes of tumor cells by enhancing antigen presentation and downregulating PD-L1 expression.36
In the two trials there were a different rate of histological diffuse type (40% in the KEYNOTE-062 and 29% in the CheckMate 649 trials), which could be an immune-resistant gastric cancer subtype.
Finally, rates of patients with GEJ adenocarcinomas were different (about 18% in the CheckMate 649 and about 33% in the KEYNOTE-062) and this different site could be less immunogenic.
The benefit of immuno-chemotherapy combinations has been assessed in two other studies. The first is the phase III KEYNOTE-590 trial, enrolling patients with previously untreated advanced/unresectable or metastatic esophageal adenocarcinoma, esophageal squamous cell carcinoma (SCC), or EGJ Siewert type 1 adenocarcinoma regardless of PD-L1 overexpression.37 The OS benefit of pembrolizumab plus chemotherapy was seen in the overall population (6.3 months versus 5.8 months, HR 0.65, 95% CI 0.55–0.76), although the results were mainly driven by the CPS ≥ 10 SCC cohort (13.9 months versus 8.8 months, HR 0.57, 95% CI 0.43–0.75). While FDA-approved pembrolizumab in combination with chemotherapy in first-line treatment of metastatic or locally advanced esophageal or EGJ carcinoma (including adenocarcinoma) regardless of PD-L1 expression, EMA has restricted it to those with a PD-L1 CPS ≥ 10.
The second study is the randomized phase III ATTRACTION-4 trial, in which 724 Asian patients with HER2-negative advanced or recurrent gastric or EGJ adenocarcinoma were randomly assigned to chemotherapy (oxaliplatin plus either S-1 or capecitabine) plus placebo, or the same chemotherapy plus nivolumab.38 When stratified according to PD-L1 overexpression, neither the PD-L1 expressors nor the PD-L1-negative patients had a survival benefit from the immune-chemotherapy combination. This trial used the tumor proportion score (TPS) instead of the CPS to designate the PD-L1 expression. W-811hile CPS represents the total number of PD-L1-positive cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells and multiplied by 100; TPS is the total number of PD-L1-positive tumor cells divided by the total number of viable tumor cells and multiplied by 100. Considering the results of the ATTRACTION-4 trial, TPS may not be as predictive as CPS in upper gastrointestinal tract adenocarcinomas. Furthermore, when analyzing the outcomes of immunotherapy-involving clinical trials, it should be considered that the PD-L1 parameter has some limitations: it is a dynamic marker that can change with local cytokines and the thresholds that separate “positive” and “negative” expression remains under debate.39
Beyond PD-L1, the status of mismatch repair (MMR) proteins/microsatellite instability (MSI) remains the most robust and validated biomarker predictive of immunotherapy response. To this end, the 50 patients with deficient MMR/high-MSI (dMMR/MSI-H) tumours and CPS ≥ 1 enrolled in the KEYNOTE-062 were included in a subgroup analysis. Compared to chemotherapy alone, combined therapy provided a significant benefit in OS (mOS not reached versus 8.5 months, 24-month survival 65 versus 26%) and PFS (mPFS not reached versus 6.6 months), and a twofold higher objective response rate (ORR) (65 versus 37%).40 Furthermore, compared with chemotherapy alone, pembrolizumab monotherapy was also associated with a higher ORR (57 versus 37%), longer duration of response (21.2 versus 7 months), higher PFS (11.2 versus 6.6 months), and prolonged OS (mOS not reached versus 8.5 months, 12-month OS 79 versus 47%). Enhanced benefit for combined therapy among patients with dMMR/MSI-H tumours was also suggested in a subgroup analysis of the CheckMate 649 trial (mOS 38.7 versus 12.3 months, HR 0.38, 95% CI 0.17–0.84).31
HER2-Positive Patients
In the phase III ToGA trial, the combination of the anti-HER2 trastuzumab with standard first-line chemotherapy (six courses of cisplatin plus 5-fluorouracil) was assessed.13 Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) were both used to determine the HER2 status of all tumors, and patients with IHC positive (IHC 3+) or FISH positive tumors were eligible. At a median follow-up of 18 months, a significant increase in OS was found (13.8 versus 11.1 months, HR 0.74, 95% CI 0.60–0.91), and the ORR was greater with trastuzumab (47% versus 35%). Of note, the subgroup analysis showed that in patients with IHC 3+ tumors, trastuzumab was more effective (HR for death 0.66, 95% CI 0.50–0.87), with low efficacy in patients with IHC 2+ tumors (HR 0.78, 95% CI 0.55–1.10), and no activity in those with HER2 gene-amplified (FISH-positive) but non-protein-expressing (IHC 0 or 1+) tumors.13 Therefore, platinum/fluoropyrimidine-based chemotherapy associated with trastuzumab represents the standard first-line therapy for HER2-positive (IHC 3+ and IHC 2+ with FISH positive) AGC.
Finally, the recent results on the use of immunotherapy in the HER2-positive setting should be mentioned. The safety and efficacy of combining pembrolizumab with first-line trastuzumab plus platinum-based chemotherapy were first shown in a single-arm open-label phase II trial of 37 previously untreated patients with HER2-positive esophageal, gastric, or EGJ cancer.41 At a median follow-up of 13 months, 26 out of 37 patients (70%) did not progress after 6 months. Positive preliminary results were reported in the subsequent multicenter phase III KEYNOTE-811 study, in which 692 patients with HER2-positive AGC or EGJ adenocarcinoma were randomly assigned to pembrolizumab or placebo in association with trastuzumab plus chemotherapy.42 In the interim analysis conducted after the first 264 patients (87% with PD-L1 CPS ≥ 1), the ORR was significantly higher in the experimental arm with the addition of pembrolizumab (74% versus 52%, complete responses 11% versus 3%). The median duration of response was 10.6 months with pembrolizumab versus 9.5 months, and more patients in the pembrolizumab group had an ongoing response of more than 6 months (70 versus 61%). Mostly based on this interim analysis, the FDA authorized the combination of pembrolizumab, trastuzumab, fluoropyrimidine, and platinum-based chemotherapy for treating patients with locally advanced or metastatic gastric or EGJ cancer who are ineligible for surgical resection or final chemoradiation. As of today, the Italian Drug Agency (AIFA) has not yet deliberated on the possible future indications of immunotherapy in AGC. However, similarly to EMA, we hope ICIs will be chosen in combination with chemotherapy in patients with high expression of PD-L1 (PD-L1 ≥5), regardless of HER2 status.
Second- and Later-Line Setting
Only in recent years, second-line therapy has become the standard of care for AGC progressing on or after platinum/fluoropyrimidine-based regimens due to the results of several randomized clinical trials16,43–46 and a Cochrane review47 showing improved OS and symptom control over best supportive care (BSC) alone. Convincing evidence from both clinical trials and real-world evidence has demonstrated that subsequent treatment favorably impacts on survival. Interestingly, post-progression survival has a stronger correlation with OS than PFS.
Of note, the uptake of sequential treatments has increased over time so that 40–60% of Western AGC remain candidate to further lines.48–50 This is mainly the result of a rationale delivery of cytotoxics, more effective options, and early implementation of supportive measures.
In this section, we discuss more in detail the evolving field of second- and later-line treatment in AGC, highlighting the main drivers of treatment decision. In Table 2 major phase III trials in this setting are shown.
 |
Table 2 Major Randomized Phase III Trials in Second- and Later Lines Setting |
Chemotherapy
Second-Line monochemotherapy
Several randomized trials showed that monochemotherapy might be beneficial in selected patients compared to BSC alone. A German phase III trial45 compared a 3-week schedule of irinotecan with BSC in patients who had a Eastern Cooperative Oncology Group (ECOG) PS of 0–2. Patients in this trial had received prior fluoropyrimidine/platinum combination and progressed during or within 6 months following first-line therapy. Due to poor accrual, the study terminated prematurely; however, a significantly longer mOS in the irinotecan arm than in the BSC arm was shown among 40 enrolled patients (4 versus 2.4 months, HR 0.48, p = 0.023).
Taxanes are well-recognized cytotoxics endowed with clinically meaningful antitumor activity in AGC. The randomized controlled COUGAR-02 study compared docetaxel and BSC in patients with a progression on a platinum plus fluoropyrimidine first-line regimen. In the docetaxel group, mOS was 5.2 months versus 3.6 months in the BSC group (HR 0.67, p = 0.01). Despite docetaxel was associated with a higher incidence of grade 3–4 neutropenia and infections, patients in this group reported less pain and gastrointestinal symptoms (nausea/vomiting or constipation).46
When used in second-line treatment, some phase II trials showed the usefulness of three-weekly paclitaxel, which improved mOS and PFS in AGC patients who failed a prior line of chemotherapy.56,57 A Japanese multicenter trial investigated the efficacy of biweekly paclitaxel in a cohort of AGC that received prior S-1-based treatment. Among the 41 patients enrolled, paclitaxel was administered at the dose of 140 mg/m2, intravenously on days 1 and 15 of a 28-day lasting cycle. The study showed a 70% disease control rate, mPFS was 111 days, and mOS was 254 days. Major adverse events (grade 3 or 4) were neutropenia (27.5%), anemia (12.5%), diarrhea (2.5%) and sensory neuropathy (2.5%).58 Several phase II studies investigated the efficacy and safety of weekly paclitaxel at the dose of 70–80 mg/m2 h in AGC whose disease progressed after a platinum plus fluoropyrimidine (mostly S-1) or irinotecan first-line therapy.59–62 The schedule had both a good safety profile and efficacy with a response rate (RR) of about 20%. Even though there are no comparison data between three-weekly paclitaxel, BSC and weekly regimen, this latter schedule seems to be more effective and tolerable in patients with poor PS in later lines of therapy. Due to the incidence of infusion-related reactions related to solvent-based paclitaxel, the efficacy and safety profile of nanoparticle albumin-bound paclitaxel (nab-paclitaxel), were investigated in a phase III trial.63 In this study, the efficacy and safety of three-weekly or weekly nab-paclitaxel versus weekly solvent-based paclitaxel were compared and intravenous nab-paclitaxel (260 mg/m2 on day 1 of a 21-day cycle) was noninferior to weekly solvent-based paclitaxel in terms of mOS (10.3 months in the 3-weekly nab-paclitaxel group, 11.1 months in the weekly nab-paclitaxel group, and 10.9 months in the weekly solvent-based paclitaxel group). The most frequent grade 3–4 adverse event was neutropenia. The study suggested that nab-paclitaxel could be a reasonable choice in patients at risk of hypersensitivity reaction to taxanes.
Second-Line Polichemotherapy
Polichemotherapy, especially the combination of paclitaxel and ramucirumab, is considered the second-line standard of care in AGC. The randomized, placebo-controlled, phase III RAINBOW trial, enrolled patients with AGC or GEJ adenocarcinoma whose disease progressed on or within 4 months after first-line chemotherapy (platinum plus fluoropyrimidine with or without an anthracycline). They were randomly assigned to receive ramucirumab 8 mg/kg or placebo intravenously on days 1 and 15, plus paclitaxel 80 mg/m2 intravenously on days 1, 8, and 15 of a 28-days cycle. The primary endpoint was OS. Efficacy analysis was by intention to treat (ITT). OS was longer in the ramucirumab plus paclitaxel group than in the placebo plus paclitaxel group (mOS 9.6 months versus 7.4, HR 0.807, p = 0.017). Grade 3 or higher adverse-events rate was more than 5% in the ramucirumab plus paclitaxel group and included neutropenia, hypertension, fatigue, anemia, and abdominal pain.51 Besides, real-world data confirmed the efficacy and tolerability of this combination in unselected Western and Asian populations.64–66 Altogether, these findings made paclitaxel and ramucirumab the gold standard second-line regimen, which should be considered for every fit patient.
Later-Line Chemotherapy
The TAGS study was a randomized, double-blind, placebo-controlled, phase III trial, which investigated the role of trifluridine/tipiracil in third-line therapy.67 The trial enrolled patients with metastatic gastric adenocarcinoma (including adenocarcinoma of the GEJ) who progressed after at least two previous lines of therapy and randomized them to oral trifluridine/tipiracil (35 mg/m2 twice daily on days 1–5 and days 8–12 every 28 days) or placebo. mOS was 5.7 months in the trifluridine/tipiracil group and 3.6 months in the placebo group. Regarding the safety profile, an increase in hematological toxicity was reported (34% grade 3 neutropenia and 19% grade 3 anemia in the experimental drug versus 8% and 0% in the placebo arm). However, an early interruption of the treatment was reported in 13% of patients in the trifluridine/tipiracil group versus 17% of patients treated with placebo. The survival benefit was confirmed in subsequent subgroup analyses regardless of age and previous gastrectomy and was particularly evident in the third-line setting (mOS 6.8 months versus 3.2 months, HR 0.68, 95% CI 0.47–0.97), with also a significant benefit on time to deterioration of general conditions (4.8 months versus 2 months, HR 0.60, 95% CI 0.42–0.86).54,68 The subsequent quality of life assessment by two different questionnaires (EORTC QLQC30 and QLQ-STO22) displayed interesting results:69 no worsening was reported in the trifluridine/tipiracil cohort, with indeed a median time to deterioration in participants with ECOG PS > 2 of 4.3 months in the experimental group versus 2.3 months in the placebo group (HR 0.69, 95% CI 0.562–0.854) and maintenance of good PS (ECOG 0–1) in 74% of patients at the time of treatment discontinuation.69 For these reasons, trifluridine/tipiracil should be considered the standard of care in the third-line setting for patients able to swallow pills.
Targeted Agents: Antiangiogenics
Ramucirumab is a fully human immunoglobulin IgG1 monoclonal antibody which targets vascular endothelial growth factor receptor 2 (VEGFR2). It showed improvement in OS when used in monotherapy in the phase III double-blind, placebo-controlled REGARD trial.43 In this study, 355 patients whose disease progressed within 4 months on a fluoropyrimidine and platinum-containing first-line chemotherapy or within 6 months of completion of adjuvant therapy were randomized to ramucirumab 8 mg/kg intravenously every 2 weeks or placebo. mOS was 5.2 months in the ramucirumab group and 3.8 months in the placebo group (HR 0.776, p = 0.047). The survival benefit with ramucirumab was not related to other prognostic factors after a multivariable adjustment. The main adverse event was hypertension, which was higher in the ramucirumab group than in the placebo group (16% versus 8%). The mOS benefit was observed even in the sub-group of patients older than 65 years old, so this targeted therapy could be a good option for elderly patients not eligible for chemotherapy.
In a Japanese phase III trial, the VEGFR2 tyrosine kinase inhibitor apatinib showed a significant benefit in OS (6.5 versus 4.7 months) and PFS (2.6 versus 1.8 months) compared to placebo in patients with AGC and EGJ cancer who received at least two prior lines of treatment.55
It is worth mentioning that at the recent ASCO GI, preliminary data from the Integrate IIa phase III trial were presented.70 After 238 events, mOS was 4.5 months for the anti-angiogenic tyrosine kinase inhibitor regorafenib and 4.0 months for placebo (HR 0.70, 95% CI 0.53–0.92, p = 0.011), with almost one out of five patients alive in the study cohort at 12 months (19% versus 6%).70
Second-Line Therapy After Taxane-Based Perioperative Treatment
It is worth to mention that about 13% of the participants in the FLOT4 trial discontinued peri-operative chemotherapy due to progression or lack of efficacy.71 The correct approach to patients who progress on perioperative chemotherapy is yet to be defined and a schedule that does not contain a used drug is to be considered. Monotherapy with ramucirumab could be a reasonable option, as it showed advantages even in the subgroup of patients who progressed during adjuvant or neoadjuvant therapy, with good tolerability, even in those with ECOG PS ≥ 1 or older than 65 years.43
In the phase II RAMIRIS trial, the combination of FOLFIRI and ramucirumab obtained an ORR of 25% in patients pretreated with docetaxel-based chemotherapy.72 To conclude, ramucirumab alone or in combination with FOLFIRI, if feasible, could represent an effective treatment option in patients with early progression during perioperative strategies. Irinotecan alone could be an alternative option for those who have contraindications to antiangiogenic treatment.
Second-Line Immunotherapy
For dMMR/MSI-H AGC that never received immunotherapy in previous lines, pembrolizumab could be a good option, as suggested in the subgroup analysis of the phase II KEYNOTE-158 study (ORR 45.8%, mPFS 11 months, mOS and median duration of response not reached).52 Furthermore, an exploratory subgroup analysis of the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials suggested a benefit in MSI-H AGC that received pembrolizumab with or without chemotherapy in later lines.40
Role of Radiotherapy and Surgery in AGC
As seen above, AGC also needs local treatments to improve palliation of symptoms or to actively support patients through cycles of systemic therapy.
Radiotherapy
Radiotherapy could help reduce localized symptoms, such as bleeding, pain, and obstruction.73 There are no clear data about the efficacy of increased radiation dose.74 Furthermore, concomitant chemoradiotherapy could also lead to better symptom control.75
Surgery
Surgery could help symptoms control and its palliative role is recommended by principal guidelines76,77 . Active role of surgery in terms of improved mOS was investigated in the phase III REGATTA trial that showed no benefit in AGC with a single non-curable factor, which underwent gastrectomy followed by systemic chemotherapy.78 These data showed a clear futility in terms of survival for gastrectomy in patients treated with chemotherapy, therefore this approach is not considered in clinical practice. Another surgical approach that has no clear benefit in terms of survival in AGC patients is Hyperthermic Intraperitoneal Chemotherapy (HIPEC) combined with surgery in patients with peritoneal metastasis only.79–81
Active Supportive Care Measures
Supportive care is a crucial part in AGC management and a key step for the continuum of care optimization, as underlined by a recent phase III randomized controlled trial, in which early integration of interdisciplinary supportive care conferred a 3 months survival benefit compared to standard oncologic care (mOS 14.8 versus 11.9 months, HR 0.68, 95% CI 0.51–0.9, p = 0.021).82 Optimal supportive care should include not only symptom palliation, but also nutritional support, since early skeletal muscle mass depletion has a negative prognostic impact during chemotherapy.83,84 Hence, early nutritional screening and prompt oral, parenteral, or enteral support are necessary to improve anti-cancer treatment which should be administered to every fit patient tolerability, quality of life, and disease outcomes in actively treated patients.18
Weight loss is a multifactorial process and is caused by both disease- and chemotherapy-related symptoms. Gastrointestinal symptoms, such as dysphagia, dyspepsia, nausea, and vomiting, are often linked to mechanical obstruction and can be handled either with a medical or interventional approach. Anti-emetic agents like prokinetics, dopamine antagonists, antihistamines, anticholinergics, and serotonin antagonists, are mainstays in clinical practice.85 Dexamethasone and octreotide are also widely used, especially in bowel obstruction.85 Dysphagia due to proximal masses might also be relieved through endoscopic stenting or palliative radiotherapy.86 The choice between these two approaches should be aided by life expectancy since symptom relief is expected immediately with the first and within 4 to 6 weeks with the latter.87 Finally, palliative surgery may be considered in cases of severe and conservatively unsolvable bowel obstruction in patients with adequate life expectancy.88
Chemotherapy-related anorexia and gastrointestinal symptoms are other common issues that could compromise access to active treatment. Low dose of daily olanzapine showed promising results in terms of weight gain and appetite improvement, which could help increase nutritional and PS.89
Finally, palliative care as defined by the World Health Organization (WHO) should also consider emotional and spiritual needs.90 Early integration of professional psychological and spiritual support should therefore be offered to every AGC diagnosis.
New Treatment Options
HER2-Negative Patients
The therapeutic algorithm of locally advanced and metastatic GC has recently been enriched by ICIs, with nivolumab and pembrolizumab approved in mono- and combination therapy in first- and third-line settings. Considering the positive results in advanced and metastatic GC that pembrolizumab has obtained in the phase II and III KEYNOTE-059,91 KEYNOTE-06192 and KEYNOTE-06230 trials, the randomized phase III KEYNOTE-859 study aims to strengthen the evidence that adding pembrolizumab to standard-of-care first-line chemotherapy improves survival in HER2-negative patients.93 Other novel anti-PD-1 agents, such as sintilimab and tislelizumab, are under investigation. The randomized phase III ORIENT-16 trial is currently evaluating the efficacy of sintilimab plus chemotherapy in the first-line setting. Preliminary results in 650 patients with AGC showed superior OS in combination therapy regardless of PD-L1 expression (mOS 15.2 versus 12.3 months, HR 0.77, 95% CI 0.63–0.94, p = 0.0090), with better outcomes in patients with CPS ≥ 5%.32 The randomized phase III BEIGENE-305 trial aims to compare the addition of tislelizumab to chemotherapy versus chemotherapy plus placebo in 997 patients with AGC94 and results are awaited soon. Promising results might also be obtained from the combination of ICI with anti-VEGF therapies since pre-clinical and clinical data hint that concurrent blockade of VEGFR-2 and PD-1 or PD-L1 enhances antigen-specific T-cell migration and antitumor activity with favorable toxicity.95 A phase I trial showed that adding anti-VEGFR-2 ramucirumab to anti-PD-1 pembrolizumab had a manageable safety profile and favorable antitumor activity in patients with previously treated AGC and other malignancies.95 The combination of the PD-L1 inhibitor avelumab with paclitaxel and ramucirumab is currently under investigation in the single-arm phase II RAP trial.96 Lenvatinib and regorafenib, both antiangiogenic and oncogenic receptors multikinase inhibitors, have been evaluated in addition to immunotherapy in East Asian populations. Lenvatinib plus pembrolizumab showed safe and promising antitumor activity (ORR 69%, 95% CI 49–85) as first- and second-line treatment.97 A phase III clinical trial evaluating the efficacy of first-line pembrolizumab plus lenvatinib plus chemotherapy is currently ongoing.98 The addition of regorafenib to nivolumab had also encouraging antitumor activity and a manageable safety profile in a phase I study.99
The identification of novel targetable biomarkers is also a crucial step in GC treatment development. Currently, the most promising targets are claudin 18.2 and FGFR2. Claudin 18.2 is a tight-junction protein confined to the gastric mucosa, whose epitopes are exposed on the cancer cell surface upon malignant transformation.100 Claudin 18.2 positivity (defined as moderate-to-strong immunohistochemical staining in ≥75% of tumor cells) is found in 24–38% AGC patients.101,102 Zolbetuximab, a chimeric monoclonal antibody that binds claudin 18.2, was well tolerated and exhibited antitumor activity both alone103 and combined with chemotherapy in claudin 18.2 positive participants in the FAST phase II trial.16 At the recent ASCO GI, primary results from the phase III SPOTLIGHT (zolbetuximab plus mFOLFOX6 versus placebo plus mFOLFOX6) were reported.104 Both PFS and OS were improved with zolbetuximab + mFOLFOX6 vs mFOLFOX6 alone: mPFS 10.61 versus 8.67 months (HR 0.751, p = 0.0066) and mOS 18.23 versus 15.54 months (HR 0.750, p = 0.0053).104
The FIGHT phase II trial involved patients with FGFR2b overexpression (occurring in up to 60% of AGC cases) and demonstrated a benefit from the addition of the FGFR2b inhibitor bemarituzumab to mFOLFOX6,15 which led to the ongoing phase III trials FORTITUDE 101 (bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6) and FORTITUDE 102 (bemarituzumab + nivolumab + mFOLFOX6 versus nivolumab + mFOLFOX6) in previously untreated patients. Furthermore, in the second-line setting, the randomized K-Umbrella Gastric Cancer Study aimed to test optimal biomarker-driven targeted agent applications versus standard-of-care chemotherapy. Data presented at ASCO 2022 showed no benefit in the biomarker group over the control arm.105
Finally, given the encouraging results that the novel anti-Trop-2 ADC sacituzumab govitecan showed in the phase I/II IMMU-132-01 basket trial in various cancer cohorts, Trop-2 currently represents a viable target in solid tumours, including GC, regardless of their Trop-2 expression level.106
HER2-Positive Patients
Several preclinical and clinical analyses have suggested a synergistic activity between HER2-targeted treatments and ICI.107 Based on the first interim analysis of the KEYNOTE-811 trial,42 in May 2021 the FDA granted accelerated approval to the combination of pembrolizumab, trastuzumab and fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with advanced HER2-positive gastric or GEJ adenocarcinoma. This randomized phase III trial showed improvement of ORR by 22.7% (74.4% versus 51.9%) in the pembrolizumab arm compared to the placebo arm,42 and data of PFS and OS are awaited soon.
Another compelling approach in HER2-targeted therapy of AGC is the use of T-Dxd, a second-generation ADC consisting of a humanized, monoclonal, anti-HER2 antibody bound to a cytotoxic topoisomerase I inhibitor. In the phase II DESTINY-Gastric0117 trial, treatment with T-Dxd significantly improved OS (12,5 versus 8.4 months, HR 0.59, p = 0.01) with an impressing ORR (51% versus 14%, p < 0.001) compared with chemotherapy in third or later line eastern patients. Similar activity was confirmed also in a small group of Western patients in the phase II single arm Destiny-Gastric02 with an ORR of 39% in the second-line setting.74
Based on the results of these trials, T-Dxd recently gained FDA and EMA approval as monotherapy in HER2-positive patients who have received a prior trastuzumab-based regimen. The phase III DESTINY-Gastric04 trial is currently evaluating T-DXd compared with ramucirumab and paclitaxel in participants who have progressed on or after a trastuzumab-containing regimen and have not received any additional systemic therapy. The phase Ib/II clinical trial DESTINY-Gastric03 is also testing the efficacy of T-DXd in several combinations (chemotherapy and/or immunotherapy), with preliminary results showing tolerability and efficacy for the addition of fluoropyrimidine. Other HER2-ADC are RC48 (disitamab vedotin) and ARX788, which showed antitumor activity in phase I108 and II109 trials, and are currently being tested in phase III (NCT04714190 and ACE-Gastric-02).
Among monoclonal antibodies, margetuximab showed a synergistic effect in a single-arm, phase Ib/II trial with pembrolizumab110 and is being further tested with anti-PD-1 retifanlimab and tebotelimab with or without chemotherapy in first-line setting.111 There is also growing attention towards the HER2-targeted bispecific monoclonal antibodies ZW25 (zanidatamab) and KN026, which have proven safety and efficacy in early phase clinical trials.112,113 Moreover, the CD47 inhibitor ALX148 (evorpacept) has obtained in 2020 fast track designations from FDA for the treatment of patients with gastric/GEJ adenocarcinoma based on an open-label, multicenter phase I clinical trial of ALX148 in combination with pembrolizumab or trastuzumab.108 A phase II/III study to test evorpacept in combination with trastuzumab, ramucirumab, and paclitaxel in second- or third-line setting is ongoing (ASPEN-06).
Besides monoclonal antibodies, tucatinib, a highly selective HER2-directed tyrosine kinase inhibitor (TKI) widely approved for HER2-positive metastatic breast cancer, is being evaluated in the MOUNTAINEER-02 phase II/III study in addition to trastuzumab, ramucirumab and paclitaxel in HER2-positive GC in the second-line setting.114
Finally, future strategies might also involve vaccination against Her-2/neu115 and cellular therapy, for example CT-0508116 and CYNK-101, which lately received FDA fast-track designation.
Conclusion
Despite the recent progresses in the therapeutic algorithm of the advanced disease and the advent of target therapies and ICIs, much work still needs to be done to improve AGC patients’ survival and quality of life. Our efforts need to concentrate on raising the bar beyond the 18 months of median survival (Figure 1). Currently, less than a half of patients who receive first-line therapy manage to receive effective second-line treatment. Thus, current and future research should focus not only on the identification of new treatment options, but also on the implementation of patient selection. A particular attention should be put on the detection of new clinicopathological prognostic and predictive biomarkers. Furthermore, considering the strong impact that sarcopenia has on prognosis and chemotherapy tolerance and efficacy, efficient collaboration between oncologists and clinical nutritionists should be emphasized to reduce the percentage of malnourished patients that still receive inadequate nutritional support.
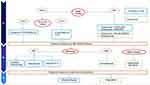
Several clinical trials are ongoing and aim at strengthening and widening the current treatment armamentarium (Table 3). We developed a new treatment algorithm that will be implemented based on the results of the upcoming clinical trials (Figure 2).
 |
Table 3 Selected Ongoing Clinical Trials in AGC |
Abbreviations
GC, gastric cancer; ICIs, immune checkpoint inhibitors; PS, performance status; AGC, advanced gastric cancer; OS, overall survival; mOS, median overall survival; PD-1, programmed cell death 1; FGFR2, fibroblast growth factor receptor 2; ADC, antibody drug conjugate; T-Dxd, trastuzumab deruxtecan; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; ORR, objective response rate; EGJ, esophagogastric junction; PD-L1, programmed cell death ligand 1; CPS, combined positive score; mPFS, median progression-free survival; FDA, Food and Drug Administration; EMA, European Medicines Agency; SCC, squamous cell carcinoma; TPS, tumor proportion score; MMR, mismatch repair; MSI, microsatellite instability; MSI-H, High-microsatellite instability; AIFA, Agenzia Italiana del Farmaco; BSC, best supportive care; ECOG, Eastern Cooperative Oncology Group; RR, response rate; VEGFR-2, vascular endothelial growth factor receptor 2; ITT, intention to treat; TKI, tyrosine kinase inhibitor.
Data Sharing Statement
All data supporting the results reported in the manuscript can be found in https://pubmed.ncbi.nlm.nih.gov
Consent for publication
The details of any images can be published, and the authors provide consent the article contents to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was not supported by any sponsor.
Disclosure
Prof. Dr. Massimo Dominici reports personal fees from Evotec Modena srl, outside the submitted work. The authors have neither financial nor non-financial competing interests.
References
1. Yan S, Gan Y, Song X, et al. Association between refrigerator use and the risk of gastric cancer: a systematic review and meta-analysis of observational studies. PLoS One. 2018;13(8):e0203120. doi:10.1371/journal.pone.0203120
2. Ford AC, Yuan Y, Forman D, Hunt R, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Coch Datab System Rev. 2020;2020:1. doi:10.1002/14651858.CD005583.pub3
3. Choi KS, Jun JK, Suh M, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the national cancer screening programme in Korea. Br J Cancer. 2015;112(3):608–612. doi:10.1038/bjc.2014.608
4. Fan X, Qin X, Zhang Y, et al. Screening for gastric cancer in China: advances, challenges and visions. Chin J Can Res. 2021;33(2):168–180. doi:10.21147/j.issn.1000-9604.2021.02.05
5. Acuna N, Park SY, Le Marchand L, et al. Diet quality and risk of gastric adenocarcinoma: the multiethnic cohort. Am J Clin Nutr. 2023;117(1):46–54. doi:10.1016/j.ajcnut.2022.11.009
6. Tayyem R, AL-Awwad N, Allehdan S, et al. Mediterranean dietary pattern is associated with lower odds of gastric cancer: a case–control study. CMAR. 2022;14:2017–2029. doi:10.2147/CMAR.S360468
7. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
8. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi:10.1002/ijc.33588
9. Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. eClin Med. 2022:47. doi:10.1016/j.eclinm.2022.101404
10. Arnold M, Morgan E, Bardot A, et al. International variation in oesophageal and gastric cancer survival 2012–2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study). Gut. 2022;71(8):1532. doi:10.1136/gutjnl-2021-325266
11. Murad AM, Santiago FF, Petroianu A, Rocha PRS, Rodrigues MAG, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72(1):37–41. doi:10.1002/1097-0142(19930701)72:1
12. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Coch Datab System Rev. 2017;2017(8). doi:10.1002/14651858.CD004064.pub4
13. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi:10.1016/S0140-6736(10)61121-X
14. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2
15. Catenacci DVT, Kang YK, Saeed A, et al. FIGHT: a randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). JCO. 2021;39(15_suppl):4010. doi:10.1200/JCO.2021.39.15_suppl.4010
16. Al-Batran SE, Schuler MH, Zvirbule Z, et al. FAST: an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. JCO. 2016;34(18_suppl):LBA4001–LBA4001. doi:10.1200/JCO.2016.34.18_suppl.LBA4001
17. Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi:10.1056/NEJMoa2004413
18. Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Anna Oncol. 2022;33(10):1005–1020. doi:10.1016/j.annonc.2022.07.004
19. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi:10.1056/NEJMoa073149
20. Hall PS, Swinson D, Cairns DA, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 Phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–877. doi:10.1001/jamaoncol.2021.0848
21. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. JCO. 2011;29(30):3968–3976. doi:10.1200/JCO.2011.36.2236
22. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi:10.1016/S1470-2045(13)70102-5
23. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):481–489. doi:10.1016/S1470-2045(13)70096-2
24. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–1482. doi:10.1016/S1470-2045(17)30566-1
25. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-Negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620–627. doi:10.1001/jamaoncol.2016.5580
26. Fuchs CS, Shitara K, Di Bartolomeo M, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–435. doi:10.1016/S1470-2045(18)30791-5
27. Shah MA, hua XR, Bang YJ, et al. HELOISE: phase iiib randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2–positive metastatic gastric or gastroesophageal junction adenocarcinoma. JCO. 2017;35(22):2558–2567. doi:10.1200/JCO.2016.71.6852
28. Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2–positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—A randomized phase III trial. JCO. 2016;34(5):443–451. doi:10.1200/JCO.2015.62.6598
29. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–1384. doi:10.1016/S1470-2045(18)30481-9
30. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571. doi:10.1001/jamaoncol.2020.3370
31. Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–948. doi:10.1038/s41586-022-04508-4
32. Xu J, Jiang H, Pan Y, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Anna Oncol. 2021;32:S1331. doi:10.1016/j.annonc.2021.08.2133
33. Moehler M, Dvorkin M, Boku N, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. JCO. 2021;39(9):966–977. doi:10.1200/JCO.20.00892
34. Stein A, Paschold L, Tintelnot J, et al. Efficacy of Ipilimumab vs FOLFOX in combination with nivolumab and trastuzumab in patients with previously untreated ERBB2-positive esophagogastric adenocarcinoma: the AIO INTEGA randomized clinical trial. JAMA Oncol. 2022;8(8):1150–1158. doi:10.1001/jamaoncol.2022.2228
35. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi:10.1038/onc.2009.356
36. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi:10.1158/2326-6066.CIR-15-0064
37. Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/S0140-6736(21)01234-4
38. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi:10.1016/S1470-2045(21)00692-6
39. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi:10.5858/arpa.2018-0043-OA
40. Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability–high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7(6):895. doi:10.1001/jamaoncol.2021.0275
41. Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–831. doi:10.1016/S1470-2045(20)30169-8
42. Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–730. doi:10.1038/s41586-021-04161-3
43. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi:10.1016/S0140-6736(13)61719-5
44. Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. JCO. 2012;30(13):1513–1518. doi:10.1200/JCO.2011.39.4585
45. Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47(15):2306–2314. doi:10.1016/j.ejca.2011.06.002
46. Ford HER, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15(1):78–86. doi:10.1016/S1470-2045(13)70549-7
47. Tomita Y, Moldovan M, Chang Lee R, Hsieh AH, Townsend A, Price T. Salvage systemic therapy for advanced gastric and oesophago-gastric junction adenocarcinoma. Coch Datab System Rev. 2020;2020:11. doi:10.1002/14651858.CD012078.pub2
48. Kanagavel D. Second-line treatment of metastatic gastric cancer: current options and future directions. WJG. 2015;21(41):11621. doi:10.3748/wjg.v21.i41.11621
49. Digklia A. Advanced gastric cancer: current treatment landscape and future perspectives. WJG. 2016;22(8):2403. doi:10.3748/wjg.v22.i8.2403
50. Cotes Sanchís A, Gallego J, Hernandez R, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PLoS One. 2020;15(7):e0235848. doi:10.1371/journal.pone.0235848
51. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi:10.1016/S1470-2045(14)70420-6
52. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. JCO. 2020;38(1):1–10. doi:10.1200/JCO.19.02105
53. Maio M, Ascierto PA, Manzyuk L, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Anna Oncol. 2022;33(9):929–938. doi:10.1016/j.annonc.2022.05.519
54. Tabernero J, Shitara K, Zaanan A, et al. Trifluridine/tipiracil versus placebo for third or later lines of treatment in metastatic gastric cancer: an exploratory subgroup analysis from the TAGS study. ESMO Open. 2021;6(4):100200. doi:10.1016/j.esmoop.2021.100200
55. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. JCO. 2016;34(13):1448–1454. doi:10.1200/JCO.2015.63.5995
56. Yamaguchi K, Tada M, Horikoshi N, et al. Phase II study of paclitaxel with 3-h infusion in patients with advanced gastric cancer. Gastric Cancer. 2002;5(2):90–95. doi:10.1007/s101200200015
57. Yamada Y, Shirao K, Ohtsu A, et al. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Anna Oncol. 2001;12(8):1133–1137. doi:10.1023/A:1011680507956
58. Koizumi W, Akiya T, Sato A, et al. Second-line chemotherapy with biweekly paclitaxel after failure of fluoropyrimidine-based treatment in patients with advanced or recurrent gastric cancer: a report from the gastrointestinal oncology group of the Tokyo Cooperative Oncology Group, TCOG GC-0501 trial. Jpn J Clin Oncol. 2009;39(11):713–719. doi:10.1093/jjco/hyp099
59. Kodera Y, Ito S, Mochizuki Y, et al. A Phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric cancer (CCOG0302 Study). Anticancer Res. 2007;2007:1.
60. Shimoyama R, Yasui H, Boku N, et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer. 2009;12(4):206–211. doi:10.1007/s10120-009-0524-9
61. Im CK, Rha SY, Jeung HC, et al. A phase II feasibility study of weekly paclitaxel in heavily pretreated advanced gastric cancer patients with poor performance status. Oncology. 2009;77(6):349–357. doi:10.1159/000265941
62. Matsuda G, Kunisaki C, Makino H, et al. Phase II study of weekly paclitaxel as a second-line treatment for S-1-refractory advanced gastric cancer. Anticancer Res. 2009;29:2863–2867.
63. Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(4):277–287. doi:10.1016/s2468-1253(16)30219-9
64. Di Bartolomeo M, Niger M, Tirino G, et al. Ramucirumab as second-line therapy in metastatic gastric cancer: real-world data from the RAMoss study. Target Oncol. 2018;13(2):227–234. doi:10.1007/s11523-018-0562-5
65. Han HS, Kim BJ, Jee HJ, et al. Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: a nationwide real-world outcomes in Korea study (KCSG-ST19-16). Ther Adv Med Oncol. 2021;13:17588359211042812. doi:10.1177/17588359211042812
66. Longo F, Jorge M, Yaya R, et al. Real-life use of ramucirumab in gastric cancer in Spain: the RAMIS study. Fut Oncol. 2021;17(14):1777–1791. doi:10.2217/fon-2020-1216
67. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–1448. doi:10.1016/S1470-2045(18)30739-3
68. Ilson DH, Tabernero J, Prokharau A, et al. Efficacy and safety of trifluridine/tipiracil treatment in patients with metastatic gastric cancer who had undergone gastrectomy: subgroup analyses of a randomized clinical trial. JAMA Oncol. 2020;6(1):e193531. doi:10.1001/jamaoncol.2019.3531
69. Tabernero J, Alsina M, Shitara K, et al. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer. 2020;23(4):689–698. doi:10.1007/s10120-020-01053-9
70. Pavlakis N, Shitara K, Sjoquist KM, et al. INTEGRATE IIa: a randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—A study led by the Australasian Gastro-intestinal Trials Group (AGITG). JCO. 2023;41(4_suppl):LBA294–LBA294. doi:10.1200/JCO.2023.41.4_suppl.LBA294
71. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/s0140-6736(18)32557-1
72. Lorenzen S, Thuss-Patience P, Pauligk C, et al. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel – results from the phase II RAMIRIS study of the German gastric cancer study group at AIO. Eur J Cancer. 2022;165:48–57. doi:10.1016/j.ejca.2022.01.015
73. Tey J, Back MF, Shakespeare TP, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67(2):385–388. doi:10.1016/j.ijrobp.2006.08.070
74. Tey J, Soon YY, Koh WY, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(15):25797–25805. doi:10.18632/oncotarget.15554
75. Kim MM, Kim MM, Rana V, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47(3):421–427. doi:10.1080/02841860701621233
76. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14(2):97–100. doi:10.1007/s10120-011-0040-6
77. National Comprehensive Cancer Network. Gastric Cancer. Version 2; 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
78. Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–318. doi:10.1016/S1470-2045(15)00553-7
79. Zhang JF, Lv L, Zhao S, Zhou Q, Jiang CG. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: a 12-year meta-analysis of this promising treatment strategy for advanced gastric cancer at different stages. Ann Surg Oncol. 2022;29(5):3170–3186. doi:10.1245/s10434-021-11316-z
80. Granieri S, Bonomi A, Frassini S, et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: a meta-analysis of randomized controlled trials. Eur J Surg Oncol. 2021;47(11):2757–2767. doi:10.1016/j.ejso.2021.05.016
81. Rau B, Feldbrügge L, Gronau F, et al. Indication of hyperthermic intraperitoneal chemotherapy in gastric cancer (Gastripec, Gastrichip). Visc Med. 2022;38(2):81–89. doi:10.1159/000522604
82. Lu Z, Fang Y, Liu C, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase iii randomized controlled trial. JCO. 2021;39(7):748–756. doi:10.1200/JCO.20.01254
83. Rimini M, Pecchi A, Prampolini F, et al. The Prognostic Role of Early Skeletal Muscle Mass Depletion in Multimodality Management of Patients with Advanced Gastric Cancer Treated with First Line Chemotherapy: a Pilot Experience from Modena Cancer Center. J Clin Med. 2021;10(8):1705. doi:10.3390/jcm10081705
84. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22(1):10–22. doi:10.1007/s10120-018-0882-2
85. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Anna Oncol. 2016;27:v119–v133. doi:10.1093/annonc/mdw270
86. Mendelsohn RB, Gerdes H, Markowitz AJ, DiMaio CJ, Schattner MA. Carcinomatosis is not a contraindication to enteral stenting in selected patients with malignant gastric outlet obstruction. Gastrointest Endosc. 2011;73(6):1135–1140. doi:10.1016/j.gie.2011.01.042
87. Bergquist H, Wenger U, Johnsson E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esoph. 2005;18(3):131–139. doi:10.1111/j.1442-2050.2005.00467.x
88. Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res. 2017;93(3):130–136. doi:10.4174/astr.2017.93.3.130
89. Sandhya L, Devi Sreenivasan N, Goenka L, et al. Randomized double-blind placebo-controlled study of olanzapine for chemotherapy-related anorexia in patients with locally advanced or metastatic gastric, hepatopancreaticobiliary, and lung cancer. JCO. 2023;41(14):2617–2627. doi:10.1200/JCO.22.01997
90. Sepúlveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the world health organization’s global perspective. J Pain Symptom Manage. 2002;24(2):91–96. doi:10.1016/S0885-3924(02)00440-2
91. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013
92. Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi:10.1016/S0140-6736(18)31257-1
93. Tabernero J, Bang YJ, Van Cutsem E, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Fut Oncol. 2021;17(22):2847–2855. doi:10.2217/fon-2021-0176
94. hua XR, Arkenau HT, Bang YJ, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line therapy in patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. JCO. 2020;38(4_suppl):TPS458–TPS458. doi:10.1200/JCO.2020.38.4_suppl.TPS458
95. Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(8):1109–1123. doi:10.1016/S1470-2045(19)30458-9
96. Högner A, Breithaupt K, Stein A, et al. RAP: a phase II trial with ramucirumab, avelumab, and paclitaxel as second line treatment in gastro-esophageal adenocarcinoma of the arbeitsgemeinschaft internistische onkologie (AIO). JCO. 2019;37(15_suppl):TPS4148–TPS4148. doi:10.1200/JCO.2019.37.15_suppl.TPS4148
97. Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057–1065. doi:10.1016/S1470-2045(20)30271-0
98. Tabernero J, Cohen D, Van Cutsem E, et al. P-154 A randomized phase 3 study evaluating the efficacy and safety of first-line pembrolizumab plus lenvatinib plus chemotherapy compared with chemotherapy in patients with advanced/metastatic gastroesophageal adenocarcinoma: LEAP-015. Anna Oncol. 2021:32:S151–S152. doi:10.1016/j.annonc.2021.05.209
99. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). JCO. 2020;38(18):2053–2061. doi:10.1200/JCO.19.03296
100. Athauda A, Chau I. Claudin 18.2-a FAST-moving target in gastric cancer? Anna Oncol. 2021;32(5):3. doi:10.1016/j.annonc.2021.02.021
101. Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133–2141. doi:10.1038/s41591-023-02465-7
102. Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open. 2023;8(1):100762. doi:10.1016/j.esmoop.2022.100762
103. Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Anna Oncol. 2019;30(9):1487–1495. doi:10.1093/annonc/mdz199
104. Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: primary results from phase 3 SPOTLIGHT study. JCO. 2023;41(4_suppl):LBA292–LBA292. doi:10.1200/JCO.2023.41.4_suppl.LBA292
105. Rha SY, Lee Kun C, Kim HS, et al. The first report of K-umbrella gastric cancer study: an open label, multi-center, randomized, biomarker-integrated trial for second-line treatment of advanced gastric cancer (AGC). JCO. 2022;40(16_suppl):4001. doi:10.1200/JCO.2022.40.16_suppl.4001
106. Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Anna Oncol. 2021;32(6):746–756. doi:10.1016/j.annonc.2021.03.005
107. Gall VA, Philips AV, Qiao N, et al. Trastuzumab Increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 2017;77(19):5374–5383. doi:10.1158/0008-5472.CAN-16-2774
108. Zhang Y, Qiu M, Wang J, et al. A phase 1 multicenter, dose expansion study of ARX788 as monotherapy in patients with HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma (ACE-Gastric-01). JCO. 2021;39(15_suppl):e16059–e16059. doi:10.1200/JCO.2021.39.15_suppl.e16059
109. Peng Z, Liu T, Wei J, et al. Efficacy and safety of a novel anti‐HER2 therapeutic antibody RC48 in patients with HER2‐overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single‐arm phase II study. Cancer Commun. 2021;41(11):1173–1182. doi:10.1002/cac2.12214
110. Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–1076. doi:10.1016/S1470-2045(20)30326-0
111. Catenacci DV, Rosales M, Chung HC, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Fut Oncol. 2021;17(10):1155–1164. doi:10.2217/fon-2020-1007
112. Meric-Bernstam F, Beeram M, Mayordomo JI, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. JCO. 2018;36(15_suppl):2500. doi:10.1200/JCO.2018.36.15_suppl.2500
113. Gong J, Shen L, Luo S, et al. 1377P Preliminary efficacy and safety results of KN026 (a HER2-targeted bispecific antibody) in combination with KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in patients (pts) with HER2-positive gastrointestinal tumors. Anna Oncol. 2021;32:S1042. doi:10.1016/j.annonc.2021.08.1486
114. Catenacci DVT, Strickler JH, Nakamura Y, et al. MOUNTAINEER-02: phase 2/3 study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—Trial in progress. JCO. 2022;40(4_suppl):TPS371–TPS371. doi:10.1200/JCO.2022.40.4_suppl.TPS371
115. Laeufle R, Maglakelidze M, Bulat I, et al. HERIZON: a phase 1B/2 open-label study of imu-131 HER2/neu peptide vaccine PLUS standard of care chemotherapy with randomization in phase 2 in patients with HER2/neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction. JCO. 2021;39(15_suppl):e16065–e16065. doi:10.1200/JCO.2021.39.15_suppl.e16065
116. Reiss K, Yuan Y, Barton D, et al. 951 A phase 1 first in human study of adenovirally transduced anti-HER2 CAR macrophages in subjects with HER2 overexpressing solid tumors: preliminary safety, pharmacokinetics, and TME reprogramming data. J Immunother Cancer. 2021;9(Suppl 2):A1000–A1000. doi:10.1136/jitc-2021-SITC2021.951
117. Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for research and treatment of cancer gastrointestinal tract cancer cooperative group. JCO. 2000;18(14):2648–2657. doi:10.1200/JCO.2000.18.14.2648
118. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) study. JCO. 2014;32(31):3520–3526. doi:10.1200/JCO.2013.54.1011
119. Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. JCO. 2008;26(9):1435–1442. doi:10.1200/JCO.2007.13.9378
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.