Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Neural Tube Defect in a Resource Limited Setting: Clinical Profile and Short Term Outcome
Authors Mengiste FG, Shibeshi MS , Gechera DY
Received 18 May 2023
Accepted for publication 11 September 2023
Published 19 September 2023 Volume 2023:14 Pages 289—299
DOI https://doi.org/10.2147/PHMT.S421868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Frezer Girma Mengiste,1 Mulugeta Sitot Shibeshi,1 Dagnachew Yohannes Gechera2
1Department of Pediatrics and Child Health, Hawassa University, Hawassa, Ethiopia; 2Department of Neurosurgery, Hawassa University, Hawassa, Ethiopia
Correspondence: Mulugeta Sitot Shibeshi, Email [email protected]
Background: There is a huge burden of neural tube defect (NTD) in Ethiopia, and surgical management is not readily available. We aimed to assess the clinical profile and hospital outcome of children with NTD that were operated in Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia.
Methods: A retrospective cross-sectional study on 250 children with NTD that were treated in a tertiary hospital from March 2016 to May 2020 was conducted to describe the clinical profile and treatment outcome at discharge. Logistic regression analysis was carried out to evaluate factors that determine mortality.
Results: Out of the 250 children, 50.4% were male. Myelomeningocele was the most common type of NTD (77.2%) followed by meningocele (10.4%). Only 3 mothers (1.2%) received periconceptional folic acid. Prenatal diagnosis of NTD was made in only 22 (8.8%) cases. 52.8% of the NTDs were ruptured at presentation and 50.8% had associated sepsis. At presentation, 42.4% were ≤ 72 hours of age and only 18 neonates (7.2%) were operated within 72 hours of admission. 54% had associated hydrocephalus, 31.6% had Chiari II malformation and 19.6% had club foot. Surgical site infection, post MMC repair hydrocephalus, and meningitis were seen in 8%, 14% and 16.8% of the participants, respectively. The mean duration of hospitalization was 24 ± 14.4 days. Twenty patients (8%) died before discharge from hospital. Prematurity [AOR: 26 (95% CI: 8.01, 86.04), P < 0.001] and the presence of meningitis [AOR: 3.8 (95% CI: 1.12,12.9), P = 0.03]were determinants of mortality.
Conclusion: NTDs are substantial health problem in this part of the country. Periconceptional folic acid supplementation is almost non-existent. Prenatal detection of NTDs is very low and management is delayed in the majority of cases. Myelomeningocele is the most common type of NTD. There is high in-hospital mortality, and prematurity and the presence of meningitis are its determinants.
Keywords: neural tube defect, hydrocephalus, outcome, Ethiopia
Introduction
Neural tube defects (NTD) are severe congenital malformations of the nervous system that result from failure of fusion of the neural tube during early embryogenesis. They result from genetic mutations or maternal exposure to environmental factors including maternal folate deficiency, infections, and exposure to drugs like antiepileptic drugs and other teratogens.1 Folic acid deficiency accounts for about 70% of cases of NTD;2 and folic acid deficiency is highly prevalent in women of reproductive age in Ethiopia.3,4 NTDs affect approximately one in every 500 births globally and their prevalence exceeds one in every 100 births in low income countries where there is no adequate provision of folic acid to women of reproductive age.5
Currently, the prenatal diagnosis of NTDs mainly relies on ultrasound evaluation and alpha-fetoprotein levels in the maternal serum and amniotic fluid. Recently, different novel serum biomarkers have been evaluated for NTD screening although they have limitations for routine clinical use.6 Serum levels of proprotein convertase subtilisin/kexin type 9 in pregnant women may be used as an additional biomarker for early prenatal diagnosis of NTDs as it is believed to have a role in the etiopathogenesis of NTDs and has a relatively high sensitivity.6
Studies from different regions of Ethiopia revealed that NTDs are the most common type of congenital malformations.7,8 According to a recent systematic review and meta-analysis, the estimated prevalence of neural tube defects among children in Ethiopia is 63.3 cases per 10,000 children.9 NTDs may involve the vertebrae, spinal cord, cranium, and/or brain; hence, the clinical presentation largely depends on the type and location of the lesion. Studies from different parts of Africa have shown that myelomeningocele is the most common type of NTD.10–13
Neonates born with NTD require surgical repair of the defect within 48 hours to improve survival and quality of life.14 However, neurosurgical interventions are lacking, surgery is often delayed in those who are operated, and mortality is still high in resource poor settings. Affected children face medical problems that require multidisciplinary care which is often not available in developing countries. Mortality reports from NTD vary considerably worldwide and ranged from none to 41%13,15–18 depending on the nature of the lesion, the health care system, the available treatment, and the duration of follow-up.19
A study from Ethiopia revealed that 26.1% of children who were operated for NTD developed wound-related complications and 41% of them died after 4 years of follow-up although there was no perioperative mortality.18 On the other hand, a 2% and 7.5% perioperative mortality were reported from Uganda13 and Turkey15, respectively.
Neurosurgical management of NTD was commenced in Hawassa University Comprehensive Specialized Hospital (HUCSH) following the establishment of a neurosurgical unit in 2016. However, the outcome of treatment was not studied and this study tried to assess the clinical presentation, risk factors, and hospital outcome of patients that were operated for NTD in HUCSH over a period of 4.2 years.
Methods and Materials
Study Area
The study was conducted in Hawassa University Comprehensive Specialized Hospital located about 275 km south of Addis Ababa, the capital city of Ethiopia. The hospital has been providing neurosurgical management to children with NTD since 2016. As the hospital is a tertiary care center, it receives referrals from other hospitals in the region.
Study Design, Subjects and Sample
The study was a retrospective cross-sectional hospital-based study on pediatric patients with NTD managed at HUCSH from March 2016 to May 2020 to describe the clinical profile and determine treatment outcome at discharge from hospital. A single proportion formula was used to determine the sample size and the following assumptions were made: 95% confidence level, 5% margin of error, and a 23% 30-day mortality reported in a study from Uganda13 making the sample size 272. However, there were only 250 infants that were operated during the study period and all of them were enrolled consecutively.
Data Collection Procedure
Patients’ medical records were reviewed and neonatal and maternal data relevant to the NTD were collected. Maternal information included sociodemographic data, obstetric history, folic acid supplementation, exposure to potential teratogens, and maternal health. Information about the child included age at admission, sex, time of NTD diagnosis, type of NTD and condition of the defect (ruptured/not ruptured), associated anomalies, presence of sepsis at admission, management the patient received, and outcome at discharge. The diagnosis of NTD was based on physical examination, radiological and intraoperative findings. Ruptured lesions were defined as NTDs with a ruptured sac usually with leakage of cerebrospinal fluid.
The primary outcome measure was inpatient mortality, while secondary outcome measures were various complications occurring after surgical intervention. Complications included CSF leakage, wound infection, meningitis and wound dehiscence.
Data Processing and Analysis
Data were entered in the Statistical Package for Social Sciences software (version 23) for windows after cleaning, and descriptive and analytic statistics were done as applicable. Sociodemographic characteristics and NTD-related variables were summarized using frequency distribution tables. Mean/median and standard deviation/inter quartile range were calculated for continuous data. Logistic regression analysis was carried out to evaluate factors that determine mortality. A P-value of <0.05 was considered statistically significant.
Ethical Consideration
The study was conducted after obtaining ethical clearance from the Institutional Review Board of Hawassa University, College of Medicine and Health Sciences. This study adhered to the standards outlined in the Helsinki Declaration and its subsequent amendments. The Institutional Review Board of Hawassa University’s College of Medicine and Health Sciences waived the requirement for parental consent due to the retrospective nature of the study. However, the study participants’ personal information was anonymized.
Results
The study was conducted to assess the clinical profile and short term management outcome of children with NTDs. A total of 250 babies (50.4% male) were included in the study. The age at admission ranged from 1 day to 14 months; and only 106 neonates (42.4%) were admitted to hospital within the first 72 hours. None of the study subjects were product of consanguineous marriage. The mean maternal age was 26.24 ± 5.35 years and more than half (62.8%) of them were multiparous (mean number of pregnancies: 2.61 ± 1.78). Five mothers reported that they had previous pregnancies complicated with NTD. The majority of mothers had antenatal care (94.8%) and obstetric ultrasound examination (87.6%) during the index pregnancy; however, antepartum diagnosis of NTD was made in only 22 study subjects (8.8%). Only 3 mothers (1.2%) received folic acid supplementation during the periconceptional period. Febrile illness during the first trimester of the index pregnancy was documented in only 9 mothers (3.6%). None of the mothers had history of diabetes and no mother took antiepileptic drugs during pregnancy. The mode of delivery was spontaneous vaginal delivery (SVD) in the majority (90.8%) of cases. Nearly 90% of cases were born at term. Out of the 49 cases with club foot, all except one (a case with lipomyelocele) had MMC. The maternal obstetric history is summarized in Table 1.
 |
Table 1 The Maternal Obstetric History and Risk Factors for NTD |
The majority of the study population (77.2%) had MMC; and most MMCs were located in the lumbosacral area (38.7%). In more than half of the cases (52.8%) the NTDs were ruptured at presentation (Figure 1) and 50.8% were treated for sepsis. Among the study subjects, 135 (54%) had associated hydrocephalus; and the hydrocephalus was identified after repair in the majority (75%) of cases. The clinical profile of children with NTD is summarized in Table 2.
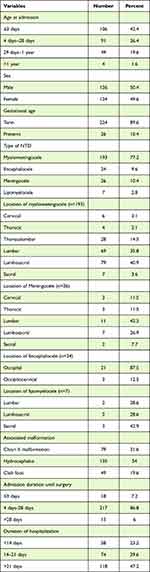 |
Table 2 Clinical Profile of Children with NTD (n = 250) |
 |
Figure 1 Status of NTDs at presentation (n = 250). Abbreviation: MMC, myelomeningocele. |
Only 18 neonates (7.2%) were operated within 72 hours of admission, and 15 patients stayed in the hospital before surgery for >28 days. Defect repair was the most common surgical procedure (96%) and ventriculoperitoneal shunt insertion along with defect repair was done in only 5 patients. The type of surgery performed is summarized in Table 3.
 |
Table 3 Types of Surgery Performed |
Post MMC repair hydrocephalus, meningitis/ ventriculitis, surgical site infection, and CSF leak were seen in 40.4%, 16.8%, 8%, and 5.2% of the participants, respectively. The mean duration of hospitalization was 24 ± 14.4 days. Twenty patients (8%) died after surgical management before discharge from hospital. Prematurity and the presence of meningitis/ventriculitis were determinants of mortality (Table 4).
 |
Table 4 Logistic Regression Analysis on Factors Associated with Mortality in Children with NTD (N = 250) |
Premature infants with NTD were 26 times more likely to die than those born at term [AOR= 26 (95% CI: 8.01, 86.04), p < 0.001)]. Similarly, infants with meningitis/ventriculitis were 3.8 times more likely to die than those without meningitis/ventriculitis [AOR=3.8(95% CI: 1.12,12.9), p = 0.03)] (Table 4).
Discussion
The study identified 250 patients with NTDs that underwent neurosurgical management in a tertiary hospital in Southern Ethiopia over a 4.2 year period. Periconceptional folic acid supplementation is almost non-existent in this setting. Although most of the mothers had obstetric ultrasound examination, the rate of prenatal diagnosis of NTD is very low. Our study revealed that MMC is the most common type of NTD. There is a significant neurosurgical management delay in the majority of the study subjects. In hospital mortality was observed in 8% of cases, and prematurity and the presence of meningitis/ventriculitis were determinants of mortality in this study.
Although both genetic and environmental risk factors are incriminated in the pathogenesis of NTDs, many of the risk factors are still undetermined. Maternal folate deficiency before and during early pregnancy, obesity, diabetes mellitus, hypertension, fever and hyperthermia in early pregnancy, anti-epileptic drugs and environmental pollutants are some of the environmental factors that influence the development of NTD.1,15 Maternal periconceptional folic acid supplementation prevents the occurrence of neural tube defects significantly;5,20 however, about 30% of NTDs are still not preventable by consumption of folic acid.2
Ethiopia faces a high level of folate deficiency among women;3 a recent study revealed that 78% of women of reproductive age in Ethiopia had low folate status.4 WHO recommends that all women, from the moment they begin trying to conceive until 3 months of gestation, should receive folic acid supplementation. Moreover, a woman who has a fetus diagnosed with NTD or has given birth to a child with NTD should receive information on the risk of recurrence, be advised on the protective effect of periconceptional folate supplementation and be offered high-dose supplementation.21
In this study, 5 women had previously given birth to children with NTD but only 3 of them received periconceptional folate to prevent recurrence – indicating a gap in the provision of optimal maternal and child health services. Fortification of the staple diet with folic acid is a safe, cost-effective, and sustainable intervention to reduce the incidence of NTDs.5
The Ethiopian Standards Council endorsed the mandatory fortification of edible oil and wheat flour in June 2022, a decision, if implemented, will significantly reduce the country’s high burden of NTD.
In this study, diagnosis of NTD was made prenatally in only 8.8% of cases although most of the mothers had antenatal care (94.8%) and obstetric ultrasound examination (87.6%). This is in contrast to a South African study in which diagnosis of NTD was made prenatally in 71% of cases.10 A study conducted in Barcelona revealed a 94% overall prenatal detection rate of NTDs.22 Measures should be taken to improve prenatal diagnosis of NTDs to provide counseling to parents, to allow better prediction of outcome, and to conduct the delivery in a setting where neurosurgical services are available.22
There is controversy regarding the optimal mode of delivery for fetuses with open NTDs. Earlier studies showed that cesarean delivery before the onset of labor had resulted in better subsequent motor function;23 however, recent studies have revealed that the mode of delivery has no impact on neurological outcome of children with open NTDs.24,25 In this study, the mode of delivery was cesarean in 9.2% of cases, a finding much lower than the 70.4% reported in a study from Turkey.15
NTDs are heterogeneous in their severity ranging from anencephaly which is not compatible to life to a potentially asymptomatic closed spina bifida.1 In this study, the most common type of NTD was myelomeningocele (77.2%), a finding in line with the result of a systematic review and meta-analysis on the magnitude of NTDs in Ethiopia9 and in Africa at large.10–13 Similarly, a study conducted in Turkey revealed that more than half (54.3%) of patients with NTDs had myelomeningocele.15 Most of the MMCs were located in the lumbosacral area, a finding similar to reports from Nigeria12 and Denmark.26
In our study, 54% of the cases had associated hydrocephalus. This finding is comparable to reports from India (58.8%)27 and Nigeria (53.8%);12 however, higher (75%) and lower (21.6%) figures were reported from Turkey15 and Ethiopia,18 respectively.
Neurosurgical management of open NTDs is recommended immediately after delivery for better outcome. A study conducted in Brazil showed that surgical management of myelomeningocele immediately after birth was associated with a lower incidence of preoperative rupture of the myelomeningocele, postoperative dehiscence and lower incidence of developmental delay at 1 year of age.28 According to a study in the US, myelomeningocele closure delayed for more than 1 day after birth was associated with an increased rate of infection and length of stay.29 However, provision of immediate surgical management to patients with NTD remains a challenge in Ethiopia. In this study, only 42.4% of the cases were admitted to hospital within the first 72 hours of birth and only 7.2% were operated within the first 72 hours of admission. Several factors may play a role for the delayed presentation to hospital. As majority of the families (61.2%) are from rural areas they may have limited awareness on the need for immediate surgical management and/or they may have financial problems to cover their transportation costs. After patients are admitted to hospital, unavailability of equipment and waiting until infections are treated are some of the causes of delayed surgical treatment in this setting. In our study, half of the patients had sepsis at admission; the delayed presentation in the majority of cases and the ruptured sac in nearly half of the cases might have contributed for the high prevalence of sepsis.
Postoperative complications that have been commonly reported in previous studies include wound dehiscence,17 CSF leaks,30 post MMC repair hydrocephalus,31 and infections.17,29 In this report, one or more postoperative complications were observed in 144 cases (57.6%), a finding higher than the 31% reported from Zambia.17
The 16.8% meningitis/ventriculitis observed in our study is similar to the finding in a Turkish study (16.4%). Moreover, the 8% who developed surgical wound infection in our setting is comparable to the 11% reported in the same Turkish study.32
Postoperative CSF leaks were observed in 5.2% of our cases and this could be partly explained by the large proportion of patients (40.4%) with post MMC repair hydrocephalus. In a study from India, a higher percentage (12.7%) of patients developed postoperative CSF leaks.30
Mortality in infants with NTD varies considerably worldwide depending on the severity of the lesion, the presence of multiple defects, the availability and use treatment, and the health care systems.19 The 1 year mortality from NTD in a 2018 Turkish cohort was 13.5%16 while a 34% mortality was reported from Uganda.13 The 30-day mortality rate of infants with NTD in a Ugandan study was 23%.13 The in hospital mortality rate in this study was 8% which was similar to the 7% from Zambia17 and the 7.5% reported from Turkey.15 However, a study conducted to evaluate the outcome of 88 children operated for NTD in a similar setting in Ethiopia revealed no perioperative mortality.18 In this report, prematurity and the presence of meningitis/ventriculitis were the determinants of mortality. According to the finding of a systematic review and meta-analysis, prematurity and LBW are the strongest predictors of NTD associated mortality.19 Similarly, a national level retrospective study in the US revealed that mortality was significantly higher in premature and low birth weight babies.33 The mean duration of hospitalization in our study (24 ± 14.4 days) is comparable to the 20 days (median) reported from Uganda.13
This study has a number of limitations. As the study was a retrospective study, some important data were missed. Since patients were not followed for a prolonged period of time, the long-term outcome was unknown.
In conclusion, NTDs are substantial health problem in this part of the country. Periconceptional folic acid supplementation is almost non-existent in this setting, calling for the immediate implementation of mandatory fortification of common foods in Ethiopia. There is low prenatal detection of NTD and delayed surgical management in the majority of cases, requiring concerted efforts by stake holders to develop capacity for prenatal detection of the problem and early surgical management of neonates with NTD. There was high in-hospital mortality, and prematurity and the presence of meningitis/ventriculitis were its determinants. We recommend a prospective study to determine the long term outcome of children with NTD in this setting.
Abbreviations
AOR, Adjusted Odds Ratio; COR, Crude Odds Ratio; CI, Confidence Interval; CSF, cerebro-spinal fluid; HUCSH, Hawassa University Comprehensive Specialized Hospital; MMC, myelomeningocele; NTD, neural tube defect; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to all individuals who participated in the data collection.
Author Contributions
All authors contributed significantly to this work in the conception, study design, execution, data acquisition, analysis, and interpretation; participated in the drafting, revising, or critical review of the article; gave final approval of the version to be published; agreed on the journal to which the article will be submitted; and agreed to be responsible for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Isaković J, Šimunić I, Jagečić D, et al. Overview of neural tube defects: gene–environment interactions, preventative approaches and future perspectives. Biomedicines. 2022;10(5):965. doi:10.3390/biomedicines10050965.
2. Botto DL, Moore AC, Khoury JM, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341(20):1509–1519. doi:10.1056/NEJM199911113412006
3. Haidar J, Melaku UPR. Folate deficiency in women of reproductive age in nine administrative regions of Ethiopia: an emerging public health problem. S Afr J Clin Nutr. 2010;23:132–137.
4. Sisay BG, Tamirat H, Sandalinas F, et al. Folate deficiency is spatially dependent and associated with local farming systems among women in Ethiopia. Curr Dev Nutr 2022; 6: 1–11. 5 10.1093/cdn/nzac088
5. Kancherla V, Botto LD, Rowe LA, et al. Preventing birth defects, saving lives, and promoting health equity: an urgent call to action for universal mandatory food fortification with folic acid. Lancet Glob Heal. 2022;10(7):e1053–e1057. doi:10.1016/S2214-109X(22)00213-3
6. Erol SA, Tanacan A, Firat Oguz E, et al. A comparison of the maternal levels of serum proprotein convertase subtilisin/kexin type 9 in pregnant women with the complication of fetal open neural tube defects. Congenit Anom. 2021;61(5):169–176. doi:10.1111/cga.12432
7. Silesh M, Lemma T, Fenta B, et al. Prevalence and trends of congenital anomalies among neonates at Jimma Medical Center, Jimma, Ethiopia: a three-year retrospective study. Pediatr Heal Med Ther. 2021;12:61–67.
8. Mekonnen D, MollaTaye Worku W, Worku W. Congenital anomalies among newborn babies in Felege-Hiwot Comprehensive specialized referral hospital, Bahir Dar, Ethiopia. Sci Rep. 2021;11(1):1–8. doi:10.1038/s41598-021-90387-0
9. Alebel A, Alemu A. Magnitude and associated factors of neural tube defects in Ethiopia: a systematic review and meta-analysis. Glob Pediatr Heal. 2020;7:1–14.
10. Krzesinski EI, Geerts L, Urban MF. Neural tube defect diagnosis and outcomes at a tertiary South African hospital with intensive case ascertainment. S Afr Med J. 2019;109(9):698–703. doi:10.7196/SAMJ.2019.v109i9.13863
11. Atlaw D, Tekalegn Y, Sahiledengle B, et al. Magnitude and determinants of neural tube defect in Africa: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21(1):1–14. doi:10.1186/s12884-021-03848-9
12. Alatise OI, Adeolu AA, Komolafe EO, et al. Pattern and factors affecting management outcome of spina bifida cystica in Ile-Ife, Nigeria. Pediatr Neurosurg. 2006;42(5):277–283. doi:10.1159/000094062
13. Xu LW, Vaca SD, He JQ, et al. Neural tube defects in Uganda: follow-up outcomes from a national referral hospital. Neurosurg Focus. 2018;45(4):1–6. doi:10.3171/2018.7.FOCUS18280
14. Spina Bifida Association. Guidelines for the Care of People with Spina Bifida; 2018.
15. Yorulmaz A, Konak M. Short-term results of patients with neural tube defects followed-up in the Konya region, Turkey. Birth Defects Res. 2019;111(5):1–9. doi:10.1002/bdr2.1462
16. Çaylan N, Yalçin SS, Tezel B, et al. Evaluation of neural tube defects from 2014 to 2019 in Turkey. BMC Pregnancy Childbirth. 2022;22(1):1–11. doi:10.1186/s12884-022-04678-z
17. Reynolds RA, Bhebhe A, Garcia RM, et al. Surgical outcomes after myelomeningocele repair in Lusaka, Zambia. World Neurosurg. 2021;145:e332–e339. doi:10.1016/j.wneu.2020.10.069
18. Getahun S, Masresha S, Zenebe E, et al. Four-year treatment outcomes of children operated for neural tube defect in Addis Ababa, Ethiopia: a Retrospective Study. World Neurosurg. 2021;148:e695–e702. doi:10.1016/j.wneu.2021.01.098
19. Ho P, Quigley MA, Tatwavedi D, et al. Neonatal and infant mortality associated with spina bifida: a systematic review and meta-analysis. PLoS One. 2021;16(5):1–23. doi:10.1371/journal.pone.0250098
20. Simkiss D. Preventing neural tube defects. J Trop Pediatr. 2017;63(2):85–86. doi:10.1093/tropej/fmw079
21. World Health Organization. Prevention of neural tube defects. In: Standards for Maternal and Neonatal Care. Department of Making Pregnancy Safer, World Health Organization; 2007:1–72.
22. Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenat Diagn. 2009;29(4):402–411. doi:10.1002/pd.2250
23. Luthy DA, Wardinsky T, Shurtleff DB, Hollenbach KA, Hickok DE, Nyberg DA. Cesarean section before the onset of labor and subsequent motor function in infants with meningomyelocele diagnosed antenatally. N Engl J Med. 1991;324(10):662–666. doi:10.1056/NEJM199103073241004
24. Tolcher MC, Shazly SA, Shamshirsaz AA, et al. Neurological outcomes by mode of delivery for fetuses with open neural tube defects: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. 2019;126(3):322–327. doi:10.1111/1471-0528.15342
25. Yachida N, Itsukaichi M, Haino K, et al. Influence of route of delivery on perinatal outcomes in fetuses with myelomeningocele. Clin Exp Obstet Gynecol. 2019;46(2):277–279. doi:10.12891/ceog4780.2019
26. Nielsen LAG, Maroun LL, Broholm H, et al. Neural tube defects and associated anomalies in a fetal and perinatal autopsy series. Apmis. 2006;114(4):239–246. doi:10.1111/j.1600-0463.2006.apm_325.x
27. Kumar R, Singhal N. Outcome of meningomyelocele/lipomeningomyelocele in children of Northern India. Pediatr Neurosurg. 2006;43:7–14. doi:10.1159/000097518
28. Pinto FCG, Matushita H, Furlan ALB, et al. Surgical treatment of myelomeningocele carried out at ‘time zero’ immediately after birth. Pediatr Neurosurg. 2009;45:114–118. doi:10.1159/000209285
29. Attenello FJ, Tuchman A, Christian EA, et al. Infection rate correlated with time to repair of open neural tube defects (myelomeningoceles): an institutional and national study. Child Nerv Syst. 2016;32(9):1675–1681. doi:10.1007/s00381-016-3165-4
30. Balasubramaniam C, Rao SM, Subramaniam K. Management of CSF leak following spinal surgery. Child Nerv Syst. 2014;30(9):1543–1547. doi:10.1007/s00381-014-2496-2
31. Sgouros S. Hydrocephalus with Myelomeningocele. Pediatr Hydroceph. 2005; 5(4):133–144.
32. Demir N, Peker E, Gülşen İ, et al. Factors affecting infection development after meningomyelocele repair in newborns and the efficacy of antibiotic prophylaxis. Child Nerv Syst. 2015;31(8):1355–1359. doi:10.1007/s00381-015-2701-y
33. Davidoff MJ, Petrini J, Damus K, et al. Neural tube defect-specific infant mortality in the United States. Teratology. 2002;66(S1):S17–S22. doi:10.1002/tera.90005.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
