Back to Journals » Cancer Management and Research » Volume 16
Negative Impact of Intra-Operative Blood Transfusion on Survival Outcomes of Hepatocellular Carcinoma Patients
Authors Teng L, Zhao L, Shao H, Dai J, Zou H
Received 17 November 2023
Accepted for publication 11 April 2024
Published 25 April 2024 Volume 2024:16 Pages 385—393
DOI https://doi.org/10.2147/CMAR.S448629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Lei Teng, Liuyuan Zhao, Hongxue Shao, Junzhu Dai, Huichao Zou
Department of Pain Medicine, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
Correspondence: Huichao Zou, Department of Pain Medicine, Harbin Medical University Cancer Hospital, 150 Haping Road, Harbin, 150081, People’s Republic of China, Tel +86-451-86298909, Email [email protected]
Background: Studies have reported that blood transfusion may have an association with survival outcomes of cancer patients. This study was aimed at finding the effect of intra-operative blood transfusion on the prognosis of patients of hepatocellular carcinoma (HCC).
Methods: This was a retrospective study. HCC patients who underwent tumor resection from January 2013 to November 2018 at Harbin Medical University Cancer Hospital were included. The survival time of patients receiving or not receiving blood transfusion during the operation were compared.
Results: Of HCC patients, 21.1% (102/484) received intra-operative blood transfusion. After propensity score matching, 87 pairs of patients were included in the study. In the subset of patients with a tumor size of > 4 cm, univariable analysis found that there were significant differences in recurrence-free survival (RFS; P=0.004) and overall survival (OS; P=0.028) between blood transfusion and non-blood transfusion groups. After multivariable Cox regression analysis, intra-operative blood transfusion was an independent risk factor for RFS (HR: 2.011, 95% CI: 1.146– 3.529, P=0.015), but not for OS (HR: 1.862, 95% CI: 0.933– 3.715, P=0.078) in the subset of patients with a tumor size of > 4 cm.
Conclusion: Intra-operative blood transfusion was associated with worse RFS in HCC patients with a tumor size of > 4 cm.
Keywords: hepatocellular carcinoma, intra-operative blood transfusion, recurrence-free survival, overall survival
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy.1 It ranks third among causes of cancer-related death.1,2 Surgical removal of the tumor remains the most effective treatment method.3 Because of the complicated anatomical structure of the liver, the percentage of patients receiving a blood transfusion is relatively high,4,5 currently 22%.6
Initiating transfusion of allogeneic red blood cells to patients with liver cancer who have experienced life-threatening bleeding during the operation is currently non-negotiable. Increasing evidence suggests, however, that transfusion of blood cells may be associated with negative outcomes. Some studies show that peri-operative transfusion could decrease survival rates and increase incidence rates of cancer recurrence and metastasis in cancer patients, while other studies show the opposite.1,7–14 It has been hypothesized that blood transfusions may promote the metastasis and recurrence of tumors through suppressing the immune function. Despite common use, the impact of blood transfusions during the operation on prognosis in patients with liver cancer remains unclear. It is necessary to validate the risks and benefits of blood transfusions for cancer patients rather than using blood transfusion as routine therapy. We conducted an updated systematic review and meta-analysis to evaluate the influence of blood transfusions on survival outcomes of lung cancer patients. Thus, we conducted a retrospective study to investigated the impact of intra-operative blood transfusion on the prognosis of HCC patients who underwent surgery.
Methods
Patient Selection
From January 2013 to November 2018, 484 patients with HCC who received transfusion of red blood cells during liver resection at Harbin Medical University Cancer Hospital were included. This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of Harbin Medical University Cancer Hospital (2021–172R). Informed consent from the patients was waived by the IRB because the nature of this retrospective study was reanalyzing of existing data, which does not involve any potential risks and benefits to the patients. Only patients who received blood transfusion during the operation were included in the study. Patients received blood transfusion before or after the operation were excluded. Patients who had a history of previous surgery, incomplete follow-up data or another type of cancer were excluded from the study.
Variables
Patient information was obtained from the electronic medical records of Harbin Medical University Cancer Hospital. According to the median tumor size (maximal diameter of the tumor), HCC patients were divided into the group with a tumor size greater than 4 cm and the group with a diameter less than or equal to 4 cm. Then the patients were divided into the blood transfusion group and non-blood transfusion group. Intra-operative blood transfusion was defined as transfusing allogeneic whole blood and/or concentrated red cells (CRCs) during surgery.12 Transfusion of albumin, fresh-frozen plasma or platelets was not included in this study.
Survival Outcomes
Recurrence-free survival (RFS) and overall survival (OS) were used as primary outcomes. RFS was defined as the time from the date of surgery to the date of first recurrence or death from any cause, whichever occurred first. OS was defined as the time from the date of surgery to the date of death from any cause. In the statistical model, those patients who remained free of disease were censored.
Statistical Analysis
Continuous variables were expressed as mean ± SD. Student’s t-test was used to analyze continuous variables. Percentages and frequency counts were defined as categorical variables. Categorical variables were analyzed with Chi-square test or Fisher’s exact test. RFS and OS were analyzed with Kaplan-Meier methods. Univariate Cox models and log rank tests were used to analyze all variables. Independent predictors of RFS and OS were analyzed with Cox proportional hazards regression models after univariate analysis. All significant factors (P<0.05) were retained in the final model. To ensure the comparability of baseline characteristics between the blood transfusion and non-blood transfusion groups, we used propensity score matching (PSM) analysis to select a nearest propensity score (caliper set to 0.2 SD of the logit of the PS) for all variables across blood transfusion or non-blood transfusion groups in a ratio of 1:1. SPSS 26.0 (IBM, Armonk, NY) were used for all analyses.
Results
Demographic and Clinical Features
A total of 484 HCC patients were included in this study after selection; 21.1% (102/484) had received a blood transfusion. The patients were classified into two sub-sets according to lesion diameter: >4 cm (n=236, 48.8%) and ≤4 cm (n=248, 51.2%). Patients’ baseline information is shown in Tables 1 and 2, respectively. After PSM, the matched cohort showed that all variables did not differ significantly between the blood transfusion group and non-blood transfusion group within each subset.
 |
Table 1 Baseline Characteristics of Patients Who Received a Blood Transfusion and Those Who Did Not in Different HCC Size Subsets in the Entire Cohort (n=484) |
 |
Table 2 Baseline Characteristics of Patients Who Received a Blood Transfusion and Those Who Did Not in Different HCC Size Subsets in the Matched Cohort (n=174) |
Impact of Blood Transfusion on Survival Outcome in the >4 Cm Subset Group
After propensity score matching, in the > 4 cm subset group, the KM curve showed that RFS (P=0.004) and OS (P=0.028) between the blood transfusion and non-blood transfusion groups (Figures 1 and 2) were different. There was no difference in RFS or OS between the blood transfusion and non-blood transfusion groups in the subset of patients with a tumor size of ≤4 cm (Figures 3 and 4). Univariable Cox regression analysis found that blood transfusion was a risk factor for both RFS (P=0.004) (Table 3) and OS (P=0.028) (Table 4). Age (P=0.005) was associated with lower RFS (Table 3). TNM stage was also associated with lower RFS (P=0.019) (Table 5) and OS (except stage II) (P=0.017) (Table 4). Multivariable analysis showed that blood transfusion was an independent predictor for lower RFS (HR: 2.011, 95% CI: 1.146–3.529, P=0.015) (Table 5) but not for OS (HR: 1.862, 95% CI: 0.933–3.715, P=0.078) (Table 4). Age (HR: 0.966, 95% CI: 0.934–0.998, P=0.040) was associated with lower RFS (Table 3). TNM stage (except stage II) was associated with both lower RFS (HR: 1.923, 95% CI: 1.097–3.371, P=0.022) (Table 3) and OS (HR: 2.222, 95% CI: 1.130–4.368, P=0.021) (Table 4).
 |
Table 3 Univariable and Multivariable Cox Regression Analysis of Recurrence-Free Survival after Resection for HCC in Propensity-Matched Patients with a Tumor Size of >4 Cm |
 |
Table 4 Univariable and Multivariable Cox Regression Analysis of Overall Survival After Resection for HCC in Propensity-Matched Patients with a Tumor Size of >4 Cm |
 |
Table 5 Univariable Cox Regression Analysis of Recurrence-Free Survival and Overall Survival Identified after Resection for HCC in Propensity-Matched Patients with a Tumor Size of ≤4 Cm |
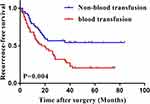 |
Figure 1 Kaplan–Meier curves for recurrence-free survival of blood transfusion and non-blood transfusion groups after PSM: >4 cm subset group. |
 |
Figure 2 Kaplan–Meier curves for overall survival of blood transfusion and non-blood transfusion groups after PSM: >4 cm subset group. |
 |
Figure 3 Kaplan–Meier curves for recurrence-free survival of blood transfusion and non-blood transfusion groups after PSM: ≤4 cm subset group. |
 |
Figure 4 Kaplan–Meier curves for overall survival of blood transfusion and non-blood transfusion groups after PSM: ≤4 cm subset group. |
Impact of Blood Transfusion on Survival Outcome in the ≤4 Cm Subset Group
In the subset of patients with a tumor size of ≤4 cm, intra-operative blood transfusion had no association with RFS (HR: 1.266, 95% CI: 0.702–2.282, P=0.427) or OS (HR: 1.533, 95% CI: 0.726–3.322, P=0.251) using univariable Cox regression analysis (Table 5).
Discussion
In the present study, we found that intra-operative blood transfusion was associated with lower RFS and OS in HCC patients who underwent surgery with a tumor size of >4 cm, but not in HCC patients who underwent surgery with a tumor size of ≤4 cm.
Previous studies have found that peri-operative blood transfusion may be associated with complications including allergic reaction, infection, immunosuppression, cancer recurrence and increased mortality.15–17 The impact of peri-operative blood transfusion on the survival outcomes of patients with HCC after hepatectomy remains controversial. Results of a meta-analysis which included 7241 HCC patients showed that blood transfusion was associated with increased incidence of post-operative complications and reduced disease-free survival time after tumor resection.18 Chen et al19 and Feng et al20 investigated the impact of blood transfusion on the survival time of HCC patients at different BCLC (Barcelona Clinic Liver Cancer) stages. They both found that peri-operative blood transfusion was associated with poor prognosis of HCC patients at the BCLC-A stage, but not for those at the BCLC-B/C stage. Peng et al21 found that peri-operative allogeneic blood transfusion negatively influenced the survival outcomes of HCC patients at AJCC (American Joint Committee on Cancer) stage I, but not for those at AJCC stages II, III and IV after hepatectomy.
In this study, we investigated the effect of blood transfusion on the survival time of HCC patients with different tumor sizes who underwent surgery. We found that the RFS and OS of patients who underwent surgery and received a blood transfusion were shorter than those of patients who underwent surgery but did not receive a blood transfusion. Moreover, our study also proved that blood transfusion is an independent predictor for the prognosis of HCC patients who undergo surgery for a tumor larger than 4 cm. Hepatocellular carcinoma involving large tumors may mean more microvascular invasion or multiple tumors,22 which may be one of the reasons for this finding.
The mechanism of blood transfusion affecting tumor prognosis may be caused by immunosuppression. Allogeneic blood transfusion may contribute to the suppression of T-cell activity, reduced activity of natural killer cells, decreased lymphocyte counts and increased release of immunosuppressive prostaglandins.12,23–25 In addition, Patel et al found that blood transfusion may stimulate proliferation and angiogenesis of endothelial cells.26
Limitations
Our research had several limitations. Firstly, the sample size in our study was relatively small after PSM. Secondly, selection bias is inevitable even after PSM since this study was retrospectively performed. Thirdly, we had no information on anti-tumor therapies received by HCC patients before and after surgery.
Conclusion
In conclusion, our study showed that intra-operative blood transfusion was an independent risk factor for the RFS and OS of HCC patients who underwent surgery for a tumor greater in size than 4 cm. Thus, it is advisable to avoid unnecessary transfusions during the operation for HCC patients with larger tumor. Well-designed prospective multi-center studies are needed to validate the exact influence of blood transfusion on the prognosis of cancer and whether the volume of blood transfusion matters.
Funding
This work was supported by the Haiyan Foundation of Harbin Medical University Cancer Hospital (JJZD2023-12).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yamashita Y-I, Hayashi H, Imai K, et al. Perioperative allogeneic blood transfusion does not influence patient survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. World J Surgery. 2019;43(11):2894–2901.
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72(2):262–276. doi:10.1016/j.jhep.2019.11.017
4. Kim JH. Pure laparoscopic right hepatectomy using modified liver hanging maneuver: technical evolution from caudal approach toward ventral approach. J Gastrointestinal Surg. 2018;22(8):1343–1349. doi:10.1007/s11605-018-3736-7
5. Nanashima A, Ariizumi S-I, Yamamoto M. Right anatomical hepatectomy: pioneers, evolution, and the future. Surgery Today. 2020;50(2):97–105. doi:10.1007/s00595-019-01809-6
6. Cescon M, Vetrone G, Grazi GL, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249(6):995–1002. doi:10.1097/SLA.0b013e3181a63c74
7. Wada H, Eguchi H, Nagano H, et al. Perioperative allogenic blood transfusion is a poor prognostic factor after hepatocellular carcinoma surgery: a multi-center analysis. Surgery Today. 2018;48(1):73–79. doi:10.1007/s00595-017-1553-3
8. Kuroda S, Tashiro H, Kobayashi T, Oshita A, Amano H, Ohdan H. No impact of perioperative blood transfusion on recurrence of hepatocellular carcinoma after hepatectomy. World j Surgery. 2012;36(3):651–658. doi:10.1007/s00268-012-1425-3
9. Yang T, Lu J-H, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: a Propensity Score Matching Analysis. J Hepatol. 2016;64(3):583–593. doi:10.1016/j.jhep.2015.10.012
10. Xu L-N, Xu -Y-Y, Gao D-W. Impact of operative and peri-operative factors on the long-term prognosis of primary liver cancer patients undergoing hepatectomy. J Huazhong Univ Sci Tech. 2016;36(4):523–528. doi:10.1007/s11596-016-1619-2
11. Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancer‐free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72(6):1866–1871. doi:10.1002/1097-0142(19930915)72:6<1866::AID-CNCR2820720613>3.0.CO;2-F
12. Sugita S, Sasaki A, Iwaki K, et al. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intra-operative allogenic blood transfusion: a retrospective study. Eur J Surgical Oncol. 2008;34(3):339–345. doi:10.1016/j.ejso.2007.02.010
13. Harada N, Shirabe K, Maeda T, Kayashima H, Ishida T, Maehara Y. Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child–Pugh class A patients. World j Surgery. 2015;39(4):1044–1051. doi:10.1007/s00268-014-2891-6
14. Peng T, Zhao G, Wang L, et al. No impact of perioperative blood transfusion on prognosis after curative resection for hepatocellular carcinoma: a propensity score matching analysis. Clin Transl Oncol. 2018;20(6):719–728. doi:10.1007/s12094-017-1773-4
15. Marcucci C, Madjdpour C, Spahn DR. Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index. Br Med Bul. 2004;70(1):15–28.
16. Okamura Y, Takeda S, Fujii T, Sugimoto H, Nomoto S, Nakao A. Prognostic significance of postoperative complications after hepatectomy for hepatocellular carcinoma. J Surgical Oncol. 2011;104(7):814–821. doi:10.1002/jso.21977
17. Elwood NR, Martin AN, Turrentine FE, Jones RS, Zaydfudim VM. The negative effect of perioperative red blood cell transfusion on morbidity and mortality after major abdominal operations. Am J Surg. 2018;216(3):487–491. doi:10.1016/j.amjsurg.2018.02.015
18. Xun Y, Tian H, Hu L, Yan P, Yang K, Guo T. The impact of perioperative allogeneic blood transfusion on prognosis of hepatocellular carcinoma after radical hepatectomy: a systematic review and meta-analysis of cohort studies. Medicine. 2018;97(43):e12911. doi:10.1097/MD.0000000000012911
19. Chen G-X, C-Y Q, W-J H, et al. Perioperative blood transfusion has distinct postsurgical oncologic impact on patients with different stage of hepatocellular carcinoma. BMC Cancer. 2020;20(1):1–12. doi:10.1186/s12885-020-06980-5
20. Xia F, Zhang Q, Huang Z, et al. Effect of Perioperative Blood Transfusion on the Postoperative Prognosis of Ruptured Hepatocellular Carcinoma Patients With Different BCLC Stages: a Propensity Score Matching Analysis. Front Surgery. 2022;9.
21. Peng T, Wang L, Cui H, et al. Impact of perioperative allogeneic blood transfusion on the long-term prognosis of patients with different stage tumors after radical resection for hepatocellular carcinoma. Eur J Surg Oncol. 2021;47(3):620–627. doi:10.1016/j.ejso.2020.09.021
22. Ishii T, Hatano E, Yasuchika K, Taura K, Seo S, Uemoto S. High risk of lung metastasis after resection of hepatocellular carcinoma more than 7 cm in diameter. Surgery Today. 2014;44(10):1900–1905. doi:10.1007/s00595-013-0792-1
23. Cata J, Wang H, Gottumukkala V, Reuben J, Sessler D. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110(5):690–701. doi:10.1093/bja/aet068
24. Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91(4):771–778. doi:10.1002/1097-0142(20010215)91:4<771::AID-CNCR1063>3.0.CO;2-9
25. Torrance HD, Brohi K, Pearse RM, et al. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Ann Surg. 2015;261(4):751–759. doi:10.1097/SLA.0000000000000653
26. Patel H, Nasir F, Nash G, Scully M, Kakkar A. Enhanced angiogenesis following allogeneic blood transfusion 1. Clin Lab Haematol. 2004;26(2):129–135. doi:10.1111/j.1365-2257.2004.00589.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
