Back to Journals » Journal of Experimental Pharmacology » Volume 16
Medicinal Plants Used by Oromo Community in Kofale District, West-Arsi Zone, Oromia Regional State, Ethiopia
Authors Nuro GB, Tolossa K, Giday M
Received 13 December 2023
Accepted for publication 23 February 2024
Published 5 March 2024 Volume 2024:16 Pages 81—109
DOI https://doi.org/10.2147/JEP.S449496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Geritu Bedasso Nuro, Ketema Tolossa, Mirutse Giday
Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Mirutse Giday, Aklilu Lemma Institute of Pathobiology, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia, Tel +251-9-11171321, Email [email protected]
Purpose: The purpose of this study was to record the utilization of medicinal plants by the Oromo people in the Kofale District, Oromia Regional State, Ethiopia, to control human and animal health problems.
Methods: Data regarding the use of medicinal plants were collected using ethnobotanical methods from 84 traditional medicine practitioners and 304 general informants sampled employing purposive and systematic random sampling methods, respectively, in the Kofale District. Data were analyzed using different indices, including a preference ranking exercise, informant consensus factor (ICF), fidelity level (FL) and relative popularity level (RPL).
Results: In the district, 106 medicinal plants were claimed to be used for the treatment of 43 human and 18 livestock illnesses, of which 75 (71%) were used to manage human health problems, 23 (21.5%) were used to treat both human and livestock ailments and eight (7.5%) were utilized to treat manage livestock health problems. Most (76.4%) plants were harvested from the wild. Leaves were the most commonly used plant part (55.6%) in remedy preparations. Skin diseases scored the highest ICF value (0.97), followed by gastrointestinal disorders (ICF = 0.95), cancer (ICF = 0.93), and hemorrhoids (ICF = 0.91). Medicinal plants that record the highest fidelity level (FL) (100%) and rank order priority (ROP) (100%) values included Justicia schimperiana, Embelia schimperi, Ekebergia capensis and Datura stramonium, which have been used to treat liver disorders, tapeworm infections, babesiosis, and rabies, respectively. There were significant differences (p< 0.05) in the mean numbers of medicinal plants claimed by different social groups: older, illiterate, and traditional medicine practitioners reported higher mean numbers of medicinal plants than younger, literate, and general informants, respectively.
Conclusion: This study indicated the richness of medicinal plant species in Kofale District. Medicinal plants with the highest FL and ROP values and those used to treat disease categories with the highest ICF values should be prioritized in future phytochemical and pharmacological investigations.
Keywords: ethnobotanical study, traditional medicine, traditional knowledge, herbal medicine practitioners
Introduction
World Health Organization (WHO) has reported that nearly 60% of people worldwide, and as far as 80% of the population in Africa, directly or indirectly, depend on traditional medicinal plants to solve their healthcare problem.1 The high reliance on medicinal plants is attributed to a number of claimed reasons that include easy accessibility, cultural acceptability, affordability (cheaper cost), fewer side effects,2 and the widespread availability of harmful pathogenic microorganisms that are resistant to existing modern drugs.3 Medicinal plants are valuable sources for the discovery of new therapeutics against different diseases,4 which may also have a wider therapeutic window than synthetic drugs, and thus prevent the development of drug resistance. Medicinal plants contain a diverse groups of phytochemical constituents such as flavonoids, triterpenoids, glycosides, saponins, carotenoids, volatile oils, amino acids, steroids, quinines, alkaloids5 and coumarins6 responsible for multifaceted biological effects.7 According to estimates, around 80% of the human population and 90% of the livestock population in Ethiopia rely on traditional medicinal plants for their day-to-day primary healthcare.8,9 Ethiopia is one of the most ethnically diverse countries in East Africa with the majority of its citizens living in rural areas and thus with limited access to modern healthcare services.10 Such condition has made the people blessed with rich traditional knowledge and practices on the use of medicinal plants, remedy preparations and administrations as well as illness diagnoses.8 Even though the majority of the populations in Ethiopia heavily depend on medicinal plants for their primary healthcare needs, very limited work has so far been done to record and analyze the associated knowledge, and validate the therapeutic values of the claimed plants.11,12 On the other hand, there is an ongoing rapid population increase, indiscriminate deforestation, overexploitation of natural resources, worldwide climate change, which has contributed to the depletion of useful medicinal plant resources and the associated indigenous knowledge.13 As a result, documenting and protecting medicinal plants and the associated knowledge is becoming a greater priority. A number of ethnobotanical studies conducted in different parts of Ethiopia have reported the common uses of medicinal and wild edible plants.12,14–27 However, only a few ethnobotanical studies have been conducted in the West-Arsi Zone of the Oromia Regional State Ethiopia28,29 which were conducted in the Negele Arsi and Nansebo districts, and no such study has been conducted in Kofale District. Like most Ethiopian communities, people in Kofale District are expected to practice traditional medicine, mainly associated with the use of medicinal plants to maintain their health, as well as that of their domestic animals. Therefore, this study was carried out to properly document traditional knowledge related to the use of medicinal plants to manage both human and livestock ailments by the people of the Kofale District, West-Arsi Zone, Oromia Regional State, Ethiopia.
Materials and Methods
Description of the Study Area
According to 2007 census conducted by the Population Census Commission (PCC) of Ethiopia, the West-Arsi Zone has a total population of 1,975,295.30 Most inhabitants in the zone belong to the Oromo ethnic group, constituting 88.52% of the total population. The Zone has 12 districts, one of which is Kofale District (Figure 1). Kofale District is geographically located between 6° 50ˈ-7° 9ˈN and 38° 38ˈ-39° 4ˈE, south of the capital Addis Ababa. According to Kofale District Healthcare Office (KDHCO), the district gets a mean annual rainfall of 1300 mm and a temperature of 10–24°C (KDHCO, 2022, unpublished data). The district has a total population of 179,508, of which 90,000 are men and 89,508 are women.30 People in the rural areas of the study district are mainly dependent on crop farming and livestock production for their livelihood. According to the Kofale District Agricultural Office (KDAO), there are 106,325 cattle heads, 112,570 sheep, 91,400 horses, 9784 goats, 9410 donkeys, and 35,901 chickens in the district (KDAO 2022, unpublished data). Based on data collected during a reconnaissance survey, the district has 43 kebeles (sub-districts) located at different distances from the administrative district center (Kofale town). The top-five human health problems in the district are dermatochalasis, gastrointestinal tract infections (diarrhea, typhoid, stomachache, abdominal pain, and internal parasite infestation), cancer, respiratory infections, and sexually transmitted diseases (KDHCO 2022, unpublished data), and the main livestock diseases in the study area include anthrax, black leg, pasteurellosis, dermatochalasis, tick infestations, equine glanders, leech infestation, and rabies (KDAO 2022, unpublished data).
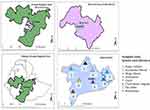 |
Figure 1 Map of Kofale District, West-Arsi Zone, Oromia Regional State, Ethiopia. |
Selection of Study Sites
A reconnaissance survey was conducted in Kofale District in January and February 2021 to select study kebeles and informants. Health and agricultural professionals in the district played key roles in the identification of herbal medicine practitioners. Of the 43 kebeles in the district, eight (Wege Abosa, Benjo Ashoka, Ilka Bebe, Sayimanna muudi, Chatimanna Jangala, Guchi, Bulchaana, and Garmaama) were purposively selected for the study, taking into consideration the history of use of traditional medicine and availability of practitioners in the area, less exposure of the community to modernization, and agro-ecological representation. Additional information regarding the sampled kebeles and traditional healers was gathered from kebele administration officers, knowledgeable elders, and other local inhabitants.
Study Design, Sample Size Determination and Sampling Techniques
A cross-sectional study design was adopted to conduct the ethnobotanical survey, and sample size was determined based on the total of household heads in the sampled kebeles30 using a standard sample size determination formula given below.31
n = N/(1+N(e)2), where n = sample size, N = total number of households in sample villages/kebeles (11,754), e = maximum variability or margin of error 5% (0.05) and 1 = the probability of event occurring
Based on this, the total sample size obtained was 388, of which 84 were locally recognized traditional health practitioners (THP) who were identified using purposive sampling method, and 304 were general informants that were sampled using a systematic random sampling method as described by Martin28 based on the total number of households each kebele (Table 1).
 |
Table 1 Number of Households and Informants in Each Sampled Kebele |
Ethnobotanical Data Collection
Ethnobotanical data were gathered from February 2022 to March 2023 through individual semi-structured interviews and guided field walks using the methods of Martin32 and Cotton.33 Data collected from informants during interviews included sociodemographic information, local name of each claimed medicinal plant, part used, condition of plant part used (fresh/dried), additive used (if any), preparation method, ailment treated, route of administration, dosage, side effects, and antidotes used. Additional data regarding habitat, abundance, and existing threats of medicinal plants were also gathered through guided field walks as described in Martin32 and Alexiades.34 Voucher specimens from all mentioned medicinal plants were collected, dried, identified by botanists at ALIPB and the National Herbarium, AAU using published volumes of the Flora of Ethiopian and Eritrea, and deposited for future reference.
Analysis of Data
Data were analyzed using quantitative tools, including preference ranking exercise, informant consensus factor (ICF), fidelity level index (FL), and rank order priority (ROP) value32,35–37 to identify the most important medicinal plants in the district. Analysis of variance (ANOVA) and t-tests were used to determine the effects of sociodemographic factors on respondents’ knowledge of traditional medicinal plants. Preference ranking exercises32 were conducted on seven medicinal plants with the highest number of informant citations to manage the most commonly reported human gastrointestinal complaints and skin diseases by ten traditional medicine practitioners sampled from those who were already involved in individual interviews. Informant consensus factor was computed to determine culturally important human and livestock ailment categories and, by doing so, identify potentially effective medicinal plant species within the respective disease categories using the formula ICF = (Nur − Nt)/(Nur − 1), where Nur is the number of use reports for each disease category and Nt is the number of species used in that category.36 Fidelity level index was computed to reveal the level of agreement among informants in selecting medicinal plants used to manage a specific ailment using the formula, FL = (Np/N) x100, where Np is the number of informants who cited or mentioned the use of a medicinal plant against a particular disease and N is the total number of informants who cited that plant for any other medical use.35 However, plants with similar FL values but known to different numbers of informants may vary in their healing potential. Thus, a correlation index known as relative popularity level (RPL) was additionally determined to compute the rank order priority (ROP) value, as given by Ali-Shtayeh et al37 by multiplying the FL value by RPL to differentiate the healing potential of plants with similar FL values.
Results
Socio-Demographic Status of Informants
In this study, 388 informants (84 traditional healers and 304 general informants) were involved, the majority (54.12%) of whom were between 41 and 60 years of age. Regarding educational status, 57.73% of informants were illiterate, and 42.27% were literate. Regarding the gender of the informants, 360 (92.79%) were males and 28 (7.22%) were females (Table 2).
 |
Table 2 Demographic Categories of Local Respondents |
Diversity of Medicinal Plants Reported and Their Growth Forms
The study conducted in Kofale District recorded 106 medicinal plant species that belonged to 90 genera and 56 families, which were reported to treat 43 human illnesses and 18 livestock diseases (Table 3). Of these, 75 (71%) were used to manage human health problems, 23 (21.5%) to treat both human and livestock ailments, and eight (7.5%) to treat livestock diseases. The family Asteraceae was represented by nine species, Fabaceae and Solanaceae by seven species each, and Euphorbiaceae and Lamiaceae by five species each. Five families (Amaranthaceae, Myrsinaceae, Cucurbitaceae, Rosaceae, and Myrsinaceae) were represented by three species each and three families (Urticaceae, Meliaceae, and Rubiaceae) were represented by two species each. Each of the remaining families was represented by single species. Regarding the growth forms of medicinal plants, shrubs contributed the most (37.7%), followed by herbs (28.3%), trees (23.5%), herbaceous climbers (5.7%), lianas (3.7%), and epiphytes (0.9%) (Figure 2).
 |
Table 3 List of Medicinal Plants Used to Treat Human and Livestock Diseases in Kofale District |
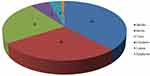 |
Figure 2 Proportions of medicinal plants growth forms in percent. |
Habitat of Medicinal Plants
Most medicinal plants (76.4%) used in traditional medicine in the district were uncultivated that were harvested from forests, riverbanks, grasslands, roadsides, life fences, and school compounds. Some were grown in homestead gardens (15%), and a few were harvested from both wild and homestead gardens (8.6%).
Medicinal Plant Parts Used in Remedy Preparations
The leaves were the most commonly used medicinal plant parts (55.6%) in the preparation of plant-based remedies in the district, followed by the bark (21.6%), root (14.15%), fruit/seed (11.3%), shoot/apex (3.8%), whole parts (2.8%), and sap (2.6%) (Figure 3).
 |
Figure 3 Proportions in percent of plant parts used for the treatment of human and livestock diseases in Kofale District, Oromia Regional State, Ethiopia. |
Conditions of Plant Parts Used and Preparation Methods
The majority (75.8%) of the medicinal plant parts were claimed to be used in their fresh form, whereas some others were used in their dry (14.15%) and dry or fresh (10.65%) forms. The highest proportion (39%) of remedies was prepared by crushing (39%), followed by squeezing (16%), pounding and squeezing (14.5%), decoction (13%), pounding and powdering (9%), pounding and mixing (7.5%), and chewing (1%) (Figure 4).
 |
Figure 4 Percentages of different methods of remedy preparations. |
Administration Routes of Remedies
Oral was the most frequently cited route of remedy administration in the district (61.3%), followed by topical/dermal (26.3%), nasal (4.7%), and ocular (3.8%) routes (Table 4).
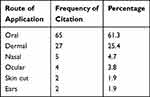 |
Table 4 Route of plant remedy application |
Dosage of Medicinal Plants and Use of Antidotes
Most frequently, traditional medicine practitioners’ prescriptions were based on patient age, gender, presence or absence of pregnancy and body condition. Different measuring materials, such as waterglass, teacup, coffee cup, teaspoon, bottle cap, handful, and between two fingertips were used to determine the dosage. Traditional medical practitioners employ different antidotes to neutralize possible adverse effects such as vomiting, nausea, diarrhea, headache, and loss of consciousness. Antidotes used mainly included fermented milk, fresh milk, honey, and coffee.
Commonly Reported Human and Livestock Diseases in the District
Of 43 human health problems occurring in Kofale District, dermatophilosis, gastrointestinal disorders, cancer, and hemorrhoids were the most prevalent reported by 35%, 31%, 18%, and 12% of the informants, respectively. Of the total 18 livestock health problems occurring in Kofale District, wound, tick infestation, leech infestation, dermatophilosis, equine glanders, anthrax, blackleg and pasteurellosis were the most reported ones with frequency of citation 26%, 22.4%, 19%, 16%, 11.3%, 7.2% and 3.3%, respectively.
Preference Ranking of Selected Medicinal Plants Used Against Human Gastrointestinal and Skin Diseases in the District
According to preference ranking exercise conducted on seven medicinal plants of the highest informant citations for their uses to treat human gastrointestinal complaints, a health problem of the second highest prevalence in the study district, Olinia rochetiana was the most preferred medicinal plant, followed by Bersama abyssinica and Vernonia amygdalina (Table 5).
 |
Table 5 Preference ranking of selected medicinal plants used to treat human gastrointestinal complaints in Kofale District |
A preference ranking exercise conducted on seven medicinal plants with the highest informant citations for their use in managing skin disorders, a health problem with the highest prevalence in the study district revealed that Euclea schimperi was the most preferred medicinal plant, followed by Maesa lanceolata and Vernonia amygdalina (Table 6).
 |
Table 6 Preference ranking of selected medicinal plants used to manage skin diseases in Kofale District |
Medicinal Plants Scoring the Highest Fidelity Level and Rank Order Priority Values
Of the medicinal plants used to manage human ailments in the district, Justicia schimperiana, Embelia schimperi, Olinia rochetiana and Euclea schimperi which have been used to treat liver disorders, tapeworm infections, general gastrointestinal complaints, and dermatological disorders, respectively, scored the highest fidelity level (FL) (100%) and rank order priority (ROP) (>81%) values (Table 7).
 |
Table 7 Rank order priority values of medicinal plants used to treat human ailments in the Kofale District with fidelity level values of above 80% |
Among the medicinal plants claimed to manage livestock health problems in the study district, Ekebergia capensis (for treatment of babesiosis), Datura stramonium (for treatment of rabies), and Millettia ferruginea (for treatment of leech infestation) had the highest fidelity level (FL) (>97%) and rank order priority (ROP) (97%) values (Table 8).
 |
Table 8 Rank order priority values of medicinal plants for livestock diseases in the Kofale District |
Informant Consensus Factor Values
Human and livestock ailments in the study district reported by informants were grouped into 12 major disease categories, and informant consensus factor (ICF) values were calculated. Accordingly, skin-related diseases scored the highest ICF value (0.97), followed by gastrointestinal tract infections (0.95), cancer (0.93), animal bites (0.92), hemorrhoids, and body swelling (0.91). Respiratory tract disorder category had the lowest ICF value (0. 62) (Table 9).
 |
Table 9 Informant consensus factor values of disease categories in study area |
Ways of Acquisition of Traditional Medical Knowledge
Traditional medicinal knowledge in the district was reported to have been acquired in different ways. Among the informants interviewed, 79.8% reported that they acquired knowledge through family lines, while the remaining (20.2%) confirmed that they acquired knowledge through observation (10.8%), mentorship with other traditional medicine practitioners (3.5%), experimentation (3.5%), and friends (2.4%).
Medicinal Plant Knowledge Comparison Between Different Social Groups
Interview data analyses showed that a significantly (p < 0.05) higher mean number of medicinal plants (6.52944 ± 0.1041) was reported by older informants (age > 60 years) compared to youngsters (20–40 years of age) (1.833 ± 0.1931) and those between the ages of 41–60 years (2.69603± 0.18031). Similarly, significantly (p < 0.05) higher mean numbers of medicinal plants were reported by illiterate (5.296 ± 3.0703) and traditional medicine practitioners (8.9058 ± 3.620) as compared with that of literate (3.6234 ± 3.122) and general informants (3.389 ± 1.661) (Table 10), respectively. But, there was no significant difference (p = 0.8789) between the mean numbers of medicinal plants reported by male (4.638 ± 3.252) and female (4.0714 ± 2.355) respondents.
 |
Table 10 Comparison of medicinal plant knowledge among different groups of informants |
Threats to Medicinal Plants and Conservation Practices
Agricultural expansion and deforestation are commonly cited threats to medicinal plants as reported by 91% and 80% of the informants, respectively. Other stated threats included drought (8%), timber and firewood production (11%), overexploitation (4%), and exotic species plantations (3%). The preference ranking exercise conducted by the informants also ranked agricultural expansion and deforestation as the major and leading threats (Table 11).
 |
Table 11 Ranking of commonly reported threats against medicinal plants in the study area |
Medicinal plant conservation practices in the study area were poor. Only a few informants (13%) reported the cultivation of medicinal plants, including Achyranthes aspera, Asparagus africanus, Ocimum lamiifolium, Rumex nepalensis, Ruta chalepensis, Withania somnifera and Aloe sp. in homestead gardens.
Ranking of Threatened Medicinal Plants
Preference ranking exercise, carried out on six threatened medicinal plants based on the interview results, revealed Hagenia abyssinica as the most threatened medicinal plant, followed by Juniperus procera and Podocarpus falcatus (Table 12).
 |
Table 12 Ranking of medicinal plants reported as threatened in the study district |
Discussion
The findings of this study showed the high dependence of the people in the Kofale District of West-Arsi Zone on traditional herbal medicine in their day-to-day primary healthcare needs, as demonstrated by the high number of medicinal plant species reported by informants. In Kofale District, 106 medicinal plant species have been claimed to be used to manage both human and livestock ailments, which is a higher number than that reported for other districts in the country. Studies by Tolossa et al,20 Ashagre and Molla,158 Yineger et al,71 and Gijan and Dalle28 reported the use of 91, 98, 101, and 102 medicinal plants, respectively. The utilization of a relatively high number of medicinal plants in the study district may be linked to people’s restricted access to modern healthcare facilities, cultural acceptability of medicinal plant-based treatments, and better vegetation cover in the area. All medicinal plants reported in the current study were found to have similar or different medicinal uses elsewhere in the country, as shown in Table 3. Of the total claimed medicinal plants, some were claimed to have been used elsewhere in the world for same or similar purpose, which include Asparagus africanus for treatment of tumor and cancer in Cote d’Ivoire,159 Calpurnia aurea as antidermatophytic in Kenya,160 Carissa spinarum against wound in India,161 Croton macrostachyus against cancer in India,162 Euclea schimperi against skin sores and rashes in Namibia,163 Ocimum gratissimum as analgesic in Nigeria,164 Physalis peruviana to treat gastro-intestinal tract disorders in Uganda,165 Podocarpus falcatus to treat cancer in China,166 Rumex abyssinicus to relieve stomachache in Africa,167 Syzygium guineense to treat stomachache in Mali168 and Verbascum sinaiticum against hepatitis in Egypt.169
The dominance of the families Asteraceae, Fabaceae, and Solanaceae in contributing high number of medicinal plants in the study district could be due linked to their diversity in species and/or richness in medically active constituents. Fabaceae and Asteraceae are among the dominant families in the Flora of Ethiopia and Eritrea in terms of species richness contributing 48650 and 440170 species, respectively. Studies conducted in other areas of country have also reported a high contribution of Asteraceae,74,125,171 Fabaceae14,161–163 and Solanaceae14 to the medicinal flora.
The majority of claimed medicinal plants was collected from the wild and semi-wild habitats is in accordance with the results of other studies conducted elsewhere in different parts of the country.23,38,67,74,81,135 The poor cultivation practice of medicinal plants in the district might be related to their easy accessibility in the wild.
This study also shown that shrubs were the most dominant medicinal flora in the study district, which might be because of their year-round availability, in contrast to trees that were exposed to selective cutting and herbs that blossom seasonally after the rainy season. The dominance of shrubby medicinal plants has been observed in other parts of the country.20,135,172
Leaves were the dominant plant parts employed in remedy preparations for the treatment of human and livestock ailments in the study district, which could be attributed to their perceived efficacy, accessibility, ease of harvesting, and simplicity of preparation. The common use of leaves in the preparation of remedies has also been reported in studies conducted elsewhere in the country.14,20,23,28,125 Harvesting leaves has been reported to have much less damaging effects on the mother plant as compared to other parts such as roots and barks, the gathering of which could seriously affect the existence of individual plants.24,135
Different techniques were employed in the preparation of remedies in the district, with the crushing method taking the lead, which is in agreement with the results of previous studies conducted elsewhere in the country.21,27 Moreover, the finding related to the condition of the plants used for preparation indicated that the majority of remedies were made from fresh plant parts, which is in agreement with the results of studies carried out elsewhere in the country.173,174 Fresh materials retain volatile bioactive compounds, such as essential oils, which may be lost upon drying.
Oral was the most popular route of remedy administration which could be due to the reason that it creates favorable environmental condition for quick physiological reaction of the preparation against the pathogens and by so doing boosts its healing power.20 Oral administration has an additional advantage in that it allows the traditional medicine practitioners to reverse complication that might happen on the clients during treatment using antidotes. Other ethnobotanical studies conducted elsewhere in Ethiopia also reported oral administration as a common route of remedy application.12,14,16,20,27,175
Skin-related and gastrointestinal tract diseases had the highest ICF values, which might imply better consensus among informants in the study district regarding the selection of plants used to manage such diseases.176,177
Of the medicinal plants employed to treat human health problems, Justicia schimperiana, Embelia schimperi, Olinia rochetiana and Euclea schimperi which were used to treat liver disorders, tapeworm infection, general gastrointestinal complaints, and skin diseases, respectively, scored the highest FL and ROP values, which are measures of therapeutic potential.35 Justicia schimperiana was reported to have shown antioxidant,178 lousicidal and acaricidal,179 anticancer180 and antimalarial181 activities. Crude extracts of Embelia schimperi exhibited anthelmintic182 and antioxidant properties.183 Investigation reported the antibacterial,184 antidiarrheal185 and anti-inflammatory186 activities of Olinia rochetiana. Leaf extracts of Euclea schimperi demonstrated antioxidant and antibacterial activities.187 Generally, there is a higher consensus among informants in the study district regarding the selection of medicinal plants for the treatment of skin infections and gastrointestinal complaints, as revealed by the highest informant consensus factor (ICF) values scored by the two disease categories, which again is a sign of their better healing potential. Similar medicinal uses of these plants have been widely reported in different parts of the country.14,17,25,54,71,86,88,98,188 Among the medicinal plants claimed to manage livestock health problems, Ekebergia capensis and Datura stramonium which are used against babesiosis and rabies, respectively, scored the highest FL and ROP values. Previous studies have also reported the anti-infectious properties.28,81
Data analysis revealed that older, illiterate, and key informants in the study district reported significantly higher mean numbers of medicinal plants than that reported by the young, literate, and general informants, respectively. The fact that the younger generation had less medicinal plant knowledge compared to the older generation could be due to the reason that the former are more prone to acculturation and modernization and thus are more reluctant to learn and practice traditional medicine. Studies conducted in other areas of the country also reported that older people have better knowledge of medicinal plants than younger people.21,74,175 The reason that key informants had better knowledge of medicinal plants in the study district compared to general informants is also in agreement with the findings of previous studies conducted elsewhere in the country.26,189 The fact that literate informants in the district had less knowledge of medicinal plants than illiterate ones could be attributed to the influence of modern education. A study conducted in other areas of the country reported similar results.118
Agricultural expansion and deforestation were identified as major threats to the survival of medicinal plants in the district. Research carried out elsewhere in the country also revealed that agricultural expansion and deforestation are main threats to medicinal plants.28,60,94,190
Conclusion
The diversity of reported medicinal plants (106 species) employed to manage human and livestock disorders is indicative of plant-related rich knowledge of traditional medicine practices in the Kofale district. Leaves have been reported to be the most commonly utilized plant part in the preparation of remedies for the treatment of various ailments. Skin and gastrointestinal disorders were the major disease categories, with the highest ICF values. Medicinal plants, including Euclea schimperi, Olinia rochetiana, Embelia schimperi, and Justicia schimperiana, were the ones having the highest FL and ROP values for their use in the treatment of skin diseases, general gastrointestinal complaints, tapeworm infections, and liver disorders. Different scientific investigations also revealed the bioactivity of these plants against a number of aetiological agents. Comparative studies conducted on sociodemographic factors in the district revealed that older, illiterate, and key informants had better knowledge of the use of medicinal plants for the treatment of various human and livestock diseases than younger, literate, and general informants, respectively. In future phytochemical and pharmacological investigations, priority needs to be given to medicinal plants that scored the highest FL and ROP values and those plants that were used to treat disease categories with the highest ICF values.
Data Sharing Statement
Data concerning this study were kept on a desktop computer at the Aklilu Lemma Institute of Pathobiology (ALIPB), Addis Ababa University (AAU). Readers may get access to the data through requests made to ALIPB. Plant voucher specimens were stored at the mini-herbarium of the Endod and Other Medicinal Plants Research Unit (ALIPB, AAU).
Ethical Consideration
The study proposal was evaluated and approved by the Ethical Review Committee of the Aklilu Lemma Institute of Pathobiology at the Addis Ababa University. Permissions were granted by the zone, district, and kebele administrations to conduct the fieldwork. Verbal consent was obtained from the study participants, which was also approved by the Review Committee. We confirm that our study complies with the Declaration of Helsinki.191
Acknowledgments
We are thank the residents of Kofale District, particularly the informants, for sharing their accumulated indigenous knowledge and use of medicinal plants. Our gratitude also goes to the Healthcare, Agricultural, and Biodiversity Conservation offices of Kofale District for their cooperation in facilitating this study. We are grateful to Addis Ababa University for its financial support to conduct this investigation.
Funding
This study was sponsored by the School of Graduate Studies of Addis Ababa University and the Office of the Vice President for Research and Technology Transfer of Addis Ababa University (grant number TR/003/2021). We confirm that the information is accurate and the grant number is correct.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. The World Health Report 2000: Health Systems: Improving Performance. World Health Organization; 2000.
2. Muzammil S, Neves Cruz J, Mumtaz R, et al. Effects of drying temperature and solvents on in vitro diabetic wound healing potential of Moringa oleifera leaf extracts. Molecules. 2023;28(2):710. doi:10.3390/molecules28020710
3. Shah NA, Khan MR, Nadhman A. Antileishmanial, toxicity, and phytochemical evaluation of medicinal plants collected from Pakistan. Biomed Res Int. 2014;2014:1–7. doi:10.1155/2014/384204
4. Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78(5):431–441. doi:10.1016/j.lfs.2005.09.012
5. Alves FS, Cruz JN, de Farias Ramos IN, Do Nascimento Brandão DL, Queiroz RN. Evaluation of antimicrobial activity and cytotoxicity effects of extracts of piper nigrum L. and piperine. Separations. 2022;10(1):21. doi:10.3390/separations10010021
6. da Silva DF, de Souza JL, da Costa DM, et al. Antiplasmodial activity of coumarins isolated from Polygala boliviensis: in vitro and in silico studies. J Biomol Struct Dyn. 2023;27:1–21.
7. Shahane K, Kshirsagar M, Tambe S, et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals. 2023;16(4):611. doi:10.3390/ph16040611
8. Abebe D, Ayehu A. Medicinal Plants and Enigmatic Health Practices of Northern Ethiopia. Addis Ababa: B.S.P.E; 1993.
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, World Health Organization. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Vol. 82. Lyon: World Health Organization; 2002.
10. Tesfahuneygn G, Gebreegziabher G. Medicinal plants used in traditional medicine by Ethiopians: a review article. J Respir Med Lung Dis. 2019;4:1–3.
11. Teklehaymanot T, Giday M. Ethnobotanical study of wild edible plants of Kara and Kwego semi-pastoralist people in Lower Omo River Valley, Debub Omo Zone, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):23. doi:10.1186/1746-4269-6-23
12. Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara region, Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):63. doi:10.1186/1746-4269-9-63
13. Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Adv Biochem Eng Biotechnol. 2015;147:59–110. doi:10.1007/10_2014_273
14. Giday M, Ameni G. An ethnobotanical survey of plants of veterinary importance in two woredas of Southern Tigray, Northern Ethiopia. SINET: Ethiop J Sci. 2003;26:123–136.
15. Awas T, Demissew S Ethnobotanical study of medicinal plants in Kafficho people, southwestern Ethiopia. In
16. Mesfin F, Demissew S, Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2009;5(1):28. doi:10.1186/1746-4269-5-28
17. Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124(1):69–78. doi:10.1016/j.jep.2009.04.005
18. Belayneh A, Asfaw Z, Demissew S, Bussa NF. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomed. 2012;8(1):42. doi:10.1186/1746-4269-8-42
19. Agize M, Demissew S, Asfaw Z. Ethnobotany of medicinal plants in Loma and Gena bosa districts (woredas) of dawro zone, southern Ethiopia. Topclass J Herb Med. 2013;2:194–212.
20. Tolossa K, Debela E, Athanasiadou S, Tolera A, Ganga G, Houdijk JG. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):32. doi:10.1186/1746-4269-9-32
21. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi:10.1186/1746-4269-11-4
22. Yohannis SW, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local people in Menz Gera Midir District, North Shewa Zone, Amhara Regional State, Ethiopia. J Med Plants Research. 2018;12(21):296–314. doi:10.5897/JMPR2018.6616
23. Kassa Z, Asfaw Z, Demissew S. An ethnobotanical study of medicinal plants in Sheka Zone of Southern Nations, Nationalities and Peoples Regional State, Ethiopia. J Ethnobiol Ethnomed. 2020;16(1):7. doi:10.1186/s13002-020-0358-4
24. Teka A, Asfaw Z, Demissew S, Van Damme P. Traditional uses of medicinal plants practiced by the indigenous communities in Gurage Zone, south central Ethiopia. Ethnobot Res Appl. 2020;19:41.
25. Eshete MA, Molla EL. Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda District, Ethiopia. J Ethnobiol Ethnomed. 2021;17(1):61. doi:10.1186/s13002-021-00487-4
26. Woldemariam G, Demissew S, Asfaw Z. An ethnobotanical study of traditional medicinal plants used for human ailments in Yem ethnic group, south Ethiopia. Ethnobot Res Appl. 2021;22:08.
27. Bekele M, Woldeyes F, Lulekal E, Bekele T, Demissew S. Ethnobotanical investigation of medicinal plants in Buska Mountain range, Hamar district, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2022;18(1):60. doi:10.1186/s13002-022-00558-0
28. Gijan M, Dalle M. Ethnobotanical study of medicinal plants in Nagelle Arsi district, West Arsi zone of Oromia, Ethiopia. J Nat Sci Res. 2019;9:13.
29. Girma Z, Abdela G, Awas T. Ethnobotanical study of medicinal plant species in Nensebo District, south-eastern Ethiopia. Ethnobot Res Appl. 2022;24:6.
30. Population Census Commission (PCC). Summary and Statistical Report of the 2007 Population and Housing Census. Population Census Commission (PCC), Federal Democratic Republic of Ethiopia: Addis Ababa. 2008.
31. Cochran WG. Sampling Techniques. New York: John Wiley & Sons; 1977.
32. Martin GJ. Ethnobotany: A Method Manual. London: Chapman and Hall; 1995.
33. Cotton CM. Ethnobotany: Principles and Applications. Chichester: John Wiley & Sons; 1996.
34. Alexiades MN. Collecting ethnobotanical data: an introduction to basic concepts and techniques. In: Alexiades MN, editor. Selected Guidelines for Ethnobotanical Research: A Field Manual. The New York Botanical Garden. 1996:53–94.
35. Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J Ethnopharmacol. 1986;16(2–3):275–287. doi:10.1016/0378-8741(86)90094-2
36. Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: healers’ consensus and cultural importance. Soc Sci Med. 1998;47(11):1859–1871. doi:10.1016/S0277-9536(98)00181-6
37. Ali-Shtayeh MS, Jamous RM, Jamous RM. Traditional Arabic Palestinian ethnoveterinary practices in animal health care: a field survey in the West Bank (Palestine). J Ethnopharmacol. 2016;182:35–49. doi:10.1016/j.jep.2016.02.005
38. Tadesse A, Kagnew B, Kebede F, Kebede M. Ethnobotanical study of medicinal plants used to treat human ailment in Guduru District of Oromia Regional State, Ethiopia. J Pharmacogn Phytother. 2018;10(3):64–75. doi:10.5897/JPP2018.0496
39. Tesfaye S, Braun H, Asres K, et al. Ethiopian Medicinal Plants traditionally used for the treatment of cancer; Part 3: selective cytotoxic activity of 22 Plants against human cancer Cell Lines. Molecules. 2021;26(12):3658. doi:10.3390/molecules26123658
40. Seid MA, Aydagnehum SG. Medicinal plants biodiversity and local healthcare management system in Chencha district; Gamo Gofa, Ethiopia. J Pharmacogn Phytochem. 2013;2:284–293.
41. Megersa M, Jima TT, Goro KK. The use of medicinal plants for the treatment of toothache in Ethiopia. Evid Based Complement Altern Med. 2019;2019:1–16. doi:10.1155/2019/2645174
42. Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiop J Health Dev. 2019;33:102–127.
43. Nibret E, Wink M. Trypanocidal and cytotoxic effects of 30 Ethiopian medicinal plants. Z Naturforsch C J Biosci. 2011;66(11–12):541–546. doi:10.1515/znc-2011-11-1202
44. Eguale T, Tadesse D, Giday M. In vitro anthelmintic activity of crude extracts of five medicinal plants against egg-hatching and larval development of Haemonchus contortus. J Ethnopharmacol. 2011;137(1):108–113. doi:10.1016/j.jep.2011.04.063
45. Bacha K, Tariku Y, Gebreyesus F, et al. Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: implication for development of potent antimicrobial agents. BMC Microbiol. 2016;16(1):139. doi:10.1186/s12866-016-0765-9
46. Fufa MF, Deressa F, Deyou T, Abdisa N. Isolation and characterization of compounds from the leaves of Melia azedarach and stem bark of Albizia schimperiana and evaluation for antimicrobial activities. Med Chem (Los Angeles). 2018;8:154–165.
47. Belayneh A, Demissew S, Bussa NF, Bisrat D. Ethno-medicinal and bio-cultural importance of aloes from south and east of the Great Rift Valley floristic regions of Ethiopia. Heliyon. 2020;6(6):e04344. doi:10.1016/j.heliyon.2020.e04344
48. Nigussie G, Wale M. Medicinal plants used in traditional treatment of malaria in Ethiopia: a review of ethnomedicine, anti-malarial and toxicity studies. Malar J. 2022;21(1):262. doi:10.1186/s12936-022-04264-w
49. Addis G, Urga K, Dikasso D. Ethnobotanical study of edible wild plants in some selected districts of Ethiopia. Human Ecolo. 2005;33(1):83–118. doi:10.1007/s10745-005-1656-0
50. Thulin M. Fabaceae. In: Hedberg I, Edwards S, editors. Flora of Ethiopia and Eritrea. Volume 3: Pittosporaceae to Araliaceae. The National Herbarium, Addis Ababa University, Addis Ababa; 1989:49–251.
51. Dikasso D, Makonnen E, Debella A, et al. In vivo anti-malarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop J Health Dev. 2006;20:112–118.
52. Asmerom D, Kalay TH, Araya TY, Desta DM, Wondafrash DZ, Tafere GG. Medicinal plants used for the treatment of erectile dysfunction in Ethiopia: a systematic review. BioMed Res Int. 2021;2021:1–12. doi:10.1155/2021/6656406
53. Yared D, Mekonnen Y, Debella A. In vivo antimalarial activities of fractionated extracts of Asparagus africanus in mice infected with Plasmodium berghei. Pharmacologyonline. 2012;3:88–94.
54. Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E. Antiviral activity against human immunodeficiency virus type 1 (HIV‐1) and type 2 (HIV‐2) of ethnobotanically selected Ethiopian medicinal plants. Phytother Res. 2001;15(1):62–69. doi:10.1002/1099-1573(200102)15:1<62::AID-PTR956>3.0.CO;2-X
55. Kifle ZD, Abdelwuhab M, Melak AD, Meseret T, Adugna M, Adugna M. Pharmacological evaluation of medicinal plants with antidiabetic activities in Ethiopia: a review. Metabol Open. 2022;13:100174. doi:10.1016/j.metop.2022.100174
56. Kifle ZD, Anteneh DA, Atnafie SA. Hypoglycemic, anti-hyperglycemic and anti-hyperlipidemic affects of Bersama abyssinica fresen (melianthaceae) leaves’ solvent fractions in normoglycemic and streptozotocin-induced diabetic mice. J Exp Pharmacol. 2020;12:385–396. doi:10.2147/JEP.S273959
57. Ayalew M, Bekele A, Mengistie MG, Atnafie SA. Evaluation of the antidiarrheal activity of 80% methanol extract and solvent fractions of the leaf of Bersama abyssinica fresen (Melianthaceae) in mice. BMC Complement Med Ther. 2022;22(1):8. doi:10.1186/s12906-021-03498-6
58. Zewdie KA, Bhoumik D, Wondafrash DZ, Tuem KB. Evaluation of in-vivo antidiarrhoeal and in-vitro antibacterial activities of the root extract of Brucea antidysenterica JF Mill (Simaroubaceae). BMC Complement Med Ther. 2020;20(1):201. doi:10.1186/s12906-020-03001-7
59. Ragunathan M, Solomon M. The study of spiritual remedies in orthodox rural churches and traditional medicinal practice in Gondar Zuria district, Northwestern Ethiopia. Pharmacogn J. 2009;1.
60. Bogale M, Sasikumar JM, Egigu MC. An ethnomedicinal study in tulo district, west hararghe zone, Oromia region, Ethiopia. Heliyon. 2023;9(4):e15361. doi:10.1016/j.heliyon.2023.e15361
61. Birhanu Z, Wuhab MA, Abula T. Antimalarial activity of Calpurnia aurea hydro alcoholic leaf extract in mice infected with Plasmodium berghei. Pharmacologyonline. 2015;2:73–79.
62. Feyisa M, Kassahun A, Giday M. Medicinal Plants Used in Ethno veterinary Practices in Adea Berga District, Oromia Region of Ethiopia. Evid Based Complement Altern Med. 2021;2021:1–22. doi:10.1155/2021/5641479
63. Berhanu M, Tintagu T, Fentahun S, Giday M. Research Article Ethnoveterinary Survey of Medicinal Plants Used for Treatment of Animal Diseases in Ambo District of Oromia Regional State of Ethiopia. Evid Based Complement Altern Med. 2020;2020:1–12. doi:10.1155/2020/8816227
64. Suleman S, Tufa TB, Kebebe D, et a. Treatment of malaria and related symptoms using traditional herbal medicine in Ethiopia. J Ethnopharmacol. 2018;213:262–279. doi:10.1016/j.jep.2017.10.034
65. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525. doi:10.1016/j.jep.2006.10.011
66. Assefa B, Megersa M, Jima TT. Ethnobotanical study of medicinal plants used to treat human diseases in Gura Damole District, Bale Zone, and Southeast Ethiopia. Asian J Ethnobiol. 2021;4(1):42–52. doi:10.13057/asianjethnobiol/y040105
67. Mesfin F, Seta T, Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda, Ethiopia. Ethnobot Res Appl. 2014;12:341–354. doi:10.17348/era.12.0.341-354
68. Saka A, Tesfaye JL, Gudata L, et al. Synthesis, characterization, and antibacterial activity of ZnO nanoparticles from fresh leaf extracts of Apocynaceae, Carissa spinarum L.(Hagamsa). J Nanomater. 2022;2022:1–6. doi:10.1155/2022/6230298
69. Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95(1):57–61. doi:10.1016/j.jep.2004.06.017
70. Khalil OS, Ismail HA, Elkot WF. Physicochemical, functional and sensory properties of probiotic yoghurt flavored with white sapote fruit (Casimiroa edulis). J Food Sci Technol. 2022;59(9):3700–3710. doi:10.1007/s13197-022-05393-5
71. Yineger H, Kelbessa E, Bekele T, Lulekal E. Plants used in traditional management of human ailments at Bale Mountains National Park, Southeastern Ethiopia. J Med Plant Res. 2008;2:132–153.
72. Mekonnen AG, Gebeyehu BT, Woldearegay M. Experience of patients with breast cancer with traditional treatment and healers’ understanding of causes and manifestations of breast cancer in North Shewa zone, Ethiopia: a phenomenological study. BMJ open. 2022;12(12):e063726. doi:10.1136/bmjopen-2022-063726
73. Yineger H, Kelbessa E, Bekele T, Lulekal E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. J Ethnopharmacol. 2007;112(1):55–70. doi:10.1016/j.jep.2007.02.001
74. Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124(3):513–521. doi:10.1016/j.jep.2009.05.009
75. Woldeab B, Regassa R, Alemu T, Megersa M. Medicinal plants used for treatment of diarrhoeal related diseases in Ethiopia. Evid Based Complement Altern Med. 2018;2018:1–20. doi:10.1155/2018/4630371
76. Issa M, Chandel S, Singh HP, et al. Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind Crops Prod. 2020;145:112083. doi:10.1016/j.indcrop.2019.112083
77. Asfaw A, Lulekal E, Bekele T, Debella A, Debebe E, Sisay B. Medicinal plants used to treat livestock ailments in Ensaro District, North Shewa Zone, Amhara Regional State, Ethiopia. BMC Vet Res. 2022;18(1):235. doi:10.1186/s12917-022-03320-6
78. Osman A, Sbhatu DB, Giday M. Medicinal plants used to manage human and livestock ailments in Raya Kobo District of Amhara Regional State, Ethiopia. Evid Based Complement Altern Med. 2020;2020:1–19. doi:10.1155/2020/1329170
79. Getachew S, Medhin G, Asres A, Abebe G, Ameni G. Traditional medicinal plants used in the treatment of tuberculosis in Ethiopia: a systematic review. Heliyon. 2022;8(5):e09478. doi:10.1016/j.heliyon.2022.e09478
80. Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;5(5):370–375. doi:10.1016/S2221-1691(11)60082-8
81. Yirga G. Assessment of traditional medicinal plants in Endrta district, south-eastern Tigray, northern Ethiopia. Afr J Plant Sci. 2010;4:255–260.
82. Yirga G, Teferi M, Kasaye M. Survey of medicinal plants used to treat human ailments in Hawzen district, Northern Ethiopia. Int J Biodivers Conserv. 2011;3:709–714.
83. Fentahun M, Ayele Y, Amsalu N, Alemayehu A, Amsalu G. Antibacterial evaluation and phytochemical analysis of selected medicinal plants against some pathogenic enteric bacteria in Gozamin District, Ethiopia. J Pharmacovigil. 2017;5:5.
84. Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. Afr J Plant Sci. 2014;8(7):366–379. doi:10.5897/AJPS2013.1041
85. Kewessa G, Abebe T, Demessie A. Indigenous knowledge on the use and management of medicinal trees and shrubs in Dale District, Sidama Zone, Southern Ethiopia. Ethnobot Res Appl. 2015;14:171–182. doi:10.17348/era.14.0.171-182
86. Debebe Y, Tefera M, Mekonnen W, et al. Evaluation of anthelmintic potential of the Ethiopian medicinal plant Embelia schimperi Vatke in vivo and in vitro against some intestinal parasites. BMC Complement Altern Med. 2015;15(1):187. doi:10.1186/s12906-015-0711-7
87. Lulekal E, Rondevaldova J, Bernaskova E, et al. Antimicrobial activity of traditional medicinal plants from Ankober district, north Shewa Zone, Amhara region, Ethiopia. Pharm Biol. 2014;52(5):614–620. doi:10.3109/13880209.2013.858362
88. Animaw Z, Asres K, Tadesse S, et al. Teratogenic evaluation of 80% ethanol extract of Embelia schimperi vatke fruits on rat embryo and fetuses. J Toxicol. 2022;2022:1–12. doi:10.1155/2022/4310521
89. Usman KA, Egigu MC, Mahalingam JS. Ethnobotanical study on traditional medicinal plants used by Oromo ethnic people of Goro district, Bale zone of Oromia region, Ethiopia. Ethnobot Res Appl. 2022;24:1–21.
90. Fadipe LA, Yaro AH, Haruna AK, Ilyas M. Anticonvulsant activity of the methanolic extract of Entada abyssinica Steud. Ex a Rich Biol Environ Sci J Trop. 2007;5:76–80.
91. Aerts R, November EJ, Rayyan M. Improvised Hand Injury Treatment Using Traditional Veterinary Medicine in Ethiopia. Wilderness & Environm Med. 2017;28(4):322–326. doi:10.1016/j.wem.2017.06.012
92. Seifu T, Mehari B, Atlabachew M, Chandravanshi B. Polyphenolic content and antioxidant activity of leaves of Urtica simensis grown in Ethiopia. Lat Am Appl Res. 2017;47:35–40.
93. Gemechu A, Giday M, Worku A, Ameni G. In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med. 2013;13(1):291. doi:10.1186/1472-6882-13-291
94. Tefera BN, Kim YD. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15(1):25. doi:10.1186/s13002-019-0302-7
95. Alemayehu G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J Med Plants Stud. 2015;3:01–11.
96. Gebre T, Addis G, Lemma H, et al. In-vitro antibacterial activity of Cymbopogon citratus (DC.) Stapf. and Eucalyptus globulus Labill. Ethiop J Public Health and Nutr. 2020;2.
97. Teka A, Maryo M. Ethiopian Medicinal Plants Used for Respiratory Tract Disorders: ethnomedicinal Review. Evid Based Complement Altern Med. 2023;2023:1–9. doi:10.1155/2023/7612804
98. Gebremariam T, Abula T, Gebremariam MG. Antibacterial and phytochemical screening of root extracts of Euclea racemosa subsp. schimperi. Int J Pharmacogn. 2015;2:66–70.
99. Tesfaye S, Tadesse S, Engidawork E, et al. Screening of Twenty-one Ethiopian Medicinal Plants for their Antiproliferative Activity against Human Acute Myeloid Leukemia (MV4-11) Cell Line. Ethiop Pharm J. 2019;35(2):143–148. doi:10.4314/epj.v35i2.7
100. Gadisa E, Tadesse E. Antimicrobial activity of medicinal plants used for urinary tract infections in pastoralist community in Ethiopia. BMC Complement Med Ther. 2021;21(1):74. doi:10.1186/s12906-021-03249-7
101. Feyisa K, Feyisa W, Girma T, Kemal T. Traditional medicinal plants used for the treatment of urological and urogenital diseases in Ethiopia: a Review. Pharmacogn J. 2022;14.
102. Demie G, Negash M, Awas T. Ethnobotanical study of medicinal plants used by indigenous people in and around Dirre Sheikh Hussein heritage site of South-eastern Ethiopia. J Ethnopharmacol. 2018;220:87–93. doi:10.1016/j.jep.2018.03.033
103. Girma Z, Abdela G, Awas T. Ethnobotanical study of medicinal plant species in Nensebo District, south-eastern Ethiopia. Ethnobot Res Appl. 2022;24:1–25.
104. Megersa M, Tamrat N. Medicinal plants used to treat human and livestock ailments in Basona Werana District, North Shewa Zone, Amhara region, Ethiopia. Evid Based Complement Altern Med. 2022;2022:1–18. doi:10.1155/2022/5242033
105. Desalegn A, Egigu MC, Sasikumra JM. Ethnobotanical study on medicinal plants used by ethnic people of Gechi District, South West Oromia, Ethiopia. Nus Biosci. 2022;14(1):104–116.
106. Andualem D, Negesse T, Tolera A. Chemical composition, in vitro organic matter digestibility and kinetics of rumen dry matter degradability of morphological fractions of stinging nettle (Urtica simensis). Adv Biol Res. 2016;10:183–190.
107. Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646. doi:10.1016/j.jep.2012.12.002
108. Birhan YS, Kitaw SL, Alemayehu YA, Mengesha NM. Ethnoveterinary medicinal plants and practices in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. Int J Anim Sci. 2018;2:1014.
109. Ayele TT. A review on traditionally used medicinal plants/herbs for cancer therapy in Ethiopia: current status, challenge and future perspectives. Org Chem Curr Res. 2018;7(02):2. doi:10.4172/2161-0401.1000192
110. Makonnen E, Debella A, Abebe D, Teka F. Analgesic properties of some Ethiopian medicinal plants in different models of nociception in mice. Phytother Res. 2003;17(9):1108–1112. doi:10.1002/ptr.1306
111. Fikadu Y, Yaya EE, Chandravanshi BS. Chemical composition and antioxidant activities of the essential oils of Lippia adoensis Hochst ex. Walp and Ocimum sanctum Linn. Bull Chem Soc Ethiop. 2022;36(1):95–108. doi:10.4314/bcse.v36i1.9
112. Gemeda N, Woldeamanuel Y, Asrat D, Debella A, Belete Y. Assessment of Lippia adoensis Hochst. var. koseret, Rosmarinus officinalis L. and Ruta chalepensis L. essential oils as a potential source of fungitoxic and mycosporicidal activity against toxigenic Aspergillus species. Pharmacologyonline. 2015;2:85–94.
113. Degu S, Berihun A, Muluye R, et al. Medicinal plants that used as repellent, insecticide and larvicide in Ethiopia. Pharm Pharmacol Int J. 2020;8(5):274–283. doi:10.15406/ppij.2020.08.00306
114. Buli GA, Duga AG, Dessalegn E. Antimicrobial activity of Lippia adoensis var. koseret against human pathogenic bacteria and fungi. Am J Clin Exp Med. 2015;3(3):118–123. doi:10.11648/j.ajcem.20150303.18
115. Tekalign E, Tadege G, Fisseha N, Nureye D. Suppressive, Curative, and Prophylactic Effects of Maesa lanceolata Forssk. Against Rodent Malaria Parasite Plasmodium berghei. BioMed Res Int. 2022;2022:1–18. doi:10.1155/2022/8901555
116. Tadesse D, Eguale T, Giday M, Mussa A. Ovicidal and larvicidal activity of crude extracts of Maesa lanceolata and Plectranthus punctatus against Haemonchus contortus. J Ethnopharmacol. 2009;122(2):240–244. doi:10.1016/j.jep.2009.01.014
117. Duresa LW, Manaye D. Phytochemical screening and antioxidant activity of selected Mango (Mangifera indica L.) and avocado (Persea Americana) fruits in Illu Ababor zone, Oromia Regional sSate, Ethiopia. Indo Am J Pharm Res. 2017;7:67.
118. Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethnopharmacol. 2007;111(2):271–283. doi:10.1016/j.jep.2006.11.019
119. Fenta M, Kahaliw W. Evaluation of antimalarial activity of hydromethanolic crude extracts and solvent fractions of the leaves of Nuxia congesta R. Br. Ex Fresen (Buddlejaceae) in Plasmodium berghei infected mice. J Exp Pharmacol. 2019;11:121–134. doi:10.2147/JEP.S230636
120. Wubetu M, Sintayehu M, Aeta MA. Ethnobotany of medicinal plants used to treat various mental illnesses in Ethiopia: a systematic review. Asian J Plant Sci Res. 2018;8:9–33.
121. Desalegn A, Egigu M, sasikumar JM. Ethnobotanical study on medicinal plants used by ethnic people of Gechi District, South West Oromia, Ethiopia. Nus Biosci. 2022;14(1):104–116. doi:10.13057/nusbiosci/n140113
122. Amuka O, Tarus PK, Machocho AK, Ruttoh EK. Plant Extracts as Solutions to Multi Drug Resistant, Indigofera homblei Bak. f and Martin, Fabaceae, a Plant used in Kenya against Microbial Related Conditions. Adv Tech Biol Med. 2015;3(03):2379. doi:10.4172/2379-1764.1000140
123. Esser KB, Semagn K, Wolde-Yohannes L. Medicinal use and social status of the soap berry endod (Phytolacca dodecandra) in Ethiopia. J Ethnopharmacol. 2003;85(2–3):269–277. doi:10.1016/S0378-8741(03)00007-2
124. Tuasha N, Petros B, Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J Ethnobiol Ethnomed. 2018;14(1):15. doi:10.1186/s13002-018-0213-z
125. Agize M, Asfaw Z, Nemomissa S, Gebre T. Ethnobotany of traditional medicinal plants and associated indigenous knowledge in Dawuro Zone of Southwestern Ethiopia. J Ethnobiolo Ethnomed. 2022;18(1):48. doi:10.1186/s13002-022-00546-4
126. Tekle Y. Medicinal plants in the ethno veterinary practices of Bensa woreda, Southern Ethiopia. Open Access Lib J. 2015;2:e1258.
127. Yeshiwas D, Mekonnen A. Comparative study of the antioxidant and antibacterial activities of two guava (Psidium guajava) fruit varieties cultivated in Andasa Horticulture Site, Ethiopia. Chem Int. 2018;4:154–162.
128. Gashe F, Belete A, Gebre-Mariam T. Evaluation of antimicrobial and anti-inflammatory activities and formulation studies on the leaf extracts of Psidium guajava L. Ethiop Pharm J. 2010;28:131–142.
129. Dilebo T, Feyissa T, Asfaw Z. In-vitro propagation of multi-use enset [Ensete ventricosum (Welw.) Cheesman] landraces using bulla as gelling agents. Plant Cell Tissue Organ Cult. 2023;155(3):693–708. doi:10.1007/s11240-023-02590-8
130. Abew B, Sahile S, Moges F. In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus. Asian Pac J Trop Biomed. 2014;4(10):816–820. doi:10.12980/APJTB.4.201414B16
131. Asressu KH, Tesema TK. Chemical and antimicrobial investigations on essential oil of Rosmarinus officinalis leaves grown in Ethiopia and comparison with other countries. J Appl Pharm. 2014;6(3):132–142. doi:10.21065/19204159.6.3.112
132. Manilal A, Sabu KR, Shewangizaw M, et al. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon. 2020;6(1):e03303. doi:10.1016/j.heliyon.2020.e03303
133. Emam JA, Yaya EE, Choudhary MI, Yousuf S, Gebremedhin TM. In Vitro Antioxidant and Anti-inflammatory Activities of Twenty-two Ethiopian Medicinal Plants. Ethiop Pharm J. 2021;37(1):89–94. doi:10.4314/epj.v37i1.8
134. Yrga Adugna B, Mequanint Adinew G, Ayalew Getahun K, et al. Evaluation of the antidiabetic activity of hydromethanolic roots extracts of Rumex abyssinicus jacq:(Polygonaceae) in Swiss albino mice. Evidence-Based Complementary and Alternative Medicine Evid Based Complement Altern Med. 2022;2022.
135. Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):10. doi:10.1186/1746-4269-4-10
136. Alemeye M, Gebre-marima T, Asres K. Antimicrobial activities and formulations of the extracts of chewing sticks commonly used in Ethiopia for oral cleansing. Ethiop Pharmaceutical J. 2018;34(2):95–108. doi:10.4314/epj.v34i2.3
137. Mekuriaw E, Mengistu E, Erdedo A, Mamo H. In vitro antibacterial activity, preliminary phytochemical screening profile, and In vivo toxicity of seven traditional medicinal plants in Ethiopia. Trad Integr Med. 2021;6:398–414.
138. Meragiaw M, Asfaw Z. Review of antimalarial, pesticidal and repellent plants in the Ethiopian traditional herbal medicine. Research Rev. 2014;3:21–45.
139. Abdeta D, Abay SM, Giday M, Kebede N, Terefe G. Antitrypanosomal effect of hydromethanolic extract of Solanum anguivi Lam on field Isolates of Trypanosoma congolense Infected Mice. J Parasitol Res. 2021;2021:1–8. doi:10.1155/2021/1239379
140. Tegegne AA, Mulugeta A, Genetu B, Endale A, Elias A. Perception towards COVID-19 related symptoms and traditional medicine used for their management among patients and their attendants in Ethiopian comprehensive specialized hospitals: a cross-sectional study. Infect and Drug Resist. 2022;15:5023–5034. doi:10.2147/IDR.S380211
141. Hishe M, Asfaw Z. Review on ethnobotanical studies on traditional medicinal plants used to treat livestock and human ailments in Tigray region, Ethiopia. Adv J Biol Sci Res. 2015;3:008–0036.
142. Teka A, Rondevaldova J, Asfaw Z, et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complement Altern Med. 2015;15(1):286. doi:10.1186/s12906-015-0822-1
143. Zemene M, Geta M, Huluka SA, Birru EM. Anti-malarial activity of the 80% methanol leaf extract and solvent fractions of Stephania abyssinica (Dill. & A. Rich.)Walp. against Plasmodium berghei infection in mice. Ethiop Pharm J. 2020;36(2):109–120. doi:10.4314/epj.v36i2.4
144. Alemneh D. Ethnobotanical Study of Ethno-veterinary medicinal plants in Yilmana Densa and Quarit Districts, West Gojjam Zone, Amhara Region, Ethiopia. Ethnobot Res Appl. 2021;22:1–16.
145. Ayele Y, Urga K, Engidawork E. Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of Syzygium guineense (willd) D.C. Phytother Res. 2010;24(10):1457–1462. doi:10.1002/ptr.3141
146. Esubalew ST, Belete A, Lulekal E, Gabriel T, Engidawork E, Asres K. Review of ethnobotanical and ethnopharmacological evidences of some Ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiop J Health Dev. 2017;31:161–187.
147. Bussa NF, Belayneh A. Traditional medicinal plants used to treat cancer, tumors and inflammatory ailments in Harari Region, Eastern Ethiopia. S Afr J Bot. 2019;122:360–368. doi:10.1016/j.sajb.2019.03.025
148. Abeje BA, Bekele T, Getahun KA, Asrie AB. Evaluation of Wound Healing Activity of 80% Hydromethanolic Crude Extract and Solvent Fractions of the Leaves of Urtica simensis in Mice. J Exp Pharmacol. 2022;14:221–241. doi:10.2147/JEP.S363676
149. Sisay W, Andargie Y, Molla M. Antimalarial Efficacy of Hydromethanolic Root Extract and Solvent Fractions of Urtica simensis Hochst. ex. A. Rich. (Urticaceae): an Experimental Study on Plasmodium berghei-Infected Mice. Evid Based Complement Altern Med. 2022;2022:1–16. doi:10.1155/2022/6702733
150. Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48(4):461–468. doi:10.3109/13880200903173593
151. Alemneh D. Ethnobotanical study of plants used for human ailments in Yilmana densa and Quarit districts of west Gojjam Zone, Amhara region, Ethiopia. BioMed Res Int. 2021;2021:1–18. doi:10.1155/2021/6615666
152. Bihonegn T, Giday M, Yimer G, Animut A, Sisay M. Antimalarial activity of hydromethanolic extract and its solvent fractions of Vernonia amygdalina leaves in mice infected with Plasmodium berghei. SAGE Open Med. 2019;7:1–10. doi:10.1177/2050312119849766
153. Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera Hiern (Asteraceae) in mice. J Exp Pharmacol. 2021;13:677–692. doi:10.2147/JEP.S308303
154. Haile F, Kifle ZD, Dejenie TA, Berhane N, Berhane N. Synergetic antibacterial activity of Vernonia auriculifera Hiern and Buddleja polystachya Fresen on selected human pathogenic bacteria. Metabol Open. 2022;16:100210. doi:10.1016/j.metop.2022.100210
155. Ashenafi E, Abula T, Abay SM, Arayaselassie M, Taye S, Muluye RA. Analgesic and Anti-Inflammatory Effects of 80% Methanol Extract and Solvent Fractions of the Leaves of Vernonia auriculifera Hiern. J Exp Pharmacol. 2023;15:29–40. doi:10.2147/JEP.S398487
156. Shilema A, Zerom K, Mussa A. Ethnoveterinary practices against animal trypanosomosis in Amaro district, Southern Ethiopia. Int J Med Plants Res. 2013;2:238–241.
157. Wondimu T, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants around ‘Dheeraa’town, Arsi Zone, Ethiopia. J Ethnopharmacol. 2007;112(1):152–161. doi:10.1016/j.jep.2007.02.014
158. Ashagre ME, Molla EL. Cultural Significance of Medicinal Plants in Healing Human Ailments Among Guji Semi-Pastoralist People, Suro Barguda District, Ethiopia. J Ethnobiol Ethnomed. 2021;17(1):61.
159. Akissi ZL, Yao-Kouassi AP, Magid AA, Koffi JK, Voutquenne-Nazabadioko L. Chemical constituents and antioxidant capacities of Asparagus africanus Lam. Phytochem Lett. 2023;53:22–30. doi:10.1016/j.phytol.2022.11.004
160. Wanga LA, Indieka AS, Matasyoh JC. Antidermatophytic quinolizidine alkaloids from Calpurnia aurea subsp. aurea (Aiton) Benth. Fitoterapia. 2023;171:105698. doi:10.1016/j.fitote.2023.105698
161. Sanwal R, Chaudhary AK. Wound healing and antimicrobial potential of Carissa spinarum Linn. In albino mice. J Ethnopharmacol. 2011;135(3):792–796. doi:10.1016/j.jep.2011.04.025
162. Maroyi A. Ethnopharmacological Uses, Phytochemistry, and Pharmacological Properties of Croton macrostachyus Hochst. Ex Delile: a Comprehensive Review. Evid Based Complement Alternat Med. 2017;2017:1–17. doi:10.1155/2017/1694671
163. Chinsembu KC, Hijarunguru A, Mbangu A. Ethnomedicinal plants used by traditional healers in the management of HIV/AIDS opportunistic diseases in Rundu, Kavango East Region, Namibia. S Afr J Bot. 2015;100:33–42. doi:10.1016/j.sajb.2015.05.009
164. Ugbogu OC, Emmanuel O, Agi GO, et al. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon. 2021;7(11):e08404. doi:10.1016/j.heliyon.2021.e08404
165. Kasali FM, Tusiimire J, Kadima JN, Tolo CU, Weisheit A, Agaba AG. Ethnotherapeutic uses and phytochemical composition of Physalis peruviana L.: an overview. Sci World J. 2021;2021:1–22. doi:10.1155/2021/5212348
166. Deng Z, Sheng F, Yang SY, Liu Y, Zou L, Zhang LL. A comprehensive review on the medicinal usage of Podocarpus species: phytochemistry and pharmacology. J Ethnopharmacol. 2023;310:116401. doi:10.1016/j.jep.2023.116401
167. Bussmann RW, Paniagua-Zambrana NY, Njoroge GN. Rumex abyssinicus Jacq. Rumex usambarensis (Dammer) Dammer Polygonaceae. In: Ethnobotany of the Mountain Regions of Africa 2021 Jan 23. Cham: Springer International Publishing. 919–926; 2021.
168. Nguyen D, Rieu I, Mariani C, van Dam NM. How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol. 2016;91(6):727–740. doi:10.1007/s11103-016-0481-8
169. Mahmoud SM, Abdel-Azim NS, Shahat AA, Ismail SI, Hammouda FM. Phytochemical and biological studies on Verbascum sinaiticum growing in Egypt. Nat Prod Sci. 2007;13:186–189.
170. Tadesse M. Asteraceae. In: Hedberg I, Friis I, Edwards S, editors. Flora of Ethiopia and Eritrea. Volume 4 Part 2: Asteraceae. The National Herbarium, Addis Ababa University, Addis Ababa; 2004:1–408.
171. Tadesse M, Hunde D, Getachew Y. Survey of medicinal plants used to treat human diseases in Seka Chekorsa, Jimma Zone, Ethiopia. Ethiop J Health Sci. 2005;15:89–106.
172. Eshete MA, Kelbessa E, Dalle G. Ethnobotanical study of medicinal plants in Guji agro-pastoralists, Blue Hora District of Borana Zone, Oromia region, Ethiopia. J Med Plants Stud. 2016;4:170–184.
173. Gebrehiwot M. An Ethnobotanical Study of Medicinal Plants in Seru Wereda, Arsi Oromia Zone of Ethiopia (Msc Thesis). Addis Ababa: Addis Ababa University; 2010.
174. Beyene T. Ethnobotany of Medicinal Plants in Erob and Gulomahda Districts, Eastern Zone of Tigray Region, Ethiopia (Phd Thesis). Addis Ababa: Addis University; 2015.
175. Kidane L, Gebremedhin G, Beyene T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern Zone of Tigray, northern Ethiopia. J Ethnobiol Ethnomed. 2018;14(1):64. doi:10.1186/s13002-018-0266-z
176. Heinrich M. Ethnobotany and its role in drug development. Phytother Res. 2000;14(7):479–488. doi:10.1002/1099-1573(200011)14:7<479::AID-PTR958>3.0.CO;2-2
177. Leonti M, Vibrans H, Sticher O, Heinrich M. Ethnopharmacology of the Popoluca, Mexico: an evaluation. J Pharm Pharmacol. 2001;53(12):1653–1669. doi:10.1211/0022357011778052
178. Abate L, Mengistu T. Phytochemical screening and peroxide value determination of methanolic extract of four traditional medicinal plants from Debre Tabor Town, Ethiopia. J Med Plant Res. 2018;12(16):203–208. doi:10.5897/JMPR2018.6579
179. Gizaw A, Kena B, Babele DA, et al. Phytochemical screening and in vitrolousicidal and acaricidal activities of Justicia schimperiana (Hochst. ex Nees) T. Anderson leaf in West Showa Zone, Ethiopia. Ann Phytomedicine. 2022;11:725–731.
180. Tesfay D, Endale M, Getaneh E, Abdisa E, Guta L, Melaku Y. Chemical composition and antibacterial activity of essential oils from various parts of Gladiolus Candidus, Ranunculus Multifidus, Artemisia Abyssinica and Crinum Abyscinicum. Bull Chem Soc Ethiop. 2022;36(4):865–878. doi:10.4314/bcse.v36i4.12
181. Komlaga G, Agyare C, Dickson RA, et al. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol. 2015;172:333–346. doi:10.1016/j.jep.2015.06.041
182. Muthee JK. Anthelmintic efficacy of selected medicinal plants against gastrointestinal nematodes in naturally infected sheep in Kenya. J Phytopharmacol. 2018;7(2):111–115. doi:10.31254/phyto.2018.7202
183. Zebeaman M, Gebeyehu R. Phytochemical Screening and Antioxidant Activity of the fruit of Embelia Schimperi V. (family Myrsinaceae). Int JPhotochem. 2018;4:27–32.
184. Kimutai N, Ogutu PA, Mutai C. Antibacterial Activities of Selected Medicinal Plants from Nandi County, Kenya. Asian Jr Microbiol Biotech Env Sc. 2020;22:541–547.
185. Terefe L, Nardos A, Debella A, et al. Antidiarrheal Activities of the Methanol Leaf Extracts of Olinia rochetiana (Oliniaceae) Against Castor Oil-Induced Diarrhea in Mice. J Exp Pharmacol. 2023;2023:485–495. doi:10.2147/JEP.S441555
186. Tadeg H, Mohammed E, Asres K, Gebre-Mariam T. Performance evaluation of topical formulations of the crude extracts of L. adoensis and O. rochetiana. Ethiop Pharm J. 2004;22:15–26.
187. Gebremariam T, Abula T, Gebremariam MG. Antibacterial and phytochemical screening of root extracts of Euclea racemosa subsp. Schimperi Int J Pharmacog. 2015;2:66–70.
188. Bazezew AM, Emire SA, Sisay MT. Bioactive composition, free radical scavenging and fatty acid profile of Ximenia americana grown in Ethiopia. Heliyon. 2021;7(6):e07187. doi:10.1016/j.heliyon.2021.e07187
189. Regassa R. Useful plant species diversity in home gardens and its contribution to household food security in Hawassa city, Ethiopia. Afr J Plant Sci. 2016;10(10):211–233. doi:10.5897/AJPS2016.1439
190. Gonfa N, Tulu D, Hundera K, Raga D. Ethnobotanical study of medicinal plants, its utilization, and conservation by indigenous people of Gera district, Ethiopia. Cogent Food Agric. 2020;6(1):1852716. doi:10.1080/23311932.2020.1852716
191. World Health Organization. World Medical Association Declaration of Helsinki. Bull World Health Org. 2001;79(4):373–374.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
