Back to Journals » Journal of Asthma and Allergy » Volume 17
New Insights into Mechanisms Traditional Chinese Medicine for Allergic Rhinitis by Regulating Inflammatory and Oxidative Stress Pathways
Authors Qin Z, Xie L, Li W, Wang C, Li Y
Received 16 October 2023
Accepted for publication 23 January 2024
Published 19 February 2024 Volume 2024:17 Pages 97—112
DOI https://doi.org/10.2147/JAA.S444923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Zhu Qin,1 Liangzhen Xie,1,2 Wentao Li,2 Chao Wang,1 Yan Li2
1Department of Otolaryngology, Graduate School of Heilongjiang University of Traditional Chinese Medicine, Harbin, Heilongjiang, People’s Republic of China; 2Department of Otolaryngology, First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin, Heilongjiang, People’s Republic of China
Correspondence: Yan Li; Liangzhen Xie, No. 26 Heping Road, Harbin, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Allergy rhinitis (AR) is becoming more common and has serious medical and societal consequences. Sneezing, paroxysmal nasal blockage, nasal itching, mucosal edema, coughing, and rhinorrhea are symptoms of this type I allergic immunological illness. Immunoglobulin E-mediated inflammation is the cause of it. Because AR is prone to recurrent attacks, extended medication therapy may impair its effectiveness. In addition to negatively affecting the patients’ physical health, this can also negatively impact their mental health. During AR development, there are inflammatory and oxidative stress responses that are linked to problems in a number of signal transduction pathways. By using the terms “allergic rhinitis”, “traditional Chinese medicine”, “inflammation”, and “oxidative stress”, we screened for pertinent research published over the previous five years in databases like PubMed. We saw that NF-KB, TLR, IL-33/ST2, PI3K/AKT, MAPK, and Nrf2 are some of the most important inflammatory and oxidative stress pathways in AR. Studies have revealed that antioxidant and anti-inflammatory therapy reduced the risk of AR and was therapeutic; however, the impact of the therapy varies widely. The Chinese medical system places a high value on traditional Chinese medicine (TCM), which has been there for virtually all of China’s 5000-year history. By influencing signaling pathways related to inflammation and oxidative stress, Chinese herbal medicine and its constituent compounds have been shown to prevent allergic rhinitis. This review will focus on this evidence and provide references for clinical treatment and scientific research applications.
Keywords: allergic rhinitis, traditional Chinese medicine, inflammatory, oxidative stress, signaling pathways
Introduction
AR is a common disease characterized by nasal congestion, sneezing, runny nose, and itching, with a prevalence of up to 40% and more than 500 million individuals around the world.1 According to a 6-year study of AR in China, the prevalence of adult AR grew from 11.1% in 2005 to 17.6% in 2011. Appropriate prevention and treatment of allergic rhinitis are vital due to the condition’s negative consequences on the body, high prevalence, and significant economic costs. So it is important to explore the mechanism of AR. Previous research has focused on the fact that oxidative stress and inflammation have played an important role in the pathogenesis of asthma. Allergy rhinitis is associated with asthma, so oxidative stress in allergic rhinitis has also been investigated.
The allergenic substance is the direct cause of the disease. Dendritic cells gather breathing allergens inside the epithelial barrier and deliver them to T lymphocytes as allergenic peptides.2 This initiates the generation of Th2 cytokines such as interleukin (IL)-4, IL-5, IL-9, and IL-13 by T helper 2 (Th2) cells. B lymphocytes are transformed into plasma cells that generate allergen-specific IgE. IgE molecules that are specific to the allergen that was released attach to mast cells in the tissue and basophils that are moving through the blood. The allergen adheres to the surface IgE on mast cells and basophils upon repeated exposure, triggering the cells. Histamine and leukotriene, among other neuroactive and vasoactive mediators, are released from the cells. A diagram of the pathophysiology of allergic rhinitis is shown in Figure 1.In the early stage of allergic rhinitis, it is mainly inflammatory in nature and characterized by the influx of inflammatory cells mainly composed of T lymphocytes, basophils, and eosinophils. In the late stage, eosinophils are the main participants, releasing a series of pro-inflammatory mediators, and inflammation plays an important role in the pathogenesis of allergic rhinitis. Inflammation and oxidative stress are closely associated. Asthma and AR are inflammatory reactions triggered by oxidative stress and the formation of ROS from this stress.3,4 Figure 2 illustrates the interaction between oxidative stress and inflammation. Oxidative stress is hypothesized to promote oxidative damage by causing a mismatch between oxidative activity and the endogenous antioxidant system, which is related to the progression of asthma and AR.5,6 In allergic rhinitis (AR), the epidemiological and immunopathological background is similar to that of asthma, and many reports suggest that oxidative stress and the ROS it produces play an important role in its inflammatory response. By reducing the quantity and effectiveness of epithelial cilia, excessive ROS can damage the respiratory tract’s epithelial cell layer, increase mucus secretion, and bring about an influx of inflammatory cells. Oxidative stress breaks down the nasal mucosa’s barrier function, sets off proinflammatory mediators and inflammatory responses, makes rhinitis symptoms worse, and is a key regulatory mechanism in allergic rhinitis.7
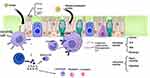 |
Figure 1 Pathogenesis of allergic rhinitis. Created with Biorender.com. |
 |
Figure 2 Crosstalk between oxidative stress and inflammation. |
The complex pathophysiology of AR requires a wide range of signaling pathways and regulatory mechanisms.8 Inflammatory factors and proinflammatory mediators exhibit unusual expression because of the aberrant regulation of several signaling pathways. These cause oxidative stress and the ongoing creation of reactive oxygen species (ROS), which leads to the development of AR. ROS are important signaling mechanisms in allergic rhinitis, although several redox-sensitive pathways are also activated as the disease progresses. Apoptosis, ER stress, altered cell migration, proliferation/senescence, apoptosis, and autophagy are all factors that lead to increased oxidative stress, which in turn alters signaling pathways that eventually influence allergic rhinitis. It has previously been reported that the NF-KB signaling pathway mediates AR inflammation,9 MAPK inhibitors can alleviate olfactory dysfunction by inhibiting OSN apoptosis in AR mice,10 and the expression of TLR4 was upregulated in the OVA-induced AR mouse model. TLR4 alleviated the allergic symptoms of AR mice by regulating the production of proinflammatory mediators. Downregulation of the TLR4/NF-KB pathway can significantly inhibit the inflammatory response, reduce nasal mucosal damage in AR rats, and improve their rhinitis symptoms, indicating that the TLR4/NF-KB signal is an important therapeutic target of AR.11 IL-33/ST2 functions in AR by binding to multiple cells and signaling pathways, and it is a key signaling pathway for AR development. Nasal epithelial cells mediate cellular inflammation via the IL-33/ST2 axis and IL-33-mediated inflammation of nasal mucosal epithelial cells through the ST2 receptor.12 Th17/Treg cell immune imbalance is an important mechanism leading to the pathogenesis of AR, and LA therapy has been shown to improve the number of Treg cells and inhibit Th17 cell differentiation, as well as antioxidant activity via the Nrf2 signaling pathway, and have a positive effect on allergic inflammation in an AR mouse model.13 During immune emergencies, the PI3K/Akt signaling pathway controls the differentiation of Th17 and Treg cells. It can also lower IgE levels by controlling the levels of the PI3K/Akt signaling pathway, which also lowers the release of inflammatory factors and mucus. Therefore, the use of drugs targeting the PI3K/Akt signaling pathway to treat AR has broad development prospects.14 In this study, we will stress the structure, function, and implications of ROS on AR from the perspective of these pathways.
Allergic reactions are mainly mediated by histamine, and inhibiting the histamine pathway is therefore fundamental for AR. Oral antihistamines and intranasal corticosteroids are currently employed as common interventions.15 Antihistamines could inhibit the release of histamines by hindering the binding of histamine to the H1 receptor.16 Intranasal corticosteroids reduce inflammation by reducing the release of mediators.17 Traditional Chinese medicine has a long history, with its characteristics of multiple components, targets, and pathways that are in line with the pathogenic mechanism of AR caused by multiple factors. It has unique advantages in the prevention and treatment of AR, and the pharmacological effects of related traditional Chinese medicine monomers and formulas have also received great attention in the pharmaceutical industry. In recent years, scholars have increasingly studied the mechanism of traditional Chinese medicine intervention in the AR signaling pathway. Many studies have shown that traditional Chinese medicine can inhibit the occurrence and development of AR by reducing histamine content, reducing the production of inflammatory metabolites, and reducing vascular permeability. However, there are currently few reports on the systematic summary of the regulation of AR signaling pathways by traditional Chinese medicine. Through searching the databases of China National Knowledge Infrastructure, Wanfang, PubMed, and Google Scholar from 2014 to 2021, the author found multiple reports exploring the possible mechanisms of action between traditional Chinese medicine and AR, including multiple signaling pathways that may be related to nuclear transcription factors: KB (NF-KB), Toll-like receptors (TLR), interleukin-33/growth stimulating expression factor 2 (IL-33/ST2), phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), mitogen activated protein kinase (MAPK), etc. This article provides a review of the mechanisms by which traditional Chinese medicine regulates the AR signaling pathway in order to provide references and ideas for in-depth research on the intervention of traditional Chinese medicine in related signaling pathways.
Signal Pathways Related to Oxidative Stress and Inflammation in AR
NF-κB Signaling Pathway
The nuclear factor NF-KB pathway has been regarded as a model proinflammatory signaling pathway based on NF-KB’s function in the regulation of proinflammatory genes such as cytokines, chemokines, and adhesion molecules. Nuclear factor kappa B is also a transcription factor that responds to oxidative stress. Translocation of NF-κB is a key feature of molecular inflammation and plays a part in the production and expression of cytokines and mediators that cause inflammation.18 Once target genes have translocated into the nucleus, NF-KB dimers start their transcription.19 When NF-KB is activated, IκB phosphorylation is increased.20,21 Activated NF-KB then enters the nucleus and starts an inflammatory response.22 These then trigger oxidative stress and inflammatory reactions that propel the development of AR. Some research found that there was a close association between the NF-KB signal pathway and AR. By modulating Th1/Th2/Th17 cells and their associated transcription factors, NF-KB was blocked, and the nasal allergic inflammation was reduced by OVA.9
TLR Signaling Pathway
Toll-like receptors (TLRs) are information-processing receptors that are essential for both innate and adaptive immune responses to pathogens.23 TLRs are mediated by a number of inflammatory proteins, or peptides. One study found that HMGB1 stimulation of neutrophils, monocytes, or macrophages required activation of both TLR2 and TLR4, increased nuclear translocation of NF-KB, and upregulated the production of proinflammatory cytokines.24 TLR-mediated signal transduction pathways could include the MyD88-reliant pathway. The mechanism that positively regulates an inflammatory response is TLR4/MyD88/NF-KB.25 The inflammatory response mediated by lipopolysaccharide was inhabited by blocking the TLR4/MyD88/NF-KB signaling pathway.26 Previous research has demonstrated that TLR4 plays a significant regulatory role in AR. Particularly, there were higher mRNA and protein levels of TLR4 in the nasal mucosa of patients with pollen-induced allergic rhinitis.27 It is generally known that TLR signaling has a significant role in inflammation.28 In the AR murine model, one study revealed that DAP alleviated oxidative stress, an inflammatory response, and nasal symptoms caused by OVA. Additionally, it inhibited TLR4/NF-B signaling in the nasal mucosa of mice.29
IL-33/ST2 Signaling Pathway
A brand-new member of the IL-1 family of cytokines, IL-33, has been shown to promote the expression of Th2 cytokines. It is typically released by inflamed or injured tissues and functions as an alarm in identifying damage in a variety of inflammatory conditions, including allergic rhinitis and skin conditions.30 When basophils and eosinophils are activated in allergic rhinitis, IL-33 is important for inflammation.31 Normal nasal epithelial cells contain the IL-33 protein in their nucleus, and it is quickly released into the nasal fluid in response to allergen exposure.32,33 The IL-33/suppression of tumorigenicity 2 (ST2) signaling pathway is now known to play a critical role in mast cell-mediated allergy infections in recent years.34 The IL-33/ST2 signaling pathway and thymic stromal lymphopoietin (TSLP) are very important in many allergic and inflammatory responses. Several studies demonstrated that the pathogenesis of AR was influenced by the interactions between the TSLP and IL-33/ST2 signaling pathways. Inhibiting IL-33/ST2 expression by downregulating TSLP could have a therapeutic impact on preventing the pathologic effects of hypoxia.35 IL-33/ST2 has an influence on the growth and development of AR.
P13K/AKT Signaling Pathway
A crucial master upstream route of Nrf2 was thought to be PI3K/Akt signaling, mediating oxidative stress by the Nrf2/HO-1 pathway.36–38 One study found that β-asarone might regulate oxidative stress through the P13K/AKT/Nrf2 signaling pathway.39 Some previous research demonstrated that the serum level of leptin was much higher in AR patients and positively associated with the clinical symptoms.40,41 MAPK, JAK2-STAT3, and PI3K/AKT pathways are activated when leptin and its receptor are present.42,43 Meanwhile, leptin mediated the activity of ILC2 and enhanced ILC2 inflammation by the PI3K/AKT pathway in AR.44
MAPK Signaling Pathway
The activation of compensatory mechanisms, response to allergies, and new insights into MAPK as a complex cell signaling system were all covered in a recent paper.45 A range of triggers, the majority of which are connected by their capacity to mediate oxidative stress, can generate HO-1.46 It has been revealed that HO-1 activation involves MAPK pathways. MAPK activation by GSTD was found to increase Nrf2 phosphorylation levels and reduce the oxidative stress/proinflammatory response.47 The three main parts of the MAPK pathway are ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal protein kinase), and p38MAPK (p38 mitogen-activated protein kinase). The p38MAPK signaling pathway is active in the progression of AR. Meanwhile, the p38MAPK signaling pathway may have a significant role in the loss of olfaction in AR animals.10 By protein blotting, allergic rhinitis rats had considerably higher p38 MAPK mRNA expression and activity than control rats. It has been determined that p38 MAPK is crucial in the development of allergic rhinitis.48
Nrf2 Signaling Pathway
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is crucial in regulating oxidative stress. Additionally, Nrf2 protects mitochondria from oxidative damage by reducing excessive ROS generation.49 The intracellular antioxidant defense mechanism, which guards against ROS-mediated cell death, is considered to be modulated by antioxidant response element (ARE) activation. The signaling pathway of Nuclear Factor Erythroid 2 Related Factor 2 activates ARE. A cytoplasmic transcription factor called Nrf2 that is redox-sensitive promotes the production of genes that protect cells from oxidative stress and chronic inflammation.50 The Nrf2 and NF-KB signaling pathways are closely involved in regulating the balance between the cellular redox state and inflammatory responses.51 Nrf2 downregulation may lead to a rise in NF-KB activity and the production of inflammatory markers. When Nrf2 works with NF-KB to coordinate inflammatory and antioxidant stress responses, it may be a good target to stop AR from developing in a way that is not normal.
Traditional Chinese Medicine Exerts Protective Effects on AR by Regulating Oxidative Stress and Inflammation Related Signaling Pathways
Traditional Chinese medicine (TCM) has been widely used in China for more than 3000 years. Presently, it has been determined that traditional Chinese herbal compounds and potent active substances may successfully treat AR. Meanwhile, it is considered that TCM could maintain a healthy balance between inflammatory interactions and suppress oxidative stress. The pathophysiology of AR involves signaling pathways. The harmony of the chronic inflammatory interaction can be maintained by TCM, which can also influence signal pathways linked to the pathogenic evolution of AR (Table 1 and Table 2). This can also inhibit oxidative stress damage.
 |
Table 1 Regulatory Effect of Traditional Chinese Medicine Monomers on Signal Pathway of AR |
 |
Table 2 Regulatory Effect of Traditional Chinese Medicine Compound on Signal Pathway of AR |
Modulation of Allergic Rhinitis Through NF-KB Pathway by Traditional Chinese Medicine Constituents
Chinese Herbal Compound
Professor Huang ShouLin recommends that Xingbi gel could dredge the airflow, clear heat, and pass the orifice. Lihong Nan suggests that the expression of inflammatory factors may be associated with the inhibition of NF-KB signaling pathway activity utilizing low-medium-high-dose nasal gel treatment.64
Monomer of Traditional Chinese Medicine
One of the most significant and active saikosaponins extracted from Bupleurum falcatum is saikosaponin A (SSA),71 a triterpenoid saponin that promotes apoptotic processes in breast cancer cell lines from humans.72–74 SSA has been demonstrated in vivo to prevent experimental sepsis by blocking NOD2-mediated NF-KB activation and to have a critical point impact in LPS-induced acute lung injury by blocking NF-KB and NLRP3 signaling pathways.75,76 SSA effectively decreased allergic inflammation of the nasal mucosa in OVA-induced AR mice by regulating Th2 and Th17 responses and suppressing the IL-6/STAT3/ROR-t and NF-KB pathways.52 These findings show that SSA might be an effective therapy for AR suppression.
Salvia miltiorrhiza Bunge’s roots contain a natural substance called tanshinone IIA (TIIA).77 TIIA has antioxidative, anti-cancer, and anti-inflammatory properties.78,79 An investigation found that TIIA could prevent oxidative stress from causing cardiac fibrosis in HF.80 According to the study, Tanshinone IIA could lower serum IgE levels, lower histamine release, inhibit intracellular Ca2+ influx, and decrease the expression of the inflammatory molecules’ tumor necrosis factor (TNF) and IL-4. This indicated that Tanshinone IIA could regulate the activity of the NF-KB pathway and enhance mast cell AR response.53
Glycyrrhizin is a triterpene glycoside. It is a key component of licorice root (Glycyrrhiza glabra) and has pharmacological properties, including anti-inflammatory and antioxidant.81,82 Glycyrrhizin has recently been related to a number of immunomodulatory benefits, including a reduction in the frequency of asthma and AR attacks, an improvement in semen quality with treatment, and a modulation of allergic inflammation in a mouse model of asthma.83,84 In the blood and nasal mucosa of AR mice, a study showed that glycyrrhizin therapy increased the antioxidant state, lowered the incidence of free radical-induced lipid peroxidation, and improved immune activity.85 Glycyrrhizin inhibited the His-induced inflammatory response by deactivating the NF-KB pathway in NECs. This suggests that glycyrrhizin could be utilized as a complementary and alternative therapy for AR.54
Astragalus membranaceous is an herb that has been used for a very long time in China to cure a wide range of illnesses, including cardiovascular ailments, hepatitis, allergic rhinitis, and skin conditions.86,87 The main bioactive component produced from Astragalus membranaceous is known as astragaloside IV (As-IV).88 As-IV possesses a variety of pharmacologic activities, including anti-inflammatory, immunomodulatory, and antiapoptotic properties.89 AS-IV partially turned off the NF-KB signaling pathway in NECs, which lowered the inflammatory response that histamine causes. By inhibiting the NF-KB pathway in vitro, we deduced that AS-IV might be thought of as a possible candidate medication for AR.55
There have been some pharmacological actions associated with baicalin, a flavonoid molecule derived from Scutellaria baicalensis Georgi, including anti-allergic, anti-inflammatory, and antioxidant effects.56 Baicalin is frequently used to treat a variety of allergic and inflammatory illnesses, including asthma, atopic dermatitis, and AR.54 Baicalin efficiently suppressed JAK2-STAT5 and NF-KB signaling pathways in LPS-stimulated human mast cells to limit the inflammatory response and prevent an allergic response in guinea pigs with OVA-induced allergy rhinitis. When taken as a whole, the data imply that baicalin may be an appropriate treatment for allergic rhinitis.90
Modulation of Allergic Rhinitis Through TLR Pathway by Traditional Chinese Medicine Constituents
Monomer of Traditional Chinese Medicine
TwHF polyglycosides are the main active ingredients extracted from the traditional Chinese medicine TwHF and have strong anti-inflammatory and antioxidant effects.91 When exploring the mechanism of triptolian polyglycosides in the intervention of the TLR-NF-κB pathway in OVA-induced AR rats, it was found that the ATL group of triptolian polyglycosides was found in AR rats. The expression levels of IgE, TLR4, and NF-KB p50 decreased significantly compared with the model group, and the production of eosinophils in the nasal mucosa was reduced, suggesting the presence of triptolian polyglycosides. Inhibition of the TLR/NF-KB signaling pathway, thereby reducing the expression of TLR4 and NF-KB and improving allergic reactions.57
Luteolin is a flavonoid, first extracted from the wood rhinoceros family. Modern research shows that it is widely present in a variety of plants and has anti-inflammatory, antioxidant, reducing inflammatory factors, protecting the nervous system, and other pharmacological effects.92 It restrained oxidative stress by preventing the generation of ROS and MDA and increasing the activity of the antioxidant enzymes CAT, T-SOD, GSH-Px, and T-AOC(1).93 Luteolin reduced inflammation and the Th1/Th2 imbalance in allergic rhinitis rats by controlling the TLR4/NF-KB pathway. This study offered fresh proof that luteolin might be a viable therapy option for allergic rhinitis.58
Modulation of Allergic Rhinitis Through IL-33/ST2 Pathway by Traditional Chinese Medicine Constituents
Chinese Herbal Compound
Xiaoqinglong decoction (XQLT), as one of the traditional herbal medications, has long been utilized as a natural remedy for allergic disorders like AR and asthma. XQLT has been used to alleviate AR patients’ chronic nasal obstruction symptoms as well as acute symptoms such as rhinorrhea and sneezing.94 Tanaka et al also noted that XQLT had the ability to reduce allergic reaction-related inflammation. In blood serum taken from individuals with persistent allergic rhinitis induced by Dermatophagoides farinas, XQLT reduced the production of IgE and IL-10.95 XQLT reduces the signs and symptoms of AR by avoiding the production of IL-33 from nasal mucosal cells.65 XQLT is probably going to be applied in clinical practice increasingly in the future.
Compound Xinyi nasal drop liquid is a traditional Chinese medicine prepared by the otolaryngology department of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine. It primarily consists of Xinyi and geese herbivores. One study reported that treated AR patients with budesonide and compound Xinyi nasal drops.66 It was discovered that IL-33 expression in the compound Xinyi nasal drop group was significantly higher than that of budesonide. This suggests that IL-33 is involved in the pathogenesis of AR and can regulate the abnormal reaction, making it a useful therapeutic drug.
Modulation of Allergic Rhinitis Through PI3K/AKT Pathway by Traditional Chinese Medicine Constituents
Chinese Herbal Compound
AR is commonly treated by Mahuang Fuzi Xixin Decoction (MFXD). Herba Ephedrae (Mahuang), Radix aconiti lateralis praeparata (Fuzi), and Asarum (Xixin) are the members of MFXD (Radix Rhizoma Asari). Pharmacological investigations have demonstrated that MFXD has anti-inflammatory and antiallergic effects by suppressing the synthesis of interferon gamma and interleukin (IL)-4, as well as the mediator release from mast cells and macrophages.96 By regulating important pathways, including the PI3K-AKT and AMPK signaling pathways, MFXD has been proven to be beneficial for AR.67
A significant herbal remedy, Rhizoma coptidis, also known as “Huang Lian” in China, includes alkaloids such as berberine, coptisine, palmatine, and jatrorrhizine.97 Coptisine showed a wide range of pharmacological capabilities, including antibacterial, anti-hyperlipidemic, anti-inflammatory, and anticancer effects.98,99 Previous research has shown that coptisine can decrease the inflammatory response caused by LPS by blocking the activation of the nuclear factor KB (NF-KB), PI3K/Akt, and mitogen-activated protein kinase (MAPK) pathways in macrophages.100 Coptisine prevented mast cell activation by suppressing the IgE-induced phosphorylation of PI3K and Akt, and it decreased nasal symptom ratings due to its antioxidant and anti-inflammatory actions on AR.101
Yupingfeng San (YPFS) is mainly composed of Astragali Radix, Atractylodes macrocephala, and Radix Saposhnikoviae. It has been discovered to treat asthma and lead to fewer side effects.68,102,103 YPFS has anti-inflammatory properties. Pro-inflammatory cytokine transcript and protein expression levels were decreased to achieve these properties.104,105 The active ingredient in YPFS’s antioxidant activity is polysaccharide.106,107 Superoxide anion (O2), hydroxyl radical (-OH), and hydrogen peroxide are examples of ROS that YPFS can neutralize (H2O2). It can also produce complex metal ions, which the body needs to produce live oxygen to activate the body’s antioxidant defenses.108 YPFS exhibited positive therapeutic effects on respiratory illnesses, inflammation, and immunological conditions. Widespread use of therapy for allergic rhinitis has been reported.109 By alleviating the symptoms of allergic rhinitis, YPFP may promote the proliferation of CD4+CD25+Foxp3+Treg cells and other mechanisms to control the body’s immune response.110 By activating the hif-1 PCR/PI3K/Akt pathway, house dust mite extract increased the release of signaling pathways in the nasal mucosa.111 The way that YPFP works to treat AR was studied using network pharmacology prediction along with other methods. We showed that YPFP may cure AR by regulating the signaling pathways for PI3K-Akt, oxidative stress and atherosclerosis, TNF, and IL-17.112
Monomer of Traditional Chinese Medicine
Fructus Xanthii was used as a traditional herbal remedy for rhinitis. FX has anti-inflammatory, anti-oxidative, and anti-hyperglycemic characteristics. We discovered that microglia in glial cells can control the proliferation of T lymphocytes.113 The induced factors of allergic rhinitis are T lymphocytes and epithelial cells.114,115 Based on GO enrichment and KEGG enrichment, Fructus Xanthii may regulate epithelial cells and control glial apoptosis through thyroid hormone signaling, p53 signaling, and the PI3K-Akt signaling pathway.59
Modulation of Allergic Rhinitis Through MAPK Pathway by Traditional Chinese Medicine Constituents
Chinese Herbal Compound
The Guizhi Decoction is a well-known set of TCM decoctions that has been used for AR for more than 1800 years in China. According to TCM theory, the effectiveness of Guizhi Decoction and associated formula corresponds to the pathogenesis of AR, and research revealed that Guizhi Decoction-associated formula could inhibit cholinergic transdifferentiation of sympathetic nerves and enhance sympathetic nerve anatomical and functional denervation.116 Guizhi Decoction affects rhinitis effectiveness via numerous components, several targets, and different pathways. According to the KEGG pathway enrichment study, signaling pathways such as TNF, T cell receptor, MAPK, and Th17 were involved in the mechanism of action.69
Cool blood and stop sneezing soup is the experience of Professor Gan Zuwang, which has the effects of cooling blood and thinning wind, desensitization, and stopping sneezing. In an AR model, it can alleviate immune inflammation in the nasal mucosa of AR rats by downregulating IL-4 and IL-5 levels in serum, balancing Th1/Th2, weakening the phosphorylation of ERK and p38 MAPK, and regulating immune function by inhibiting the activation of the MAPK signaling pathway.70
Monomer of Traditional Chinese Medicine
Curcuma longa L., a naturally occurring yellow polyphenolic pigment called curcumin, was discovered in the plant’s rhizomes. The biological activities of curcumin are thought to be based on its anti-inflammatory characteristics, which are crucial in the treatment of diseases.117 By producing reactive oxygen species (ROS), curcumin can also encourage the activation of the MAPK pathway.60 Through oxidative alteration of intracellular kinases and inhibition of the MAPK phosphatases, the generation of intracellular ROS can cause the activation of the p38 MAPK pathway.118,119 Curcumin decreased allergy-related symptoms such as sneezing, frequent nose rubbing, lacrimation, and nasal congestion in a guinea pig model of induced allergic rhinitis. It also decreased inflammatory cell infiltration of the nasal mucosa.120 Additionally, in vivo production of IL-2, IL-5, GM-CSF (granulocyte macrophage colony stimulating factor), and IL-4 was decreased by curcumin in response to home dust mites.121 The potential of curcumin to scavenge ROS through regulation of cellular antioxidant enzyme activity and GSH levels has been linked to its antioxidant mechanisms.122–125
Magnolol is a naturally occurring extract and the primary bioactive element of Magnolia used in Chinese medicine. The study found that magnolol reduced inflammation by turning on signaling pathways for MAPK, NF-KB, and immune-regulatory phagocytosis. The findings offer theoretical support for studies on the anti-inflammatory properties of magnolol as a prospective anti-inflammatory medication candidate, as well as experimental evidence for those effects.61 In a prior study, magnolol at 300 M dramatically reduced cytokine and eosinophil infiltration, reducing the symptoms of mice with OVA-induced AR.126
Psoralen (PSO), a molecule that belongs to the class of furanocoumarins, is one of the most major functional substances in the psoralen family.127 PSO contains anti-inflammatory, antioxidant, and other pharmacological characteristics, according to modern pharmacology.128 The MAPK family, of which AP-1 is a part, contains crucial signal transducers. Modifications in cellular tension, ionization effects, DNA damage, oxidative stress, and UV radiation, in addition to bacterial and viral infection, can all activate the MAPK/AP-1 signal transduction pathway.129 Because MAPK/AP-1 can act as a signaling molecule for transcription factors, it stops the AP-1 pathway and the expression of CST1 further down the line. PSO was proven to lessen the inflammatory response and mucus formation in AR.62
Modulation of Allergic Rhinitis Through Nrf2 Pathway by Traditional Chinese Medicine Constituents
Monomer of Traditional Chinese Medicine
Formononetin is a component of a traditional Chinese herb. Radix astragali can also be extracted from the entire leaf of Ononis spinosa L.130,131 Studies on its potential to treat different kinds are also becoming more prevalent. In an animal model of murine allergic asthma, formononetin, for instance, was discovered to protect against airway inflammation and oxidative stress.132 The current study found that formononetin can inhibit IL-13-induced proinflammatory cytokine release and improve nasal mucosal sensitization through boosting the Nrf2 signaling pathway.63
Conclusion and Outlook
Allergic rhinitis has been linked to inflammatory reactions. The formation of ROS from harmed cells or inflammatory responses causes an imbalance in allergic inflammation conditions that is characterized by increased oxidative stress.133 Oxidative stress and inflammatory mechanisms make up the pathophysiology of AR.133–136 Through altering proteins, encouraging inflammation, stimulating apoptosis, inhibiting autophagy, and reducing mitochondrial activity, oxidative stress can disrupt several signaling pathways and affect numerous biological processes. These effects typically quicken pathological development and make the symptoms of disorders like AR worse. The signaling pathways NF-KB, TLR, IL-33/ST2, PI3K/AKT, MAPK, and Nrf2 all contribute to the amplification of AR development, and lowering the phosphorylation of molecules in these pathways helps to reduce the production of inflammatory substances, hence preventing AR.According to the present investigation, Chinese medicine offers distinct benefits in the prevention and treatment of AR because of its high level of safety, stable curative impact, multiple targets, and overall regulation. The constituent chemicals of traditional Chinese medicine, such as monomers and Chinese medicinal compounds, were discussed in this article. These substances all operate on these pathways and have positive therapeutic effects on AR. We discovered that traditional Chinese medicine not only limits the activation of inflammatory factors by acting on signaling pathways and maximizing its therapeutic benefits, but also performs an anti-AR effect from an anti-inflammatory and antioxidant perspective. In summary, oxidative stress is a multifaceted process with numerous contributing elements. Numerous processes connected to numerous signaling pathways need additional investigation.
In addition to its significant therapeutic effects on inflammation and oxidative stress in treating AR, traditional Chinese medicine’s main mechanism of action is to regulate the body’s Th1/Th2 immune balance, Th17/Treg immune balance, macrophage M1/M2 immune balance, alleviate excessive infiltration of eosinophils and mast cells in the nasal mucosa, and reduce the release of histamine-based active mediators by mast cells in the nasal mucosa, thereby inhibiting inflammation and protecting the nasal mucosa to achieve the goal of treating AR.
There are still some issues with how Chinese medicine regulates the AR anti-inflammatory and anti-oxidation signaling pathways. There is a discrepancy between clinical practice and basic research. The question of how to balance good research between the two is important. In the basic research of AR, TCM monomer research is more than Chinese medicine compounds, and in clinical practice treatment, AR is more in Chinese medicine compound intervention, rarely a single application of Chinese medicine monomers. Clinical practice of Chinese medicine compounds lacks basic composition and mechanism research. In many basic studies of AR, drug efficacy is the gold standard for experiments; the average metabolic cycle of drugs in vivo is not evaluated, and there is a lack of clinical efficacy evaluation. When conducting basic research, most of the extracted monomers and compound components of traditional Chinese medicine are crude extracts without standardization or chemical characteristics, and most of the active chemical markers have not been identified. The bioactive metabolites beneficial for the anti-AR effect have not been well determined.
We make the following recommendations based on the definition of compound medicinal compounds and the impressive clinical practice effects of Chinese medicine compounds. The mechanism of the AR signaling pathway was investigated, and basic research was carried out after carefully examining the relationships between the TCM compound, TCM monomer, and active components. Future research can standardize the quality of TCM extract using current science and cutting-edge technology and quickly and efficiently find the biological activity targets of TCM monomers and compound components to contribute to the stability of clinical efficacy.
To conclude, oxidative stress is critical for AR pathogenesis, while Chinese medicine can inhibit the occurrence of oxidative stress to enhance the body’s antioxidant ability, thereby delaying disease progression.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article is supported by the National Natural Science Foundation of China (NO.81874495), the Chunhui Project Foundation of the Education Department of China (NO.HLJ2019036), Heilongjiang Province Traditional Chinese Medicine scientific research project(NO.ZHY19-033).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
2. van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 2009;339:b2433. doi:10.1136/bmj.b2433
3. Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. JAllergy Clin Immunol. 2002;110(3):349–356. doi:10.1067/mai.2002.126780
4. Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14(6):409–420. doi:10.1006/pupt.2001.0319
5. Ercan H, Birben E, Dizdar EA, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol. 2006;118(5):1097–1104. doi:10.1016/j.jaci.2006.08.012
6. Marple BF. Allergic rhinitis and inflammatory airway disease: interactions within the unified airspace. Am J Rhinol Allergy. 2010;24(4):249–254. doi:10.2500/ajra
7. Han M, Lee D, Lee SH, et al. Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants. 2021;10(8):1266. doi:10.3390/antiox10081266
8. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–1151. doi:10.1111/cea.12780
9. Piao CH, Fan YJ, Nguyen TV, et al. Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-KB signaling pathways. Int J Mol Sci. 2020;21:3415. doi:10.3390/ijms21103415
10. Gao X, Li N, Zhang J. a p38MAPK inhibitor, attenuates olfactory dysfunction by inhibiting OSN apoptosis in AR mice (activation and involvement of the p38 mitogen-activated protein kinase in olfactory sensory neuronal apoptosis of OVA-induced allergic rhinitis). Brain Behav. 2019;9:e01295. doi:10.1002/brb3.1295
11. Xu H, Shu H, Zhu J, et al. Inhibition of TLR4 inhibits allergic responses in murine allergic rhinitis by regulating the NF-KB pathway. Exp Ther Med. 2019;18(1):761–768. doi:10.3892/etm.2019.7631
12. Li YQ, Zhong Y, Xiao XP, et al. IL-33/ST2 axis promotes the inflammatory response of nasal mucosal epithelial cells through inducing the ERK1/2 pathway. Innate Immun. 2020;26(6):505–513. doi:10.1177/1753425920918911
13. Van Nguyen T, Piao CH, Fan YJ, et al. Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Sci Rep. 2020;10(1):12528. doi:10.1038/s41598-020-69234-1
14. Jiaru F, Minggang W, Lin Feng P, et al. Research progress of phosphatidylinositol 3-kinase/protein kinase/mammalian rapamycin target pathway in allergic rhinitis. Guang Med J. 2022;44(08):902–906.
15. May JR, Dolen WK. Management of allergic rhinitis: a review for the community pharmacist. Clin Ther. 2017;39(12):2410–2419. doi:10.1016/j.clinthera.2017.10.006
16. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1Suppl):S1–43. doi:10.1177/0194599814561600
17. Beard S. Rhinitis. Prim Care. 2014;41(1):33–46. doi:10.1016/j.pop.2013.10.005
18. Guo LY, Hung TM, Bae KH, et al. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur J Pharmacol. 2008;591:293–299. doi:10.1016/j.ejphar.2008.06.074
19. Chen F, Castranova V, Shi X, et al. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45(1):7–17. doi:10.1093/clinchem/45.1.7
20. Bui TT, Piao CH, Song CH, et al. Bupleurum chinense extract ameliorates an OVA-induced murine allergic asthma through the reduction of the Th2 and Th17 cytokines production by inactivation of NF kappaB pathway. Biomed Pharmacother. 2017;91:1085–1095. doi:10.1016/j.biopha.2017.04.133
21. Wei DZ, Guo XY, Lin LN, et al. Effects of Angelicin on Ovalbumin (OVA)-induced airway inflammation in a mouse model of asthma. Inflammation. 2016;39:1876–1882. doi:10.1007/s10753-016-0423-2
22. Wu Y, Su SA, Xie Y, et al. Murine models of vascular endothelial injury: techniques and pathophysiology. Thromb Res. 2018;169:64–72. doi:10.1016/j.thromres.2018.07.014
23. Chen K, Le Y, Liu Y, et al. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184(7):3331–3335. doi:10.4049/jimmunol.0903022
24. Jiang D, Liang J, Li Y, et al. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16(8):693–701. doi:10.1038/sj.cr.7310085
25. Kong F, Ye B, Lin L, et al. Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB signaling in PMA-stimulated THP-1 monocytes. Biomed Pharmacother. 2016;82:167–172. doi:10.1016/j.biopha.2016.04.043
26. Hu N, Wang C, Dai X, et al. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J Ethnopharmacol. 2020;248:112361. doi:10.1016/j.jep.2019.112361
27. Fransson M, Adner M, Erjefält J, et al. Up-regulation of Toll-like receptors 2, 3 and 4 in allergic rhinitis. Respir Res. 2005;6(1):100. doi:10.1186/1465-9921-6-100
28. Yoshimura A, Ohishi HM, Aki D, et al. Regulation of TLR signaling and inflammation by SOCS family proteins. J Leukoc Biol. 2004;75(3):422–427. doi:10.1189/jlb.0403194
29. Tian B, Ma X, Jiang R. Daphnetin mitigates ovalbumin-induced allergic rhinitis in mice by regulating Nrf2/HO-1 and TLR4/NF-kB signaling. Am J Rhinol Allergy. 2023;37(1):19–25. doi:10.1177/19458924221124363
30. Gangemi S, Franchina T, Minciullo PL, et al. axis: a new pathological mechanisms for EGFR tyrosine kinase inhibitors-associated skin toxicity. J Cell Biochem. 2013;114(12):2673–2676. doi:10.1002/jcb.24614
31. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3):110. doi:10.4172/2155-9899.1000110
32. Haenuki Y, Matsushita K, Futatsugi-Yumikura S, et al. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130(1):184–94.e11. doi:10.1016/j.jaci.2012.02.013
33. Carriere V, Roussel L, Ortega N, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104(1):282–287. doi:10.1073/pnas.0606854104
34. Ho LH, Ohno T, Oboki K, et al. induces IL-13 production by mouse mast cells independently of IgE-Fc RI signals. J Leuko Biol. 2007;82(6):1481–1490. doi:10.1189/jlb.0407200
35. Huang R, Mao W, Wang G, et al. Synergistic relationship between TSLP and IL-33/ST2 signaling pathways in allergic rhinitis and the effects of hypoxia. Int Forum Allergy Rhinol. 2020;10(4):511–520. doi:10.1002/alr.22504
36. Xi YD, Yu HL, Ding J, et al. Flavonoids protect cerebrovascular endothelial cells through Nrf2 andPI3K from β-amyloid peptide-induced oxidative damage. Curr Neurovasc Res. 2012;9(1):32–41. doi:10.2174/156720212799297092
37. Zhang Y, Liu B, Chen X, et al. Ameliorates behavioral dysfunction and neurological deficits in a d-galactose-induced aging mouse model through activation of PI3K/Akt/Nrf2 pathway. Rejuvenation Res. 2017;20(6):462–472. doi:10.1089/rej.2017.1960
38. Zhao M, Tang X, Gong D, et al. Bungeanum improves cognitive dysfunction and neurological deficits in D-galactose-induced aging mice via activating PI3K/Akt/Nrf2 signaling pathway. Front Pharmacol. 2020;11:71. doi:10.3389/fphar.2020.00071
39. Meng M, Zhang L, Ai D, et al. Ameliorates β-amyloid-induced neurotoxicity in PC12 cells by activating P13K/Akt/Nrf2 signaling pathway. Front Pharmacol. 2021;12:659955. doi:10.3389/fphar.2021.659955
40. Hsueh KC, Lin YJ, Lin HC, et al. Serum leptin and adiponectin levels correlate with severity of allergic rhinitis. Pediatr Allergy Immunol. 2010;21(1 Pt 2):155–159. doi:10.1111/j.1399-3038.2009.00878.x
41. Ciprandi G, De Amici M, Tosca MA, et al. Serum leptin levels depend on allergen exposure in patients with seasonal allergic rhinitis. Immunol Invest. 2009;38(8):681–689. doi:10.3109/08820130903107965
42. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174(6):3137–3142. doi:10.4049/jimmunol.174.6.3137
43. Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68(4):437–446. doi:10.1189/jlb.68.4.437
44. Zeng Q, Luo X, Tang Y, et al. Leptin regulated ILC2 Cell through the PI3K/AKT pathway in allergic rhinitis. Mediators Inflamm. 2020;2020:4176082. doi:10.1155/2020/4176082
45. Braicu C, Buse M, Busuioc C, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers. 2019;11(10):1618. doi:10.3390/cancers11101618
46. Haines DD, Lekli I, Teissier P, et al. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardio vascular, lung, neurological and kidney disorders. Acta Physiol. 2012;204(4):487–501. doi:10.1111/j.1748-1716.2011.02387.x
47. Qu LL, Yu B, Li Z, et al. Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway. Phytother Res. 2016;30(3):402–411. doi:10.1002/ptr.5541
48. Liu J, Liu L, Cui Y, et al. p38 MAPK regulates Th2 cytokines release in PBMCs in allergic rhinitis rats. J Huazhong Univ Sci Technolog Med Sci. 2010;30(2):222–225. doi:10.1007/s11596-010-0218-x
49. Brandes MS, Gray NE. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro. 2020;12:1759091419899782. doi:10.1177/1759091419899782
50. Jayaram S, Krishnamurthy PT. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson’s disease: the therapeutic role of Nrf2 activators. Neurochem Int. 2021;145:105014. doi:10.1016/j.neuint.2021.105014
51. Sharma V, Kaur A, Singh TG. Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer’s disease. Biomed Pharma Cother. 2020;129:110373. doi:10.1016/j.biopha.2020.11037
52. Piao CH, Song CH, Lee EJ, et al. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced, allergic rhinitis. Chem Biol Interact. 2020;315:108874. doi:10.1016/j.cbi.2019.108874
53. Shuo H, Shuang L, Zhe C, et al. Effect of Tanshinone IIA on mast cell-mediated allergic rhinitis through modulation of the NF- κ B pathway. J Wuhan Univ Med Edition. 2018;39(2):223.
54. Li H, Guo D, Zhang L, et al. Glycyrrhizin attenuates histamine-mediated MUC5AC upregulation, inflammatory cytokine production, and aquaporin 5 downregulation through suppressing the NF-κB pathway in human nasal epithelial cells. Chem Biol Interact. 2018;285:21–26. doi:10.1016/j.cbi.2018.02.010
55. Guo J, Xu S. Astragaloside IV suppresses histamine-induced inflammatory factors and mucin 5 subtype AC overproduction in nasal epithelial cells via regulation of inflammation-related genes. Bioengineered. 2021;12(1):6045–6056. doi:10.1080/21655979.2021.1965813
56. Zhou YJ, Wang H, Sui HH, et al. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis Guinea pigs and lipopolysaccharide-stimulated human mast cells. Inflamm Res. 2016;65(8):603–612. doi:10.1007/s00011-016-0943-0
57. Zhang M, Wang S, Liu L. Triptolide intervention in the TLR-NF-KB pathway to play an immunosuppressive role. Chin Herbs. 2017;45:9.
58. Dong J, Xu O, Wang J, et al. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol. 2021;43(3):319–327. doi:10.1080/08923973.2021.1905659
59. Wang K, Meng Y, Wang Y, et al. Molecular mechanism of the treatment of allergic rhinitis. J Transl Med. 2021;10(05):330–334.
60. Gersey ZC, Rodriguez GA, Barbarite E, et al. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer. 2017;17(1):99. doi:10.1186/s12885-017-3058-2
61. Chen H, Fu W, Chen H, et al. Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-κB signal pathways in vitro and in vivo. Mol Immunol. 2019;105:96–106. doi:10.1016/j.molimm.2018.11.008
62. Gao W, Jin Z, Zheng Y, et al. Psoralen inhibits the inflammatory response and mucus production in allergic rhinitis by inhibiting the activator protein 1 pathway and the downstream expression of cystatin‑SN. Mol Med Rep. 2021;24(3):652. doi:10.3892/mmr.2021.12291
63. Huang J, Chen X, Xie A. Formononetin ameliorates IL‑13‑induced inflammation and mucus formation in human nasal epithelial cells by activatingtheSIRT1/Nrf2 signaling pathway. Mol Med Rep. 2021;24(6):832. doi:10.3892/mmr.2021.12472
64. Lihong N, Qing X, Wei X, et al. Effect of a nasal nasal gel agent on the nuclear transcription factor- KB signaling pathway in Guinea pigs with allergic rhinitis. Chin J Trad Chin Med. 2020;31(02):441–444.
65. Fukuoka S, Adachi N, Hisamitsu T, et al. Shoseiryuto Ameliorated TDI-induced allergic rhinitis by suppressing IL-33 release from nasal epithelial cells. Pharmaceutics. 2022;14(10):2083. doi:10.3390/pharmaceutics14102083
66. Song Renjie IN. IL-33 and Allergic Rhinitis [D]. Hefei: Anhui University of Traditional Chinese Medicine; 2015.
67. Liang X, Liu CS, Xia T, et al. Identification of active compounds of mahuang fuzi xixin decoction and their mechanisms of action by LC-MS/MS and network pharmacology. Evid Based Complement Alternat Med. 2020;2020:3812180. doi:10.1155/2020/3812180
68. Zhong Y, Wang X, Xu G, et al. Modified Yupingfeng formula for the treatment of stable chronic obstructive pulmonary disease: a systematic review of randomized controlled trials. Afr J Tradit Complement Altern Med. 2013;11(1):1–14.
69. Sun Y, Liu H, Xiao X, et al. Study the mechanism of the treatment of rhinitis based on network pharmacology technology. Chin Patent Med. 2021;43(10):2893–2898.
70. Xing X, Liang Y, Wang H. Based on MAPK signaling pathway, the mechanism of Chinese medicine in immune regulation in allergic rhinitis rats. Liaon J Trad Chin Med. 2019;46(12):2647–2650+2686.
71. Liang Z, Oh K, Wang Y, et al. Cell type-specific qualitative and quantitative analysis of saikosaponins in three Bupleurum species using laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry. JPharm Biomed Anal. 2014;97:157–165. doi:10.1016/j.jpba.2014.04.033
72. Hsu MJ, Cheng JS, Huang HC. Effect of saikosaponin, a triterpene saponin, on apoptosis in lymphocytes: association with c-myc, p53, and bcl-2 mRNA. Br J Pharmacol. 2000;131(7):1285–1293. doi:10.1038/sj.bjp.0703559
73. Chen JC, Chang NW, Chung JG, Chen KC. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 2003;31(3):363–377. doi:10.1142/S0192415X03001065
74. Hsu YL, Kuo PL, Chiang LC, et al. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett. 2004;213(2):213–221. doi:10.1016/j.canlet.2004.03.044
75. Zhao H, Li S, Zhang H, et al. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-κB activation. Exp Ther Med. 2015;10(2):823–827. doi:10.3892/etm.2015.2558
76. Du ZA, Sun MN, Hu ZS. Saikosaponin a Ameliorates LPS-induced acute lung injury in mice. Inflammation. 2018;41(1):193–198. doi:10.1007/s10753-017-0677-3
77. Xu QQ, Xu YJ, Yang C, et al. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. Biomed Res Int. 2016;2016:9852536. doi:10.1155/2016/9852536
78. Li Q, Shen L, Wang Z, et al. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–114. doi:10.1016/j.biopha.2016.09.014
79. Wang X, Wei Y, Yuan S, et al. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer. 2005;116(5):799–807. doi:10.1002/ijc.20880
80. Chen R, Chen W, Huang X, et al. Tanshinone IIA attenuates heart failure via inhibiting oxidative stress in myocardial infarction rats. Mol Med Rep. 2021;23(6):404. doi:10.3892/mmr.2021.12043
81. Sato H, Goto W, Yamamura J, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30(2–3):171–177. doi:10.1016/0166-3542(96)00942-4
82. Rahman S, Sultana S. Chemo preventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity in Wistar rats. Chem Biol Interact. 2006;160(1):61–69. doi:10.1016/j.cbi.2005.12.003
83. Iida R, Otsuka Y, Matsumoto K, et al. Pseudoaldosteronism due to the concurrent use of two herbal medicines containing glycyrrhizin: interaction of glycyrrhizin with angiotensin-converting enzyme inhibitor. Clin Exp Nephrol. 2006;10(2):131–135. doi:10.1007/s10157-006-0415-x
84. Jayaprakasam B, Doddaga S, Wang R, et al. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi:10.1021/jf802601j
85. Li XL, Zhou AG, Zhang L, et al. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int J Mol Sci. 2011;12(2):905–916. doi:10.3390/ijms12020905
86. Ren S, Zhang H, Mu Y, et al. Pharmacological effects of Astragaloside IV: a literature review. J Tradit Chin Med. 2013;33(3):413–416. doi:10.1016/s0254-6272(13)60189-2
87. Guo H, Liu MP. Mechanism of traditional Chinese medicine in the treatment of allergic rhinitis. Chin Med J. 2013;126(4):756–760. doi:10.3760/cma.j.issn.0366-6999.20121844
88. Li L, Hou X, Xu R, et al. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31(1):17–36. doi:10.1111/fcp.12232
89. Zhang J, Wu C, Gao L, et al. Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. Adv Pharmaco. 2020;l(87):89–112. doi:10.1016/bs.apha.2019.08.002
90. Xu L, Li J, Zhang Y, et al. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J Ethnopharmacol. 2017:208:199–206. doi:10.1016/j.jep.2017.07.013
91. Miao J, Haibo Z, Ying D. Research progress on pharmacological effects and clinical application of triptera gontoline polyglycosides. Chin J Trad Chin Med. 2021;39(03):59–63.
92. Longyue H, Hongxin N, Xuechao Y, et al. Research progress of luteolin extraction and purification process. Chin Traditional Herbal Drugs. 2021;52(04):1185–1192.
93. Rajput SA, Shaukat A, Wu K, et al. Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants. 2021;10(8):1268. doi:10.3390/antiox10081268
94. Das AK, Mizuguchi H, Kodama M, et al. Sho-seiryu-to suppresses histamine signaling at the transcriptional level in TDI-sensitized nasal allergy model rats. Allergol Int. 2009;58(1):81–88. doi:10.2332/allergolint.O-07-526
95. Tanaka A, Ohashi Y, Kakinoki Y, et al. The herbal medicine shoseiryu-to inhibits allergen-induced synthesis of tumour necrosis factor alpha by peripheral blood mononuclear cells in patients with perennial allergic rhinitis. Acta Otolaryngol Suppl. 1998;538:118–125.
96. Ikeda Y, Iijima OT, Iizuka A, et al. Anti-inflammatory effects of mao-bushi-saishin-to in mice and rats. Am J Chin Med. 1998;26(2):171–179. doi:10.1142/S0192415X98000221
97. Chai FN, Ma WY, Zhang J, et al. Coptisine from Rhizoma coptidis exerts an anti-cancer effect on hepatocellular carcinoma by up-regulating miR-122. Biomed Pharmacother. 2018;103:1002–1011. doi:10.1016/j.biopha.2018.04.052
98. Han B, Jiang P, Li Z, et al. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Aktand mitochondrial- associated apoptotic pathway. Phytomedicine. 2018;48:152–160. doi:10.1016/j.phymed.2017.12.027
99. Zhou L, Yang F, Li G, et al. Coptisine induces apoptosis in human hepatoma cells through activating 67-kDa laminin receptor/cGMP signaling. Front Pharmacol. 2018;9:517. doi:10.3389/fphar.2018.00517
100. Wu J, Zhang H, Hu B, et al. Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur J Pharmacol. 2016;780:106–114. doi:10.1016/j.ejphar.2016.03.037
101. Fu S, Ni S, Wang D, et al. Coptisine suppresses mast cell degranulation and ovalbumin-induced allergic rhinitis. Molecules. 2018;23(11):3039. doi:10.3390/molecules23113039
102. Wang HZ, Hong M, Gui LL, et al. Effect of Yupingfeng San against OVA-induced allergic asthma in mice. Zhongguo Zhong Yao Za Zhi. 2013;38(7):1052–1055.
103. Chan PH, To CY, Chan EY, et al. A randomized placebo-controlled trial of traditional Chinese medicine as an add-on therapy to oral montelukast in the treatment of mild persistent asthma in children. Complement Ther Med. 2016;29:219–228. doi:10.1016/j.ctim.2016.10.010
104. Du CY, Choi RC, Zheng KY, et al. Yu Ping Feng San, an ancient Chinese herbal decoction containing astragali radix, atractylodis macrocephalae rhizoma and saposhnikoviae radix, regulates the release of cytokines in murine macrophages. PLoS One. 2013;8(11):e78622. doi:10.1371/journal.pone.0078622
105. Sun H, Ni X, Zeng D, et al. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res Vet Sci. 2017;110:22–28. doi:10.1016/j.rvsc.2016.10.015
106. Lingzi H, Wenliang L, Wenhui Z. Research progress of prevention and treatment of liver disease by Yupingfeng powder. World Chin Med. 2018;13(9):2357–2361.
107. Zhao Z, Gao Y, Liu W. Modern pharmacology of Yupingfeng Sanand its research progress in dermatology. Chin J Dermatovenereol Integr Tradit Western Med. 2018;17:2.
108. Mingyong Y, Zhonglin Z, Dan W. Study on the antioxidant activity of Yupingfeng powder. Hebei Med. 2014;20(6):901–904.
109. Chen H, Feng W, Lu Y, et al. Effects and mechanism of Chinese medicine Jiawei Yupingfeng in a mouse model of allergic rhinitis. J Integr Med. 2021;19(4):354–361. doi:10.1016/j.joim.2021.01.012
110. Luo Q, Zhang CS, Yang LZ, et al. Potential effectiveness of Chinese herbal medicine Yu ping feng san for adult allergic rhinitis: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med. 2017;17(1):485. doi:10.1186/s12906-017-1988-5
111. Zhou T, Shi J, Li X. Role of PI3K/Akt signaling pathway in the innate immune of sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30(11):1091–1094. Chinese. doi:10.3760/cma.j.issn.2095-4352.2018.011.016
112. Yang S, Fu Q, Deng H, et al. Mechanisms and molecular targets of the Yu-Ping-Feng powder for allergic rhinitis, based on network pharmacology. Medicine. 2021;100(35):e26929. doi:10.1097/MD.0000000000026929
113. Xianghong Z, Ying X. Progress on the role of glial cells. Straits Pharmacy. 2013;25(3):210–213.
114. Duan S, Xinling H, Ying L, et al. Close junction expression of the nasal mucosal epithelium in allergic rhinitis. Otolaryngol Head Neck Surg China. 2021;28(05):293–296.
115. Qianhong B, Lingyun L, Weiqun Z, et al. Detection and analysis of inflammatory factors, immunoglobulin and T lymphocyte subsets in the serum of patients with allergic rhinitis. Chin J Health Inspect. 2020;2020:30.
116. Wang YC, Ma DF, Jiang P, et al. Guizhi Decoction () inhibits cholinergic trans differentiation by regulating imbalance of NGF and LIF in salt-sensitive hypertensive heart failure rats. Chin J Integr Med. 2020;26(3):188–196. doi:10.1007/s11655-019-2706-6
117. Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol. 2014;39:113–204. doi:10.1016/B978-0-12-800173-8.00003-9
118. Keshari RS, Verma A, Barthwal MK, et al. Reactive oxygen s pecies-induced activation of ERK and p38 MAPK mediates PMA-induced NE Ts release from human neutrophils. J Cell Biochem. 2013;114(3):532–540. doi:10.1002/jcb.24391
119. McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:9–10.
120. Thakare VN, Osama MM, Naik SR. Therapeutic potential of curcumin in experimentally induced allergic rhinitis in Guinea pigs. Int Immunopharmacol. 2013;17(1):18–25. doi:10.1016/j.intimp.2013.04.025
121. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem. 2009;49(9):908–911.
122. Ide N, Lau BH. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J Pharm Pharmacol. 1997;49(9):908–911. doi:10.1111/j.2042-7158.1997.tb.06134.x
123. Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011;117(3):388–402. doi:10.1111/j.1471-4159.2010.07145.x
124. Anoush M, Eghbal MA, Fathiazad F, et al. The protective effects of garlic extract against Acetaminophen-induced oxidative stress and glutathione depletion. Pak J Biol Sci. 2009;12(10):765–771. doi:10.3923/pjbs.2009.765.771
125. Nahdi A, Hammami I, Kouidhi W, et al. Protective effects of crude garlic by reducing iron-mediated oxidative stress, proliferation and autophagy in rats. J Mol Histol. 2010;41(4–5):233–245. doi:10.1007/s10735-010-9283-5
126. Phan HTL, Nam YR, Kim HJ, et al. In-vitro and in-vivo anti-allergic effects of magnolol on allergic rhinitis via inhibition of ORAI1 and ANO1 channels. J Ethnopharmacol. 2022;289:115061. doi:10.1016/j.jep.2022.115061
127. Seo E, Kang H, Oh YS, et al. Seed extract attenuates diabetic nephropathy by inhibiting renal fibrosis and apoptosis in streptozotocin-induced diabetic mice. Nutrients. 2017;9(8):828. doi:10.3390/nu9080828
128. Li X, Yu C, Hu Y, et al. New application of psoralen and angelicin on periodontitis with anti-bacterial, anti-inflammatory, and osteogenesis effects. Front Cell Infect Microbiol. 2018;8:178. doi:10.3389/fcimb.2018.00178
129. Bejjani F, Evanno E, Zibara K, et al. TheAP-1transcriptional complex: local switch or remote command? Biochim Biophys Acta Rev Cancer. 2019;1872(1):11–23. doi:10.1016/j.bbcan.2019.04.003
130. Gampe N, Darcsi A, Lohner S, et al. Characterization and identification of ISO flavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC-MS/MS and NMR. J Pharm Biomed Anal. 2016;123:74–81. doi:10.1016/j.jpba.2016.01.058
131. Ong SKL, Shanmugam MK, Fan L, et al. Focus on formononetin: anticancer potential and mol ecular targets. Cancers. 2019;11(5):611. doi:10.3390/cancers11050611
132. Yi L, Cui J, Wang W, et al. Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front Pharmacol. 2020;11:533841. doi:10.3389/fphar.2020.533841
133. Wei Choo CY, Yeh KW, Huang JL, et al. Oxidative stress is associated with atopic indices in relation to childhood rhinitis and asthma. J Microbiol Immunol Infect. 2021;54(3):466–473. doi:10.1016/j.jmii.2020.01.009
134. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30(6):620–650. doi:10.1080/01926230290166724
135. Sim CS, Lee JH, Kim SH, et al. Oxidative stress in schoolchildren with allergic rhinitis: propensity score matching case-control study. Ann Allergy Asthma Immunol. 2015;115(5):391–395. doi:10.1016/j.anai.2015.07.022
136. Celik M, Tuncer A, Soyer OU, et al. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatr Allergy Immunol. 2012;23(6):556–561. doi:10.1111/j.1399-3038.2012.01294.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
