Back to Journals » International Journal of Women's Health » Volume 16
Number of Positive Lymph Nodes and Survival in Endometrial Carcinoma: A Proposal for a Modified Staging
Received 31 August 2023
Accepted for publication 28 December 2023
Published 20 January 2024 Volume 2024:16 Pages 99—109
DOI https://doi.org/10.2147/IJWH.S438064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Lianwei Li,1 Mengsi Zhang,2 Chao Na3
1Department of Gynecology, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, 150081, People’s Republic of China; 2Medical Record Statistics Department, The Second Hospital of Heilongjiang Province, Harbin, Heilongjiang, 150028, People’s Republic of China; 3Integrated Traditional Chinese and Western Medicine Rehabilitation Medical Center, Heilongjiang Provincial hospital, Harbin, Heilongjiang, 150036, People’s Republic of China
Correspondence: Chao Na, Integrated Traditional Chinese and Western Medicine Rehabilitation Medical Center, Heilongjiang Provincial Hospital, No. 82 Zhongshan Road, Xiangfang District, Harbin, Heilongjiang, 150036, People’s Republic of China, Tel +86-13313619817, Email [email protected]
Purpose: To construct a new clinical staging system including the number of lymph node metastases to supplement the International Federation of Gynecology and Obstetrics (FIGO) staging for the prognosis of endometrial carcinoma patients.
Methods: This cohort study retrieved the data of 28,824 patients confirmed as endometrial carcinoma between 2010 and 2015 in the surveillance, epidemiology, and end results (SEER) database. COX risk proportional model was established to evaluate the association between FIGO staging with the all-cause mortality of endometrial carcinoma. The diagnostic value of FIGO staging and the new staging for the mortality of patients were evaluated by receiver operator characteristic curve (ROC). Hazard ratio (HR) and 95% confidence interval (CI) were effect size.
Results: The 5-year survival rate of all participants was 77.21%. The median follow-up time was 60.00 (60.00,60.00) months. Patients at FIGO staging IB (HR=1.75, 95% CI: 1.62– 1.90), FIGO staging II (HR=2.22, 95% CI: 2.00– 2.47), FIGO staging IIIA (HR=2.74, 95% CI: 2.43– 3.09), FIGO staging IIIB (HR=4.07, 95% CI: 3.48– 4.76), FIGO staging IIIC1 (HR=3.84, 95% CI: 3.52– 4.20), FIGO staging IIIC2 (HR=4.52, 95% CI: 4.09– 4.99), FIGO staging IVA (HR=5.56, 95% CI: 4.58– 6.74), and FIGO staging IVB (HR=7.62, 95% CI: 6.94– 8.36) were associated with increased risk of all-cause mortality of endometrial carcinoma patients. After adding positive lymph nodes as another covariate in Model 3, the effect on of FIGO staging survival was reduced when the FIGO staging was higher than stage III/IV. The C-index of the new staging 0.781 (95% CI: 0.774– 0.787) was higher than FIGO staging 0.776 (95% CI: 0.770– 0.783).
Conclusion: Our new staging using the number of positive lymph nodes supplement to the FIGO staging was superior than the FIGO staging for predicting the prognosis of endometrial cancer patients, which might help more accurately identify endometrial carcinoma patients who were at high risk of mortality and offer timely treatments in these patients.
Keywords: positive lymph nodes, FIGO staging, endometrial cancer, prediction
Introduction
Endometrial carcinoma is a type of epithelial malignancy that arises from the endometrium, which ranks among the most prevalent gynecological malignancies.1 According to the epidemiological survey, the number of new cases of endometrial carcinoma exceeded 410,000 globally in 2020, making endometrial carcinoma the sixth most common cancer among women, and the morbidity and mortality of endometrial carcinoma are both on the rise.2–4 To identify more reliable biomarkers related to the prognosis of endometrial carcinoma patients was necessary. Previous evidence suggested that lymph node involvement is an important factor affecting the survival of endometrial carcinoma patients.5 The 5-year survival rate of endometrial carcinoma patients is more than 80% for those without lymph node metastasis, but only about 60% for those with lymph node metastasis.6,7
Currently, the International Federation of Obstetrics and Gynecology (FIGO) and the American Joint Committee on Cancer (AJCC) staging systems are the main tools for assessing the prognosis of endometrial carcinoma, and both staging systems assess lymph node involvement based on the site of lymph node metastasis (pelvic/para-aortic).8,9 In addition to the site of lymph node metastasis, the number of lymph node metastases is also a vital index to assess the prognosis of endometrial carcinoma patients.10 A previous study revealed that the survival of patients with FIGO IIIC1 endometrial carcinoma with multiple lymph node metastases was significantly worse than patients with only one lymph node metastasis.11 There was evidence indicated that the number of positive lymph nodes is an important supplement of AJCC stage for improving the accuracy of evaluation and prognosis stratification for breast cancer, gastric cancer and other cancers.12,13 In view of this, we speculated that the number of lymph node metastases as a supplement to FIGO staging might increase the accuracy for predicting the prognosis of endometrial carcinoma patients.
The current study planned to construct a new clinical staging system including the number of lymph node metastases to supplement the FIGO staging based on the data from the surveillance, epidemiology, and end results (SEER) database. Subgroup analysis was conducted to evaluate the prognostic value of the new staging system in endometrial carcinoma patients with different numbers of lymph node metastases.
Methods
Study Design and Population
This cohort study retrieved the data of 35,510 patients confirmed as endometrial carcinoma between 2010 and 2015 in the SEER database. SEER abstracts patient-level data from 18 geographically diverse populations that encompass rural, urban, and regional areas, which represents the US population.14 The included criteria of our study were (1) diagnosed as primary endometrial carcinoma based on the primary site code the international classification of diseases for oncology-3 (ICD-O-3), (2) age at diagnosis ≥18 years old, and (3) FIGO/AJCC stage I–III. The exclusion criteria were as follows: (1) patients with reported diagnosis source from autopsy or death certificate or only clinically diagnosed, (2) who did not examine lymph nodes, (3) unknown number of positive lymph nodes, (4) without complete clinicopathological information and survival data. Finally, a total of 28,824 patients were included.
Data Collection and Definitions
Age (years), race [Black, White and others (American Indian/AK Native, Asian/Pacific Islander)], marital status (married, never married, others and unknown), primary site [isthmus uteri, endometrium, myometrium, fundus uteri, overlapping lesion of corpus uteri, corpus uteri, uterus (not otherwise specified)], histologic type (carcinosarcoma, clear cell, endometrioid, mixed, serous and others), tumor size (<2 cm, 2–5cm, ≥5cm and unknown), T stage (T1, T2, T3 and T4), N stage (N0, N1, and N2), M stage (M0 and M1), FIGO staging (IA, IB, II, IIIA, IIIB, IIIC1, IIIC2, IVA and IVB), tumor grade (Grade I, Grade II, Grade III, Grade IV and unknown), pathologically examined lymph nodes, and positive lymph nodes (0, 1–6 and ≥7) were variables collected in our study.
FIGO staging was calculated based on TNM staging of AJCC 7th Edition. In this study, a novel staging was established based on FIGO staging and the number of positive lymph nodes. The number of positive lymph nodes was divided into three categories according to X-tile software: 0, 1–6 and ≥7. Combined FIGO staging with the number of positive lymph nodes, we obtained IA, IB, II, IIIA, IIIB, IIIC1 (1–6), IIIC1 (≥7), IIIC2 (1–6), IIIC2 (≥7), IVA (0), IVA (1–6), IVA (≥7), IVB (0), and IVB (1–6), and IVB (≥7). After combining the above stages according to the KM curve, they were eventually divided into IA, IB, II, IIIA, IIB (IIIB or IIIC+ positive lymph nodes <7), IVA (IVA or IIIC + positive lymph nodes≥7), IVB (IVB + positive lymph nodes<7), IVC (IVC + positive lymph nodes≥7). Histologic type was defined based on a previous study.15 Carcinosarcoma was defined based on ICO-O-3 codes including 8950, 8951, 8980 and 8981), clear cell was defined based on ICO-O-3 codes including 8313 and 8310, endometriosis was defined based on ICO-O-3 codes (8260, 8262, 8384, 8140, 8210, 8380, 8381, 8382, 8383, 8440, 8480, 8481, 8482, 8560 and 8570), mixed was defined based on ICO-O-3 codes including 8255 and 8323, serous was defined based on ICO-O-3 codes 8450, 8441, 8460 and 8461 and others was defined based on ICO-O-3 codes including 8000, 8001, 8012, 8013, 8014, 8015, 8020, 8021, 8022, 8032, 8033, 8045, 8046, 8051, 8072, 8120, 8130, 8141, 8143, 8230, 8261, 8263, 8211, 8246, 8320, 8340, 8490, 8574, 8575, 8576, 8933, 9080, 8041, 8070, 8071, 8076, 8005, 8010, 8050, 8082, 8083, 8240, 8244, 8370, 8510, 8800, 8890, 8891, 8895, 8896, 8900, 8901, 8902, 8910, 8920, 8930, 8931, and 8935. Surgery including no surgery (00), local tumor destruction (10–26), subtotal hysterectomy (30–32), total hysterectomy (40–67) and pelvic exenteration (75–79).
Outcome Variables
The all-cause mortality of endometrial carcinoma patients was the outcome in this study. The median follow-up time was 60.00 (60.00,60.00) months. All participants were divided into the alive group and dead group at the end of follow-up.
Statistical Analysis
Kolmogorov–Smirnov was used to evaluate the normality of quantitative measurement data. The normally distributed measurement data were described as Mean (standard deviation) [Mean (SD)]. Independent sample t-test was used for comparison between the two groups. The non-normally distributed measurement data were expressed as median and quartiles [M (Q1, Q3)], and the Mann–Whitney U rank sum test was used for comparison between groups. Enumeration data were presented as number and percentage of cases [n (%)], and Chi-square test was used for comparison between groups, and rank sum test was used for rank data. The covariates were screen out by Cox risk proportional model. COX risk proportional model was established to evaluate the association between FIGO staging with the all-cause mortality of endometrial carcinoma. Model 1 was the univariable Cox proportional-hazards regression analyses without adjusting anything. Model 2 was the multivariable Cox proportional-hazards regression analyses adjusting for age, race, marital status, primary site, histologic type, tumor size, tumor grade, examined lymph nodes, surgery, chemotherapy and radiation. Hazard ratio (HR) and 95% confidence interval (CI) were effect size. The diagnostic value of FIGO staging and the new staging for the mortality of patients were evaluated by receiver operator characteristic curve (ROC). All statistical tests were conducted using a two-sided test with the test level α= 0.05. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for model construction. R 4.0.3 software was used to calculate C-index and draw Kaplan–Meier curves.
Results
Comparisons of the Characteristics of Alive and Dead Patients with Endometrial Carcinoma
In total, the data of 35,510 endometrial carcinoma patients >18 years with FIGO staging who examined the number of lymph nodes were identified in SEER database. Among them, we excluded patients with reported diagnosis source from autopsy or death certificate or only clinically diagnosed (n=0), patients without complete clinicopathological information and survival data (n=6523), and patients without data on race (n=142) and those without information for deciding whether surgery was performed (n=21). Finally, a total of 28,824 patients were included. The screen process of the participants is exhibited in Figure 1.
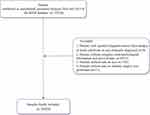 |
Figure 1 The screen process of the participants. Abbreviation: SEER, surveillance, epidemiology, and end results. |
The mean age of participants in the alive group was younger than the dead group (61.14 years vs 66.59 years). The percentages of participants with different primary sites, histologic types, tumor sizes, T stages, N stages, M stages, FIGO staging, and tumor Grades in the alive group were statistically different from the dead group. The number of examined lymph nodes in the alive group was higher than the dead group (14.00 vs 12.00). The percentages of patients with different positive lymph nodes in the alive group were statistically different from the dead group (Table 1).
 |
Table 1 Comparisons of the Characteristics of Alive and Dead Patients with Endometrial Carcinoma |
The Associations Between Different FIGO Staging and the All-Cause Mortality of Endometrial Carcinoma Patients
Compared to patients at FIGO staging IA, patients at FIGO staging IB (HR=1.75, 95% CI: 1.62–1.90), FIGO staging II (HR=2.22, 95% CI: 2.00–2.47), FIGO staging IIIA (HR=2.74, 95% CI: 2.43–3.09), FIGO staging IIIB (HR=4.07, 95% CI: 3.48–4.76), FIGO staging IIIC1 (HR=3.84, 95% CI: 3.52–4.20), FIGO staging IIIC2 (HR=4.52, 95% CI: 4.09–4.99), FIGO staging IVA (HR=5.56, 95% CI: 4.58–6.74), and FIGO staging IVB (HR=7.62, 95% CI: 6.94–8.36) were associated with increased risk of all-cause mortality of endometrial carcinoma patients after adjusting for confounding factors including age, race, marital status, primary site, histologic type, tumor size, tumor grade, examined lymph nodes, surgery, chemotherapy and radiation (Table 2). However, the HRs for all-cause mortality of endometrial carcinoma patients was not increased as the severity of FIGO staging. The survival curves of patients at different FIGO staging are shown in Figure 2. After adding positive lymph nodes as another covariate in Model 3, the effect on of FIGO staging survival was reduced when the FIGO staging was higher than stage III/IV. These results indicated that positive lymph nodes might improve the survival prediction of FIGO staging for endometrial carcinoma patients.
 |
Table 2 The Association of Survival of Patients with FIGO Staging |
 |
Figure 2 The Kaplan–Meier curve of endometrial carcinoma based on FIGO staging. Abbreviation: FIGO, The International Federation of Gynecology and Obstetrics. |
Construction of a New Staging for Survival of Endometrial Carcinoma Patients Based on FIGO Staging and Positive Lymph Nodes
FIGO staging was combined with the number of positive lymph nodes, and 15 stages were obtained including IA, IB, II, IIIA, IIIB, IIIC1 (<), IIIC1 (≥7), IIIC2 (1–6), IIIC2 (≥7), IVA (0), IVA (1–6), IVA (≥7), IVB (0), and IVB (1–6), and IVB (≥7) (Table 3). The survival curves of patients at these stages were presented in Figure 3 (left). We found that the curves were overlapped in some stages, so these stages were combined. Finally, a novel staging was constructed including IA, IB, II, IIIA, IIIB (IIIB or IIIC+ positive lymph nodes <7), IVA (IVA or IIIC + positive lymph nodes≥7), IVB (IVB + positive lymph nodes<7), IVC (IVC + positive lymph nodes≥7). As exhibited in Table 4, in the adjusted model, the HRs for the risk of all-cause mortality of endometrial carcinoma patients were elevated with the increasing of new staging. The survival probability of patients was decreased as the increased of new staging (Figure 3, right).
 |
Table 3 Construction of the New Staging System |
 |
Table 4 The Association Between the New Staging with the Survival of Patients |
 |
Figure 3 The Kaplan–Meier curve of endometrial carcinoma based on new staging before (left) and after (right) combining overlap. |
According to the results of the cindex.com () function in the survcomp package of R software, the C-index of the new staging was 0.781 (95% CI: 0.774–0.787), which was higher than FIGO staging [C-index=0.776 (95% CI: 0.770–0.783)], suggesting that the new staging had better predictive value for the survival of endometrial carcinoma patients than FIGO staging (P<0.001) (Table 5). Sensitivity analysis was conducted after excluding FIGO staging IV, and the C-index of the new staging 0.750 (95% CI: 0.742–0.757) was still higher than FIGO staging 0.744 (95% CI: 0.737–0.751) (P<0.001) (Supplementary Table 1).
 |
Table 5 Comparisons of the C-Index of FIGO Staging and New Staging |
Subgroup Analysis of the Survival of Endometrial Carcinoma Patients Using FIGO Staging and New Staging
Subgroup analysis was stratified by the number of examined lymph nodes. The C-index of FIGO staging for predicting the survival of endometrial carcinoma patients with examine lymph node≥14 was 0.755 (95% CI: 0.745–0.765) while the C-index of new staging for these patients was 0.760 (95% CI: 0.750–0.770). The C-indexes of FIGO staging and new staging for predicting the survival of endometrial carcinoma patients with examine lymph node<14 were 0.797 (95% CI: 0.789–0.805) and 0.802 (95% CI: 0.794–0.810), respectively. The C-indexes of FIGO staging and new staging for predicting the survival of endometrial carcinoma patients with examine lymph node≥21 were 0.746 (95% CI: 0.732–0.759) and 0.750 (95% CI: 0.737–0.764), respectively. As for predicting the survival of endometrial carcinoma patients with examine lymph node<21, the C-indexes of FIGO staging and new staging were 0.789 (95% CI: 0.782–0.796) and 0.793 (95% CI: 0.786–0.800), respectively. The C-indexes of new staging were all higher than FIGO staging (all P<0.001) (Table 6).
 |
Table 6 Subgroup Analysis of the Survival of Endometrial Carcinoma Patients Using FIGO Staging and New Staging |
Discussion
The present study established a new clinical staging system through combining the number of positive lymph nodes and the FIGO staging for predicting the prognosis of endometrial carcinoma. The results revealed that the HRs for the risk of all-cause mortality of endometrial carcinoma patients were elevated with the increasing of new staging. The C-index of the new staging for predicting the survival of endometrial carcinoma patients was 0.781, which was higher than FIGO staging. Subgroup analysis also demonstrated that the new staging had better diagnostic value for the survival of endometrial carcinoma patients in patients with different examine lymph nodes than FIGO staging. The new staging might provide a tool to more accurately identify endometrial carcinoma patients who were at high risk of mortality and offer timely management in these patients.
The role of lymph node assessment/dissection in endometrial cancer has been a topic of debate for decades, resulting in significant variability in practice across different centers. Whether endometrial cancer patients require lymphadenectomy or other procedures such as sentinel lymph node mapping, pelvic lymphadenectomy alone or combined pelvic and para-aortic lymphadenectomy is still controversial.16–18 Previously, the presence of lymph node metastasis was identified to influence the prognosis and treatment decisions of patients with endometrial cancer.19 The molecular subtype and preoperative cancer antigen 125> 25 were significantly associated with lymph node metastasis in patients with endometrial cancer.20 For patients with lymph nodes metastasis, lymphadenectomy was frequently reported to be correlated with prolonged survival time.21 Endometrial cancer patients who were at stage IIIC were identified to benefit from lymphadenectomy.22 These findings suggested the importance of lymph node assessment in endometrial carcinoma patients.
Accurate cancer staging is crucial for clinicians to accurately predict patient prognosis, provide appropriate interventions, and select the most effective treatment option.23 Patients with higher tumor stage might be associated with poor prognosis,24 but in our study, the survival of some patients at IIIC1 or IIIC2 might be better than IIIB. This might result in an overestimation of the prognostic risk of stage IIIC1 or IIIC2 and underestimation of the risk of stage IIIB, which may further lead to inappropriate treatments for those patients. When the number of lymph node metastases was used to supplement the traditional FIGO staging for evaluating the prognosis of endometrial carcinoma patients, the survival of patients was decreased as the increase of tumor stage. In a previous study, the count of metastatic lymph node was used to supplement the AJCC staging system, and the modified AJCC staging system showed superior performance for evaluating the prognosis of endometrial carcinoma patients.7 The optimal dissection range of lymph node needs to be discussed. A previous SEER-based study revealed that the median number of resected lymph nodes in patients with endometrial carcinoma at stages I to IV increased from 7 during the period of 1988–1992 to 12 during the period of 1998–2001,25 which suggested that the dissection range of lymph nodes appeared to have expanded gradually over time. In the current study, the median examined lymph nodes were 14. Different numbers of lymph nodes metastases were supplemented with the traditional FIGO staging, and the survival of patients was decreased as the increase of tumor stage. We also identified that the prognostic value of the count of metastatic lymph node supplement to FIGO staging was better than FIGO staging alone.
In this study, FIGO staging was improved based on the number of lymph node metastases, and the predictive values of the two staging systems for survival of endometrial carcinoma patients were compared, suggesting that the number of metastases might improve the diagnosis of the survival of endometrial carcinoma patients. The findings might provide a reference for accurate assessment and hierarchical management of the mortality risk of endometrial carcinoma patients, and hope to early identify those at high risk of poor prognosis and offer timely interventions to improve their prognosis. This study used the data from the large public SEER database, and the sample size was large and the study population was representative. Due to the requirement of exposure and outcome data, there was inevitably a certain selection bias. The retrospective cohort study design was limited to data, and other factors that might affect survival, such as lifestyles and other treatments, were not available. Although the SEER database contains multi-ethnic groups, and due to regional socioeconomic differences, the extension of our results should be with caution. The application value of our staging system to other populations needs to be further verified.
Conclusions
A new clinical staging system combined the number of positive lymph nodes and the FIGO staging for predicting the prognosis of endometrial carcinoma was established in the current study. The data delineated that the prognostic value of our new staging using the number of positive lymph nodes supplement to the FIGO staging was superior than the FIGO staging. The new staging might help more accurately identify endometrial carcinoma patients who were at high risk of mortality and offer timely treatments in these patients.
Ethics Approval and Informed Consent
The requirement of ethical approval for this was waived by the Institutional Review Board of Heilongjiang Provincial Hospital, because the data was accessed from SEER (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Heilongjiang Provincial Hospital due to retrospective nature of the study.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gao S, Zhao T, Meng F, Luo Y, Li Y, Wang Y. Circular RNAs in endometrial carcinoma (Review). Oncol Rep. 2022;48(6). doi:10.3892/or.2022.8427
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602
4. Makker V, MacKay H, Ray-Coquard I, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7(1):88. doi:10.1038/s41572-021-00324-8
5. Tran L, Christensen P, Barroeta JE, et al. Prognostic significance of size, location, and number of lymph node metastases in endometrial carcinoma. Int J Gynecol Pathol. 2022;42:376–389. doi:10.1097/PGP.0000000000000897
6. Siesto G, Romano F, Iedà NP, Vitobello D. Survival outcomes after surgical management of endometrial cancer: analysis after the first 10-year experience of robotic surgery in a single center. Int J Med Robot. 2020;16(6):1–9. doi:10.1002/rcs.2157
7. Huo X, Wang S. A lymph node count-based AJCC staging system facilitates a more accurate prediction of the prognosis of patients with endometrial cancer. Front Oncol. 2021;11:641962.
8. Kiuchi K, Hasegawa K, Ochiai S, et al. Prognostic significance of inflammatory parameters and nutritional index in clinical stage IVB endometrial carcinomas. J Obstet Gynaecol. 2019;39(2):237–241. doi:10.1080/01443615.2018.1494703
9. McCluggage WG. Pathologic staging of endometrial carcinomas: selected areas of difficulty. Adv Anat Pathol. 2018;25(2):71–84. doi:10.1097/PAP.0000000000000182
10. Ladbury C, Li R, Shiao J, et al. Characterizing impact of positive lymph node number in endometrial cancer using machine-learning: a better prognostic indicator than FIGO staging? Gynecol Oncol. 2022;164(1):39–45. doi:10.1016/j.ygyno.2021.11.007
11. Uccella S, Falcone F, Greggi S, et al. Survival in clinical stage I endometrial cancer with single vs. multiple positive pelvic nodes: results of a multi-institutional Italian study. J Gynecol Oncol. 2018;29(6):e100. doi:10.3802/jgo.2018.29.e100
12. Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi:10.1245/s10434-018-6486-6
13. Hemmert R, Schliep KC, Willis S, et al. Modifiable life style factors and risk for incident endometriosis. Paediatr Perinat Epidemiol. 2019;33(1):19–25. doi:10.1111/ppe.12516
14. Liang W, He J, Shen Y, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. 2017;35(11):1162–1170. doi:10.1200/JCO.2016.67.5140
15. Yan G, Li Y, Du Y, Ma X, Xie Y, Zeng X. Survival nomogram for endometrial cancer with lung metastasis: a SEER database analysis. Front Oncol. 2022;12:978140. doi:10.3389/fonc.2022.978140
16. Proppe L, Alkatout I, Koch R, et al. Impact of lymphadenectomy on short- and long-term complications in patients with endometrial cancer. Arch Gynecol Obstet. 2022;306(3):811–819. doi:10.1007/s00404-022-06396-5
17. Min Y, Zhang Z. Hysterectomy or/and lymphadenectomy for the survival of patients with primary endometrial cancer: a cohort study using the SEER database. Biotechnol Genet Eng Rev. 2022;1–19. doi:10.1080/02648725.2022.2162235
18. Capozzi VA, Rosati A, Maglietta G, et al. Long-term survival outcomes in high-risk endometrial cancer patients undergoing sentinel lymph node biopsy alone versus lymphadenectomy. Int J Gynecol Cancer. 2023;2023;1.
19. Pollom EL, Conklin CM, von Eyben R, Folkins AK, Kidd EA. Nomogram to predict risk of lymph node metastases in patients with endometrioid endometrial cancer. Int J Gynecol Pathol. 2016;35(5):395–401. doi:10.1097/PGP.0000000000000246
20. Jamieson A, Thompson EF, Huvila J, et al. Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol Oncol. 2022;165(2):376–384. doi:10.1016/j.ygyno.2022.01.025
21. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375(9721):1165–1172. doi:10.1016/S0140-6736(09)62002-X
22. Havrilesky LJ, Cragun JM, Calingaert B, et al. Resection of lymph node metastases influences survival in stage IIIC endometrial cancer. Gynecol Oncol. 2005;99(3):689–695. doi:10.1016/j.ygyno.2005.07.014
23. Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. doi:10.1136/ijgc-2020-002230
24. Garg V, Jayaraj AS, Kumar L. Novel approaches for treatment of endometrial carcinoma. Curr Probl Cancer. 2022;46(5):100895. doi:10.1016/j.currproblcancer.2022.100895
25. Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107(8):1823–1830. doi:10.1002/cncr.22185
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
