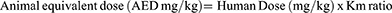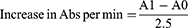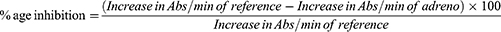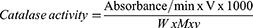Back to Journals » Journal of Experimental Pharmacology » Volume 16
Methanol Crude Peel Extract of P. granatum Prevents Oxidative Damage in Kidneys of Rats Exposed to Highly Active Antiretroviral Therapy
Authors Kwizera E , Ssekatawa K, Aja PM , Miruka CO, Wandera A, Mpumbya JR, Siida R, Shehu D, Salihu TS
Received 28 September 2023
Accepted for publication 17 December 2023
Published 6 January 2024 Volume 2024:16 Pages 1—11
DOI https://doi.org/10.2147/JEP.S438368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Eliah Kwizera,1 Kenneth Ssekatawa,2,3 Patrick Maduabuchi Aja,1 Conrad Ondieki Miruka,1 Allan Wandera,1 Jackie Rachael Mpumbya,1 Robert Siida,1 Dayyabu Shehu,1 Tijjani Shinkafi Salihu1
1Department of Biochemistry, Faculty of Biomedical Sciences, Kampala International University-Western Campus, Bushenyi, Uganda; 2Department of Science, Technical and Vocational Education, Makerere University, Kampala, Uganda; 3Africa Center Excellence in Materials Product Development and Nanotechnology (MAPRONANO ACE), Makerere University, Kampala, Uganda
Correspondence: Kenneth Ssekatawa, Email [email protected]
Background: Highly Active Antiretroviral Therapy (HAART) has been linked to oxidative damage to kidney cells leading to renal disease in people living with HIV/AIDS on HAART treatment. The toxic effects of HAART affect the patients’ quality of life leading to poor adherence to their regimen. Therefore, the purpose of this study was to investigate the nephron-protective activity of methanol crude peel extract of Punica granatum (MPEPG) in HAART-administered Wistar rats.
Methods: Thirty male albino Wistar rats weighing between 180– 200g were randomly divided into six groups of five rats each. Group one served as normal control and was given distilled water only. Group two serves as a negative control and was given HAART at a dosage of 64 mg/kg. Groups 3 and 4 were given 100 and 400 mg/kg of MPEPG, respectively, while groups 5 and 6 were given MPEPG dosages of 100 and 400 mg/kg along with HAART, respectively, for 40 days. The rats were sacrificed under halothane anaesthesia, and the kidneys were removed for histological evaluation, while blood samples were analyzed for biochemical parameters.
Results: In the HAART (TLD) treated group, there was a significantly high amount of MDA and a lower level of the antioxidant enzymes SOD and CAT. Biochemical analysis revealed that animals treated with HAART (TLD) had significantly higher levels of urea and creatinine, which are biomarkers of kidney damage than the normal control animals. In contrast, all the kidney function markers were returned to normal levels in the HAART-treated group after administration of methanol crude peel extract of P. granatum. The kidney tissues of animals given HAART had considerable structural damage as revealed by histopathological studies. When HAART-exposed rats were treated with MPEPG, both the biochemical and histological results significantly improved.
Conclusion: Methanol crude peel extract of P. granatum provided effective protection against kidney oxidative injury brought on by HAART because of its anti-oxidant and free radical scavenging properties.
Keywords: HAART, oxidative stress, nephrotoxicity, Punica granatum
Background
Highly Active Antiretroviral therapy (HAART) is considered an effective therapy for HIV viral suppression, thus aiding in the prevention of morbidity and death in HIV-positive patients.1 TLD (Tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and dolutegravir (DTG)) is a HAART drug combination that forms the first-line treatment regimen for HIV and has proven to be effective in suppressing viral replication but it has been associated with potential kidney toxicity.2
The nucleotide analog reverse transcriptase inhibitor tenofovir, which causes proximal tubule damage, is one of the most common causes of nephrotoxic side effects of TLD.3–5 Tenofovir-induced tubule malfunction or nucleoside reverse transcriptase inhibitor-induced severe mitochondrial dysfunction and lactic acidosis can both result in renal damage.3 Mitochondrial dysfunction disrupts the electron transport chain, which increases the generation of reactive oxygen species (ROS) and impairs the ability of the body’s natural antioxidant system to remove them.6,7 These free radicals cause cell death, renal damage due to lipid peroxidation, and oxidation of DNA, proteins, and carbohydrates.8
The edible fruit Punica granatum (PG), often known as pomegranate, is a member of the Punicaceae genus. Since ancient times, the pomegranate has been utilized in traditional medicine as an antiviral agent, anti-diarrheal, and anti-inflammatory.9 Active components having antibacterial and antioxidant properties can be found in the plant’s leaves, stem, roots, fruits, and seeds.9 PG fruit peel contains antioxidants that can slow plasma lipid peroxidation and halt the free radical chain reaction.10,11 The primary components in PG peel with very strong antioxidant activity are polyphenols, anthocyanins, and flavonoids, which are hypothesized to have nephro-protective effects by scavenging free radicals that cause oxidative stress.11–13 Additionally, it was discovered that PG peels contain numerous phenolic compounds with protective properties against damage to renal tubular cells and oxidative stress brought on by free radicals.14–16 Prior studies have highlighted the potent antioxidant properties found in the methanol peel extract of PG.2,17,18 These properties have demonstrated protective effects against drug-induced toxicity by bolstering cellular oxidative status.11 Yet, exploring the potential protective effects of MPEPG against the toxicities associated with HAART remains an unexplored avenue worth investigating. Hence, this study aims to explore the protective effects of MPEPG against HAART-induced nephrotoxicity-related oxidative damage in Wistar rats.
Materials and Methods
Plant Material and Extract Preparation
Fresh PG fruits were collected from Kanyanya, Kampala district. Freshly collected branches with flowers and fruits were taken to Makerere University’s herbarium for authentication. The voucher specimen was added to the Makerere University Herbarium, College of Natural Sciences, and given the accession number MHU51208. The fruits were cleaned under clean running water and skin was peeled off. The peels were allowed to dry under the shade on the laboratory bench. The dried peels were ground with a laboratory blender Saachi NL-BL-4361 and five hundred grams (500 g) of the powder transferred into a clean conical flask. To the peel powder, 2000 mL of 70% methanol was added. The mixture was continuously stirred for 3 days with an orbital shaker before being filtered through Whatman size 1 filter paper into a clean container and concentrated to dryness using a rotary evaporator. The dried extract was then stored at 4°C in the refrigerator until further use.
Preparation of Highly Active Anti-Retroviral Therapy
A fixed-dose combination of Tenofovir (300 mg), Lamivudine (300 mg), and Dolutegravir (50 mg) was employed in the study (TLD). The tablets were supplied by Comboni ART clinic in Bushenyi district. The tablets were crushed into a fine powder, dissolved in distilled water, and kept at 4°C until needed to create the TLD treatments. Using this formula, drug doses were calculated by converting human doses to animal doses;
Km is a correction factor that takes into account the connection between body weight and surface area. Rats have a Km ratio of 6.2.19 Animals were given drug dosage via oral gavage utilizing catheters throughout the trial.
Animals
The rats were purchased from the Animal House at Kampala International University-Western Campus (KIU-WC) and were kept under standard laboratory conditions of a 12-hr/12-hr light/dark cycle at room temperature with free access to food and water. All procedures and animal work were carried out in accordance with the guide for the care and use of laboratory animals.
Experimental Design
Thirty (30) male Wistar albino rats aged 12 weeks, weighing 150–200g were randomly assigned to six groups of five rats each: Throughout the experiment, Group one (Normal control) received only distilled water orally. Group two (negative control) received only TLD orally daily for 40 days. Group three received 100mg/kg MPEPG orally for 40 days. Group four received 400mg/kg MPEPG orally for 40 days. Group five received TLD concurrently with 100mg/kg body weight MPEPG given by oral gavage daily for 40 days. Group six received TLD as well as an oral dose of MPEPG (400 mg/kg body weight). The animals were euthanized on day 41 by cardiac exsanguination under anaesthesia with an overdose of halothane. TLD was a fixed-dose combination pill containing tenofovir, lamivudine, and dolutegravir in a ratio of 300mg:300mg:50mg, respectively. TLD dosage was calculated using the human equivalent dose for laboratory animals,19 while the dosage for the plant extract was calculated using LD50 (2000 mg/Kg) as reported by.20 Dosages at 5% and 20% of LD50 were chosen for use in the present study as previously used by21
Biochemical Assays
Levels of urea, creatinine, and electrolytes, which are markers of kidney damage, were measured in serum using an automatic analyser (BS-200 Chemical analyzer, Mindray, UK).
Measurement of Oxidative Stress Markers
Preparation of the Kidney Homogenates
The kidneys were collected after the abdomen was excised, rinsed twice in 0.1 M potassium phosphate with a pH of 7.5, and then blotted between two filter papers. Kidneys were each cut into two pieces, and 10% of the kidney homogenate was prepared using one piece of the kidney. The activity of antioxidant enzymes was assessed in the homogenates in addition to the amounts of MDA, a marker of lipid peroxidation brought out by oxidative stress. The remaining pieces of the kidney were used for histopathological examination. The preparation of 10% kidney homogenate was carried out according to El- Beshbishyet al.22 Briefly, 100 mg of tissue was cut into small pieces and ground in a mortar with a pestle while cold. After adding 900 µL of ice-cold buffer (0.1 M potassium phosphate; pH 7.5), the homogenate was centrifuged at 10,000 g for 15 min with a Hettich Zentrifugen D-78532 Tuttlingen centrifuge. The supernatant was transferred to a new tube and used for the MDA and anti-oxidant enzyme assays.
Measurement of MDA Levels
MDA is a byproduct of lipid peroxidation that reacts with thiobarbituric acid (TBA) to form a pink complex with a peak absorbance of 532 nm. Prabhakar et al described a method for determining MDA levels in supernatants.23 An aliquot of clear kidney homogenate (200 µL) was mixed with 2 mL of TBA-TCA reagent (0.375% and 15%, respectively). The volume was increased to 3 mL with double distilled water, then incubated in a 95°C water bath for 20 min before cooling under tap water. n-Butanol (3 mL) was used to extract the reaction product (TBA-MDA complex). A BSSUV-202 spectrophotometer was used to measure the absorbance of the pink-colored extract in n-butanol at 532 nm. To obtain the average MDA, this procedure was repeated three times. The amount of MDA formed per gram weight of the tissue was calculated using a molar extinction coefficient of 1.56×10−5 M−1cm−1 and expressed as nmoles of MDA formed per gram of the tissue as shown by formula 2.
Measurement of Antioxidant Enzymes Activities
Measurement of Superoxide Dismutase (SOD) Activity
Superoxide dismutase (SOD) catalyzes superoxide dismutation into oxygen and hydrogen peroxide. This study uses procedures outlined by Afolabi et al24 to determine superoxide dismutase activity. The assay is based on SOD’s ability to inhibit epinephrine autoxidation (adrenaline). To equilibrate the spectrophotometer, 2 ml of supernatant (liver homogenate) were added to 2.5 mL of 0.05 M carbonate buffer (pH 10.2). The reaction was started by quickly mixing 0.3 mL of freshly prepared 0.3mM adrenaline into the mixture. The reference tube was made up of 2.5 mL of 0.05 M carbonate buffer (pH 10.2), 0.3 mL of substrate (adrenaline), and 0.2 mL of water. The increase in absorbance (Abs) at 480 nm caused by the formation of adrenochrome (Adreno) was measured at 30 s and at 150 s. The SOD activity was calculated using equations 3 and 4.
A0 = Abs after 30 seconds
A1 = Abs after 150 seconds
Where one unit of SOD activity was defined as the amount of SOD required to inhibit the auto-oxidation of adrenaline to adrenochrome by 50% for 1 min.
Measurement of Catalase (CAT) Activity
Catalase activity was determined using the method described by Cohen et al,25 as cited by Afolabi et al.24 Zero-point five milliliters of supernatant were added to 15 mL ice-cold tubes. By adding 5 mL of 30 mM H2O2, a reaction was started. By inverting the tube, the contents were thoroughly mixed. After 3 min, 1 mL of 6M H2SO4 was used to stop the reaction. After adding 7 ml of 0.01 M potassium permanganate (KMnO4), absorbance at 545 nm was measured within 30–60 s.
V=total volume of the reaction mixture; W=Weight of tissue; M=molar extinction coefficient that is 40.0, v=volume of sample used.
Measurement of Serum Biomarkers of Renal Damage
The following renal function tests were performed on the sera specimens: urea, creatinine, and electrolytes using Mindray Diagnostic kits and following the manufacturer’s instructions using an automated BS-200 Chemical analyzer, Mindray (UK).
Histopathological Examination
Each animal’s kidneys were removed, blotted with normal saline between filter paper, fixed in 10% neutral buffered formalin, embedded in paraffin, and sliced into 5-µm-thick slices. Sections were mounted on glass slides, deparaffinized in xylene, rehydrated decreasing concentrations of ethanol, and then stained with hematoxylin and eosin stain. Dibutyl Phthalate in Xylene (DPX) mounting medium was used to adhere the coverslips to the sections of the glass slide. The morphological changes in the stained kidney sections were observed using a light microscope Nikon Eclipse Ci fitted with a camera.26
Statistical Analysis
The collected data were statistically analyzed using Graph pad prism version 7 software. The means of the groups were compared using one-way analysis of variance (ANOVA) and Tukey multiple comparison analysis. A p-value ≤ 0.05 indicated significant statistical variance.
Results
Effect of Methanol Crude Peel Extract of PG on the Levels of Oxidative Stress Markers
Effect of Methanol Crude Peel Extract of PG on MDA Levels
To assess the degree of stress in the kidneys, MDA levels were measured. According to the study’s results (Figure 1), the TLD-treated group (Group 2) had significantly greater MDA levels in the kidney homogenate than the normal control group (P < 0.05). Following treatment of MPEPG + TLD, MDA levels in the kidney (P < 0.0001) homogenates significantly (P < 0.05) decreased (Group 5–6). When compared to the TLD group, the MPEPG-treated groups (Groups 3–4) had considerably lower renal MDA levels. When compared to the normal control group, MDA levels did not significantly increase when MPEPG alone was utilized (Group 1). Group 2 had the highest MDA levels (4.7 ± 1.048), while Group 4 had the lowest (1.02 ± 0.36). The mean differences between the MPEPG extract groups (3–6) and the normal control group (Group 1) were statistically insignificant (p > 0.05). Group 2 exhibited a statistically significantly higher value (p < 0.05) when the mean values of the negative control (Group 2) were compared to the normal control (Group 1) and extract Groups (3–6) as shown in (Figure 1a).
Effect of Methanol Crude Peel Extract of PG on Activities of Enzymatic Antioxidants
Inhibition of epinephrine autoxidation by SOD in alkaline circumstances was measured as a percentage (%) of SOD activity, whereas CAT activity was stated as micromoles of H202 produced per litre of sample (mol of H202/l) of kidney tissue. According to the study’s findings, Group 2 (TLD treated) had significantly (P < 0.05) lower SOD and CAT activity in the kidney homogenate than the normal control group (Group 1). Administration of MPEPG + TLD (Groups 5 and 6) significantly (P < 0.05) raised SOD and CAT activity in comparison to the negative control (Group 2). The mean values of MPEPG alone at 100 mg/kg and 400 mg/kg for the kidney homogenates demonstrated an insignificant increase in SOD and CAT activity (P > 0.05) compared to the normal control (Group 1) and a significant increase compared to the negative control (Group 2) (Figure 1b and c).
Effect of Methanol Crude Peel Extract of PG on Serum Biomarkers of HAART Induced Renal Damage in Wistar Rats
When TLD was administered to rats (group 2), the mean values of renal serum biomarkers (BUN, creatinine, Na, K, and Cl) increased significantly (p < 0.05) compared to rats in the normal control group. When compared to the TLD-treated group, administration of methanol crude extract of PG plus TLD (groups 5 and 6) significantly decreased (p < 0.05) the mean values of BUN, creatinine, and electrolytes concentration (group 2). When compared to the normal control group, there were no significant changes in renal serum biomarkers of damage in the groups that received MPEPG alone (Figure 2).
Histopathological Architecture
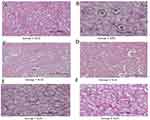
The kidneys in the control group had typical renal parenchyma morphology, with clearly defined tubules and identifiable lumen (Figure 3A). In TLD-treated rats, morphological alterations such as tubular epithelial degeneration, vacuolization, and necrosis were clearly seen (Figure 3B). In the MPEPG-TLD cocktail treated rats, the proximal tubules of the kidneys exhibited mild tubular degeneration, epithelial vacuolization, and no necrosis (Figure 3E and F). Figures 3C and D show that MPEPG administration alone had no effect on normal kidney structure.
Discussion
Although antiretroviral drugs have significantly reduced morbidity and mortality in HIV-infected people, these benefits are limited by a number of adverse clinical events, toxicities, and side effects.27 The kidneys are target organs of toxicity due to their role in excretory part of xenobiotics.28 Nephrotoxicity is a serious issue for HAART patients because it can cause necrosis and death,29 with 6% to 30% of HAART patients exhibiting significantly elevated serum levels of renal markers of damage.30
P. granatum (PG) is renowned for its many phytochemicals and strong antioxidant capacity, which can halt free radical chain reactions and postpone plasma lipid peroxidation, protecting important organs including the kidney. PG fruit peel has significant antioxidant capacity due to its high total polyphenol content, specifically flavonoids, condensed tannins, ellagitannins, and anthocyanins.31,32 As a result, the current study was designed to assess the nephroprotective effects of P. granatum methanol crude fruit extract against HAART-induced renal damage in Wistar rats.
The results of this study revealed that MDA levels in kidney homogenate were higher than the normal control group with a significance level of P < 0.0001 in TLD alone. This suggests that HAART therapy increases the formation of ROS, which are extremely unstable, reactive, and capable of oxidizing lipids, hence elevating MDA levels. Free radicals generated by biotransformation HAART are accompanied by a significant immune function decline, which in turn causes a significant increase in polyunsaturated lipid peroxidation.6 This investigation’s findings are consistent with those of Manda et al33 and Gil et al,34 who observed considerably high levels of MDA in HAART treated rats. However, TLD and MPEPG extract treatments at low and high doses had MDA levels comparable to those of normal control rats and the rats that received the extract alone. This suggests that MPEPG extract can protect against the oxidative stress caused by HAART.
The results of this investigation also showed that when TLD was administered to the rats, both SOD and CAT activity significantly decreased when compared to the normal control group. Whereas, treatment with MPEPG at both low and high doses and administration with TLD significantly boosted SOD and CAT activity. Different anti-oxidant enzymes are known to remove ROS under physiological circumstances. It has been demonstrated that the activity of these enzymes decreases after prolonged exposure to oxidative stress.35 Endogenous ROS-eliminating enzymes like SOD and CAT protect cellular macromolecules from oxidative damage by eliminating ROS. Superoxide radical is transformed by SOD into hydrogen peroxide, which is then broken down by CAT to produce water and oxygen. The results of this study are consistent with those of Offer et al,36 who showed that after exposing rats to HAART, SOD, and CAT enzyme activity decreased. The decline in CAT and SOD activity in the TLD administered group may be caused by oxidative stress, which increases ROS generation and probably causes oxidation of these enzymes or proteins involved in their regulation hence their decreased activity. Based on the findings of this study, MPEPG significantly increases enzymatic antioxidant activities by preventing oxidation of these enzymes through lowering levels of ROS, thus providing an antioxidative therapeutic benefit against HAART-induced oxidative stress.
Kidney damage can significantly affect electrolyte balance in the body. Kidneys play a crucial role in regulating the levels of various electrolytes, including sodium, potassium, calcium, and phosphate. It also deals with the elimination of nitrogenous metabolic waste products like urea, creatinine, and uric acid.37 Elevations in serum electrolytes, urea, and creatinine are regarded as useful indicators for investigating drug-induced nephrotoxicity in both people and animals.37 Serum urea and creatinine concentrations were significantly greater in the TLD group as compared to the normal control group, indicating a decline in renal function. The active buildup of tenofovir disoproxil fumarate (TDF) in the proximal renal tubule causes TLD associated renal impairment.38 TDF inhibition of mitochondrial DNA polymerase3 leads to energy deprivation and oxidative stress in the mitochondrion.39 Injury caused by ROS peroxidation of unsaturated fatty acids in kidney tubular cells would result in an increase in serum BUN and creatinine due to decreased excretion by this section of the nephron.
This is in line with the findings of this study as well as Offor et al findings, who treated rats with HAART (Triplavar).40 All rats given P. granatum methanol crude peel extract and TLD showed changed plasma levels of urea and creatinine, suggesting that PG fruit peel extract might prevent tubular injury. It has been demonstrated that, PG pre-treatment significantly reduced the levels of creatinine and BUN in rats, alleviating all symptoms of chronic renal failure.41 Additionally, the TLD-treated group’s serum Na, K, and Cl levels were significantly greater than those in the control group, indicating renal injury. By preventing a rise in blood urea, creatinine, and electrolytes, PG peel extract treatment protects renal function from adverse effects of TLD. By protecting the structural integrity of renal cells against the challenge of HAART, PG peel extract enhances renal function as shown by a considerable decrease in serum creatinine, urea, and electrolyte levels.
The study’s biochemical analysis was further supported by histopathological findings. The assessment of kidney sections from TLD treated rats indicated glomerular capillary abnormalities such as tubular necrosis, vacuolization, and tubular epithelial desquamation, indicating that disruptions between the mesangial cell and glomerular capillaries may have been dramatically affected. Glomerular abnormalities frequently lead to a reduction in the amount of surface area available for filtration, glomerular filtration rate, and metabolic activity. However, the groups that received MPEPG and TLD in combination displayed decreased vacuolization, no necrosis, and minor alterations in the cellular structure of the liver and kidney sections.
In this study, a methanol crude peel extract of P. granatum significantly reduced the levels of renal damage markers elevated by HAART, as well as preventing pathological changes in renal tubular cellular architecture. The decreased levels of renal biomarkers, as well as the maintenance of normal renal cellular architecture in the TLD and MPE PG treated groups, led to the conclusion that P. granatum methanol crude peel extract protects rats from renal damage caused by HAART. Similar research on nephron protection has also been reported.42–44 According to Alimoradian et al, antioxidant, anti-inflammatory, and nephroprotective activities have been demonstrated for anthocyanins, β-carotene, and phenolic compounds.44 In addition, it has been demonstrated that condensed tannins exhibit anti-inflammatory, antioxidant, and free radical scavenging capabilities,45 whereas saponins have been shown to have protective properties through modulation of their antioxidant and anti-inflammatory activities.40,41 The synergistic action of flavonoids, condensed tannins, and anthocyanins in medicinal plants may also account for their nephroprotective activity.46
Therefore, the anti-oxidative properties of the plant may be the cause of the nephroprotective activities shown by P. granatum methanol crude peel extract. Flavonoids, tannins, and phenolic chemicals, which are recognized for their potent antioxidant properties, have been found in PG extracts, according to earlier investigations on the phytochemistry of PG extracts.44
Conclusion
The administration of HAART (TLD) to rats caused oxidative stress, as evidenced by increased MDA levels and a decrease in SOD and CAT activity, as well resulted into renal damage as evidenced by increased levels of serum urea, creatinine, and electrolytes, as well as remarkable diffuse lipid droplets and necrosis in the micrographs, but this was prevented by daily administration of P. granatum methanol crude peel extract for 40 days. This study found that P. granatum methanol crude peel extract can protect against HAART (TLD)-induced nephrotoxicity by restoring the anti-oxidative defense system. The presence of flavonoids, tannins, and phenolic compounds in the extracts may contribute to the P. granatum peel’s nephroprotective activity via free radical scavenging and antioxidant activity.
Abbreviations
3TC, Lamivudine; ALB, Albumin; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; ANOVA; Analysis of variance; ART, Antiretroviral therapy; AST, Aspartate aminotransferase; BIL-T, Total bilirubin; CAT, Catalase; CCl4, Carbon tetrachloride; DPX, Dibutyl Phthalate in Xylene; DTG, Dolutegravir; HAART, Highly active antiretroviral therapy; HIV, Human immunodeficiency virus; KIU-WC, Kampala International University-western campus; KMnO4, potassium permanganate; MDA, Malondialdehyde; MPEP, Methanol crude peel extract of P. granatum; OECD, Organization for Economic Cooperation and Development; OS, Oxidative stress; PG, Punica granatum; ROS, reactive oxygen species; SOD, Superoxide dismutase; TCA; trichloroacetic acid; TDF, Tenofovir; TBA, thiobarbituric acid; TLD, Tenofovir, Lamivudine, Dolutegravir; TPro, Total protein.
Data Sharing Statement
All data generated by this study have been submitted with this manuscript.
Ethics Approval and Informed Consent
The study involved the use of animals and the Kampala International University-Western Campus Animal Research Ethics Committee provided ethical approval for all animal experiments (Approval number: SF/202031). The animals were treated humanely to avoid any discomfort, such as excessive pain and stress. The study adhered to the 3Rs principles of Reduce, Refine, and Replace to ensure use of the fewest number of animals possible according to Organisation for Economic Co-operation and Development (OECD) protocols.
Acknowledgments
We are thankful to the Central Diagnostic Laboratory, College of Veterinary Medicine Animal Resources and Biosecurity, Makerere University, and Biochemistry Laboratory, Kampala International University for availing laboratory space.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors acknowledge with sincere gratitude the funding from Africa Centre of Excellence in Materials Product Development and Nanotechnology (MAPRONANO)-Makerere University (Project ID: P151847).
Disclosure
Allan Wandera reports grants from MAPRONANO ACE, during the conduct of the study. The rest of the authors declare that they have no competing interests.
References
1. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017;4(1):1–7. doi:10.1136/bmjgast-2017-000166
2. World Health Organization. Update of first- and second-line antiretroviral regimens. HIV Treat. 2019;2019:1.
3. Kalemeera F, Rennie T, Godman B, Stergachis A. Tenofovir disoproxil fumarate associated nephrotoxicity: a retrospective cohort study at two referral hospitals in Namibia. Pharmacoepidemiol Drug Saf. 2020;30(2):189–200. doi:10.1002/pds.5125
4. Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol. 2009;5(10):563–573. doi:10.1038/nrneph.2009.142
5. Daugas E, Rougier JP, Hill G. HAART-related nephropathies in HIV-infected patients. Kidney Int. 2005;67(2):393–403. doi:10.1111/j.1523-1755.2005.67096.x
6. Williams AA, Meyer D. Molecular BioSystems detectable by metabonomics. Mol Biosyst. 2017. doi:10.1039/C7MB00336F
7. Lewis W, Copeland WC, Day BJ. Mitochondrial DNA depletion, oxidative stress, and mutation: mechanisms 0f dysfunction from nucleoside reverse transcriptase inhibitors. Lab Invest. 2001;81(6):777–790. doi:10.1038/labinvest.3780288
8. Mebrat Y, Amogne W, Mekasha A, Gleason RL Jr, Seifu D. Lipid peroxidation and altered antioxidant profiles with pediatric HIV infection and antiretroviral therapy in Addis Ababa, Ethiopia. J Tro. 2017;63:196–202. doi:10.1093/tropej/fmw076
9. Sharma J, Maity A. Pomegranate phytochemicals: nutraceutical and therapeutic values. Fruit Veg Cereal Sci Biotechnol. 2010;4(2):56–76.
10. Panth N, Manandhar B, Paudel KR. Anticancer activity of Punica granatum (pomegranate): a review. Phytother Res. 2017;31(4):568–578. doi:10.1002/ptr.5784
11. Shaban NZ, El-Kersh MAL, El-Rashidy FH, Habashy NH. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141(3):1587–1596. doi:10.1016/j.foodchem.2013.04.134
12. Zahin M, Aqil F, Ahmad I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat Res Genet Toxicol Environ Mutagen. 2010;703(2):99–107. doi:10.1016/j.mrgentox.2010.08.001
13. Middha SK, Usha T, Pande V. HPLC evaluation of phenolic profile, nutritive content, and antioxidant capacity of extracts obtained from Punica granatum fruit peel. Advan Pharmacol Pharmaceut Sci. 2013;2013:1.
14. El-Daly AA. Pomegranate peel extract protects cadmium-induced nephrotoxicity in albino mice. J Biosci Appl Res. 2016;2(6):362–375. doi:10.21608/jbaar.2016.108529
15. Karwasra R, Kalra P, Gupta YK, Saini D, Kumar A, Singh S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016;7(7):3091–3101. doi:10.1039/c6fo00188b
16. Emam NM, Anjum S, Okail HA, Ibrahim MAR, Ahmad T. Pomegranate peel extract protects against carbon tetrachloride-induced nephrotoxicity in mice through increasing antioxidants status. Biomed Rep. 2020;13(3):1–9. doi:10.3892/br.2020.1320
17. Kennas A, Amellal-Chibane H. Comparison of five solvents in the extraction of phenolic anti-oxidants from pomegranate (Punica granatum L.) peel. North African J Food Nutr Res. 2019;3(5):140–147. doi:10.51745/najfnr.3.5.140-147
18. Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50(1):81–86. doi:10.1021/jf010865b
19. Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27. doi:10.4103/0976-0105.177703
20. Jacinto AMT. Review of the phytochemical, pharmacological and toxicological properties of Punica granatum L. J Sci Food Agric. 2018;2(3):71–83. doi:10.26855/jsfa.2018.03.004
21. Mestry SN, Gawali NB, Pai SA, et al. J. Ayurveda Integr. Med Punica granatum improves renal function in gentamicin-induced nephropathy in rats via attenuation of oxidative stress. J Ayurveda Integr Med. 2020;11(1):16–23. doi:10.1016/j.jaim.2017.09.006
22. El-Beshbishy HA, Tork OM, El-Bab MF, Autifi MA. Antioxidant and antiapoptotic effects of green tea polyphenols against azathioprine-induced liver injury in rats. Pathophysiology. 2011;18(2):125–135. doi:10.1016/j.pathophys.2010.08.002
23. Prabhakar PV, Reddy UA, Singh SP, et al. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol. 2012;32(6):436–445. doi:10.1002/jat.1775
24. Afolabi OB, Oloyede O, Olayide I, et al. Antioxidant enhancing ability of different solvents extractable components of Talinum triangulare in some selected Tissue homogenates of Albino Rats -In vitro. J Appl Pharm Sci. 2015;5(9):056–061. doi:10.7324/JAPS.2015.50911
25. Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34(1):30–38. doi:10.1016/0003-2697(70)90083-7
26. Slaoui M, Bauchet AL, Fiette L. Tissue sampling and processing for histopathology evaluation. Methods Mol Biol. 2017;1641:101–114. doi:10.1007/978-1-4939-7172-5_4
27. Esté JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir Res. 2010;85(2010):25–33. doi:10.1016/j.antiviral.2009.10.007
28. Singh A, Bhat TK, Sharma OP. Clinical biochemistry of hepatotoxicity journal of clinical toxicology. J Clin Microbiol. 2011;S4(001):1–19. doi:10.4172/2161-0495.S4-001
29. Esté JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir Res. 2010;85(1):25–33. doi:10.1016/j.antiviral.2009.10.007
30. Baynes HW, Tegene B, Gebremichael M, Birhane G, Kedir W, Biadgo B. Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: a retrospective study. HIV/AIDS - Res Palliat Care. 2017;9:1–7. doi:10.2147/HIV.S120979
31. Rahmani AH, Alsahli MA, Almatroodi SA, Rahmani AH, Alsahli MA. Active constituents of pomegranates (Punica granatum) as potential candidates in the management of health through modulation of biological activities. Pharmacogn J. 2017;9(5):689–695. doi:10.5530/pj.2017.5.109
32. Ashoush IS. Antioxidant activity and hepatoprotective effect of pomegranate peel and whey powders in rats. Ann Agric Sci. 2013;58(1):27–32. doi:10.1016/j.aoas.2013.01.005
33. Mandas A, Iorio EL, Congiu MG, et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol. 2009;2009:7. doi:10.1155/2009/749575
34. Gil L, Tarinas A, Hernández D, et al. Altered oxidative stress indexes related to disease progression marker in human immunodeficiency virus infected patients with antiretroviral therapy. Biomed Aging Pathol. 2011;1(1):8–15. doi:10.1016/j.biomag.2010.09.001
35. Zhao L, Zhang N, Yang D, et al. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients. 2018;10(11):1754. doi:10.3390/nu10111754
36. Offor U, Coleridge E, Olalekan O, Isaac A, Imo A, Okpara O. Nephrotoxicity and highly active antiretroviral therapy: mitigating action of Momordica charantia. Toxicol Rep. 2018;5(September):1153–1160. doi:10.1016/j.toxrep.2018.09.003
37. Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AAK, Vernekar SN. Markers of renal function tests. North Am J Med Sci. 2010;2(4):2–5.
38. Perazella MA. Tenofovir-induced kidney disease: an acquired renal tubular mitochondriopathy. Kidney Int. 2010;78(11):1060–1063. doi:10.1038/ki.2010.344
39. Blas-garcía A, Apostolova N, Esplugues JV. Oxidative stress and mitochondrial impairment after treatment with anti-HIV drugs: clinical implications. Curr Pharm Des. 2011;17(36):4076–4086. doi:10.2174/138161211798764951
40. Offor U, Naidu EC, Ogedengbe OO, Jegede AI, Peter AI, Azu OO. Nephrotoxicity and highly active antiretroviral therapy: mitigating action of Momordica charantia. Toxicol Rep. 2018;5(February):1153–1160. doi:10.1016/j.toxrep.2018.09.003
41. El-habibi E. Renoprotective effects of Punica granatum (pomegranate) against adenine- induced chronic renal failure in male rats. Life Sci J. 2013;10(4):2059–2069.
42. Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by Acetaminophen in rats. Food Chem Toxicol. 2009;47(7):1480–1484. doi:10.1016/j.fct.2009.03.034
43. Al-Olayan EM, El-Khadragy MF, Metwally DM, Abdel Moneim AE. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014;14. doi:10.1186/1472-6882-14-164
44. Alimoradian A, Changizi-Ashtiyani S, Farahani AG, Kheder L, Rajabi R, Sharifi A. Protective effects of pomegranate juice on nephrotoxicity induced by captopril and gentamicin in rats. Iran J Kidney Dis. 2017;11(6):422–429.
45. Aloqbi A, Omar U, Yousr M, Grace M, Lila MA, Howell N. Antioxidant activity of pomegranate juice and punicalagin. Nat Sci. 2016;2016:235–246.
46. Bhandari P. Pomegranate (Punica granatum L). ancient seeds for modern cure? Review of potential therapeutic applications. Int J Nutr Pharmacol Neurol Dis. 2012;2(3):171. doi:10.4103/2231-0738.99469
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.