Back to Journals » Integrated Blood Pressure Control » Volume 16
Medication Related-Problems and Associated Factors Among Patients with Hypertension at a Tertiary Care Hospital in Ethiopia: A Prospective Interventional Study
Authors Garedow AW , Mamo MD , Tesfaye GT
Received 5 August 2023
Accepted for publication 25 October 2023
Published 30 November 2023 Volume 2023:16 Pages 123—136
DOI https://doi.org/10.2147/IBPC.S434072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Aster Wakjira Garedow,1 Mekonnen Damessa Mamo,1 Gorfineh Teshome Tesfaye2
1Jimma University, School of Pharmacy, Jimma, Ethiopia; 2Jimma University Medical Center, Department of Pharmacy, Jimma, Ethiopia
Correspondence: Aster Wakjira Garedow, Email [email protected]; [email protected]
Background: Hypertension affects more than 1.4 billion people worldwide currently, with that number anticipated to climb to 1.6 billion by 2025 with high mortality and morbidity effects. Medication related problems in cardiovascular disease patients, especially among hypertension patients were found to be high and a critical problem which is associated with high mortality, complication, prolonged hospital stay, compromised quality of life and increase health care cost.
Objective: To determine medication related problems and its predictors among hypertension patients on chronic follow-up at Jimma Medical Center.
Methods: A prospective interventional study was conducted among hypertension patients from November 28, 2021 to June 30, 2022 at Jimma Medical Center. Medication related problems were classified and identified based on Pharmaceutical care network Europe drug classification tool version 9.0. Interventions were done through discussion with individual prescriber and patients. Consecutive sampling technique was used. Binary Logistic regression was used to identify independent predictors of medication related problems. Variables having P-values < 0.05 were considered statistically significant.
Results: Among 384 hypertension patients included in the study, 219 (57.1%) were male. The mean (SD) age was 49.06+17.79. Two thirds of study participants had at least one medication related problem. A total of 483 MRPs were identified among 231 (60.15%) patients. Treatment effectiveness related problem (55.48%) was the most common observed medication related problems. Alcoholism (AOR; 3.15, 95% CI [1.46– 7.23]), stage II hypertension (AOR=2.77, 95% CI= [3.53– 4.66]); comorbidity (AOR=2.88, 95% CI= [1.47– 5.66]) and polypharmacy (AOR=3.07, 95% CI= [1.57– 5.99]) were the independent predictors of medication related problems.
Conclusion: The prevalence of medication related problems was high among hypertensive patients. Alcoholism, stage II hypertension, comorbidity and poly-pharmacy were the predictors of medication related problems. Therefore, to overcome the problems, clinical pharmacists, physicians and other health care professionals have to work in collaboration.
Keywords: medication related problems, interventions, hypertension, Jimma
Background
Hypertension (HTN) is a condition in which the blood pressure (BP) in arteries or veins is abnormally high and defined as a systolic blood pressure (SBP) ≥140 mm Hg and/ or diastolic blood pressure (DBP) ≥90 mm Hg.1 Worldwide, prevalence of hypertension is 31% which is almost similar with in the US adult population 31.9% (72.2 million people), defined at the SBP/DBP cutoff of >140/90 mm Hg.2 Hypertension affects more than 1.4 billion people worldwide currently, with that number anticipated to climb to 1.6 billion by 2025.3
Medication related problems (MRPs) are a consequence of medication related needs that were not met, central to pharmaceutical care practice. According to Pharmaceutical Care Network Europe (PCNE) classification version 9, MRPs is an event or circumstance involving drug therapy that interferes with desired health outcomes.4 MRPs are common in HTN patients and result in patient morbidity, mortality, increased costs, impact on patients quality of life, prolonged hospital stays and increase the overall burden of healthcare expenditures.3,5 Older age, different co-morbidities and polypharmacy may complicate management of HTN, which probably puts patients at risk for MRPs.6 Need for additional drug therapy was the most common MRP, which showed that treatment of HTN patients is still suboptimal.7 Systematic review carried out in Ethiopia showed that MRPs among HTN was higher than other medical conditions which is due to multiple medications and having a comorbid condition has been linked to adverse health outcomes including drug interaction and poor adherence to treatment.8
The clinical pharmacist, as a part of the multidisciplinary team, could reduce MRPs.9 Interventional study done in United State of America (USA) showed that the average of MRPs reduced from 2.8 to 1.95 after intervention of clinical pharmacist.10 Pharmacist-based services can empower patients with HTN to understand and manage their complex medication regimens through medication reconciliation, identification of MRPs and implementation of interventions on identified MRPs.11 In addition, pharmacist-based interventions can improve clinical outcomes of HTN patients, reduce hospital stay, fewer re-admissions and fewer complications, reduce costs of readmissions and emergency room visits.12
MRPs contribute to a high number of morbidities and mortalities worldwide and are responsible for undesirable health consequences in HTN patients.13 Studies revealed that one out of six patients with chronic conditions visit health facilities because of MRPs14 and up to 30% of hospital admissions are related to MRPs.15 MRPs are relatively common among HTN patients and can result in patient morbidity and mortality, thus the increased cost.16–18 Little is known about the extent of MRPs and the clinical pharmacist role in the management of HTN patients in Ethiopia. Knowing the extent of MRPs among HTN patients will lead healthcare professionals to optimize drug therapy that may influence health expenses, save lives, improves health, reduce morbidity and mortality and increase quality of life.19,20 Hence, this study aimed to identify MRPs and associated factors among HTN patients at Jimma University Medical Center (JUMC).
Methods and Participants
Study Area and Period
This study was conducted from November 28, 2021 to June 30, 2022 at JMC, which is located in Jimma town; 345 km Southwest of Addis Ababa, the capital. JUMC is the only teaching and medical center hospital in the south western part of the country with bed capacity of 600. It provides service for approximately 9000 inpatient and 80,000 outpatient clients per year with a catchment population of about 15 million people. The medical services provided by the JUMC include internal medicine, surgery, orthopedics, ophthalmology, pediatrics, gynecology and obstetrics, dermatology, oncology, psychiatric services, pathology, pharmacy, medical laboratory, intensive care unit, radiology, and others as both inpatient and outpatient service. Chronic follow-up clinic provides different services such as: DM, TB, HTN, neurology and AIDS. Annually, about 937 HTN patients come to HTN clinic of JUMC for follow up.
Study Design and Variable
A prospective interventional study design was conducted among HTN patients at JUMC. All HTN patients who had follow-up at chronic follow up clinic of JUMC were Source Population. Study Population was all HTN patients who had follow-up at chronic follow up clinic of JUMC during study period and fulfill the inclusion criteria. HTN patients’ age ≥18 years and willing to give written consent were included. HTN patients who died or were lossed to follow-up and with incomplete medical charts were excluded. Dependent variable was medication related problems. Independent Variables include Socio-demographic characteristics (age, sex, marital status, educational status, residence, medication belief, cost coverage, occupation, and social drug use), clinical characteristics (co-morbidity, stage of HTN, etiology of HTN, class and number of drug, duration of HTN diagnosis [years], and laboratory investigation).
Sample Size Determination and Sampling Technique
The sample size was determined by using the single population proportion formula. By considering the proportion (P) of MRP among HTN patients 50%, 95% confidence interval (CI) and 5% marginal error the final minimum sample size was 384. Consecutive sampling technique was used until the required sample size was obtained.
Data Collection Instrument
Pharmaceutical care network Europe (PCNE) version 9.00 MRP classification was used to classify and document MRPs. It has three primary domains for problems (P1-treatment effectiveness, P2-Treatment Safety and P3-others). There are nine primary domains for causes (C1-drug selection, C2-drug form, C3-dose selection, C4-treatment duration, C5-dispensing, C6-Drug use process, C7-patient related, C8-patient transfer related and C9-Others). Structured data collection tool was used to extract relevant information regarding patient demographics and clinical data. Medication belief was measured by belief about medication questionnaire (BMQ), in which the patient’s belief was considered as positive when the average sum of the 5-item patient’s medication necessity scale score exceeded the average 5-item medication concerns scale, otherwise it was considered as negative.21 ADR was assessed by Naranjo drug reaction probability scale which has also been standardized and validated.22 Lexi comb, Medscape drug interaction checker accessed to check drug-drug, and drug-disease interaction. MRPs were identified by comparing patient’s treatment with guidelines.23–25
Data Collection Procedure
Data were collected by two pharmacists and two nurses. The data collectors were trained for five days before starting data collection. Data were collected through medical record review of patients using a prepared standard checklist and structured questionnaire. Content of checklist include patient details, laboratory investigations, current and past medications and medical condition. The structured questionnaire content includes socio-demographic characteristics and clinical characteristics. After data were collected, clinical pharmacist reviewed patient’s therapy to assess MRPs. Medication related problems were identified by evaluating the appropriateness of prescription regarding indication, dosage, and safety and by assessing patients for adherence. Interventions were done by the principal investigator and three senior clinical pharmacists through discussion with individual prescriber and patients immediately. MRPs which are not accepted were further discussed with senior physicians or residents for further interventions.
Data Quality Assurance
The questionnaires were prepared in English and translated into Amharic and Vernacular language, Afan Oromo, and back-translated into English by an independent person to assure its consistency. A pre-test was conducted on 19 (5%) study participants by randomly selected patients before the actual data collection to check the consistency and validity of the structured data collection format. Data were compiled, cleared, coded, and checked for consistency. All steps in data collection and recording were closely monitored by the supervisor and any gaps identified were immediately communicated with the data collectors.
Data Processing, Analysis and Presentation
Data were entered into Epidata version 4.6.0.4 and exported to the SPSS version 25 for analysis. First, data were edited and checked for completeness and consistency, then exported into SPSS for analysis. A bivariate analysis was performed with binary logistic regression to assess association between the MRPs and independent variables. Those variables with a p value<0.25 in bivariate analysis were introduced into multivariate logistic regression analysis and those variables with a p value<0.05 were considered as spastically significant.
Ethical Consideration
The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Council on Harmonization Guidelines for Good Clinical Practice. The Jimma University institution review board (IRB) (Ref.No IRB 000238 /2021/2022) granted ethical clearance and approval, and the JUMC clinical director office was given a letter of authorization. By employing identification numbers rather than patient names, confidentiality was guaranteed. Written informed consent was obtained from HTN patients.
Operational Definition and Definition of Terms
Medication related problem: event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes.
Hypertension: Is an increase in arterial BP measured at least twice and the result in SBP and/or DBP readings are ≥ 140 and/ or ≥ 90 mm HG, respectively.2
Clinical inertia: Physicians’ failure to introduce and/or enhance drugs.
Adverse drug reaction: is a noxious and unintended response to a drug which occurs at doses normally used for the prophylaxis, diagnosis, or treatment of disease.26
Poly-pharmacy: defined as concomitant use of five or more prescription medications.27
Clinical pharmacist interventions: Is any action by a clinical pharmacist that directly results in a change in patient management or therapy.
Comorbidity: presence of other medical condition other than hypertension.
Non-compliance: the patient does not understand instruction, cannot afford drug product, prefers not to take medication, forgets to take medication timely or drug product not available.
Insurance: cost coverage of available medication provided by health institution.
Results
Socio-Demographic Characteristics of the Study Participants
Among 384 study participants included in this study, 219 (57.1%) were male. The mean ± SD age of the patients was 49.06 +17.79 years. Most 156 (40.63%) of them were in the age range of 19–47 years. Most, 268 (69.79%), of patients were residing in the rural area. More than half (202, 52.6%)of patients were farmers, while 222 (57.81%) of participants had no formal education and 133 (56.1%) patients have positive belief. Almost nearly one third of participants 211 (55.0) had family history of hypertension (Table 1).
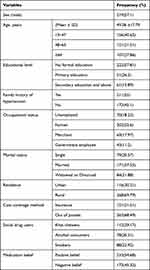 |
Table 1 Baseline Socio-Demographic Characteristics Among Hypertension Patients at JMC, from November 28, 2021 to June 2022 |
Clinical Characteristics of Study Participants
Of 384 HTN patients included in the study, most of patients (246, 64.1%)had comorbid condition. The most common causes of HTN were CKD 134 (34.89%) followed by CVD 96 (25%) and CMP 91 (23.69%). Majority of participants 221 (57.55%) were with less than five years of duration of diagnosis of hypertension. The overall proportion of controlled BP (SBP and/or DBP) in the last 7 months of follow-up period was 124 (50.8). The mean SBP and DBP readings of the participants were 143.25 (± 8.75 SD) and 88.75 (± 5.5 SD) mm HG, respectively. Slightly more than half (127, 52.0%)of the participants had comorbidity, DM 246 (64.1%) was the most encountered, followed by CKD 96 (25%) (Table 2).
 |
Table 2 Clinical Characteristics Among Hypertension Patients at JMC, from November 28, 2021 to June 2022 |
Past Medical and Medications History and Medication Involved in Medication Related Problems Among Study Participants
Total of 669 drugs were prescribed for 384 HTN patients during study period. The mean number of drug per patient was 1.18±0.82. Diuretics 252 (65.62%) were the most commonly prescribed antihypertensive medications, followed by ACEI 241 (62.72%) and CCB 102 (26.565%). The most commonly prescribed specific drugs were enalapril 241 (62.72%) followed by Hydrochlorothiazide 232 (60.41%). Nearly two thirds 202 (67.15) of participants were with high adherence to their medications. More than half 207 (53.9%) of participants were prescribed one antihypertensive drug regimens. The most frequently encountered drug classes involved in MRPs were diuretics 134 (34.72%), of which 126 (36.41%) was Hydrochlorothiazide, Angiotensin converting enzyme inhibitors (ACEIs) and calcium channel blockers (CCBs) were about 141 (40.75%) and 51 (13.74%) respectively (Table 3).
The Prevalence, Type and Causes of Medication Related Problems
From a total of 384 HTN patients, 231 (60.15%) patients experienced MRPs and 483 MRPs were identified. The average number of MRP per patient was 1.25± 1.18. Among Patients who experienced MRPs, 130 (61.93%) had 1 MRP, 56 (26.67%) had 2 MRPs and 45 (21.42%) had > 3 MRPs. The most commonly found MRPs were treatment effectiveness related (no effect of drug treatment, untreated indication, effect of drug not optimal) 268 (55.48%) followed by others (unnecessary drug treatment, compliance and cost effectiveness related) 111 (22.97%) and safety related (ADE occur or may occur) 104 (21.57%). Six hundred fifty-four causes of MRPs were identified. Drug selection 109 (16.67%), dose selection 67 (10.24%) and patient related 63 (9.63%) were the most common causes of MRPs (Table 4).
 |
Table 4 Medication Related Problems and Causes of Medication Related Problems Among Patients with Hypertension at JMC from November 28, 2021 to June 30, 2022 |
Intervention, Acceptance Rate and Outcome of Intervention of Medication Related Problems
For the identified MRPs, a total of 458 intervention were delivered at different levels, out of this 198 (43.23%) interventions were done at prescriber level, 421 (91.92%) of them were accepted. After intervention, 401 (87.55%) and 37 (10.26%) of the problems were solved and not solved respectively (Table 5).
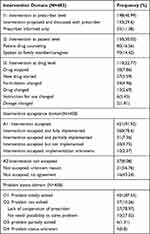 |
Table 5 Intervention, Prescriber Acceptance Rate and Outcome of Intervention for MRPs Among Hypertension Patients at JMC, November 28, 2021 to June 2022 |
Predictor’s of Medication Related Problems
In crude analysis using binary logistic regression: Sex, age, chat chewers, alcohol drinkers, comorbidity, stage of hypertension and polypharmacy were found to predispose HF patients for MRPs with statistically significant association. Independent predictors for encountered MRPs were identified using multivariate logistic regression. Finally alcohol drinker (AOR; 3.25, 95% CI (1.46–7.23), P=0.004), stage II HTN (AOR; 2.77, 95% CI 1.93–7.37, P=0.001), presence of comorbid condition (AOR: 2.59, CI 1.35–4.96, P=0.004) and polyp-harmacy (AOR; 2.94, 95% CI 1.54–5.61, P=0.02) were found to be independent predictors of MRPs.
According to our finding HTN patients with stage II HTN are 2.77 (AOR; 2.45, 95% CI 2.32–5.34) times more at risk for MRP than those pre-HTN, HTN patients who had comorbid condition were 2.59 (AOR: 2.59, CI 1.35–4.96) times more likely to encounter MRP than those without comorbid condition. HTN patients who are Alcohol drinkers were 3.25 (AOR; 3.25, 95% CI (1.46–7.23) times more likely to encounter MRPs than those not khat chewers, with smokers (Table 6).
 |
Table 6 Predictors of MRPs Among Hypertension Patients at JMC, November 28, 2021 to June 2022 |
Discussion
Out of 231 (60.15%) patients who experienced MRPs, 62% of MRPs were found in males, which is similar with the result of two studies done in India.28,29 This might be due to increased medication use because of comorbid condition was higher in males and other various risk factors like smoking, alcoholism and chewing Khat compared to females. The prevalence of MRP was found to be 60.15% and average of MRPs per patient was 1.25± 1.18, which was lower than study conducted at JUMC (83.5%) and 2.6 ± 1.8, the difference could be due to setting difference where our study conducted chronic follow up patients in which senior physicians and clinical pharmacists are available more frequently.20 However, it is almost in line with study conducted at TASH (65.5%)26 and GUH 63.4% or average 1.17 ± 1.1 per patient.30
The most common MRPs in our study were treatment effectiveness related problems 268 (55.48%) and the least was ADE occurrence (21.57%). Of the treatment effectiveness related problems, suboptimal drug treatment and untreated indication were about 28% and 25% respectively. This finding was in line with a study conducted in Barcelona which showed suboptimal drug therapy (31%) and probability of ADE occurrence (16%).31 In contrary to this finding, a study conducted at JUSH in 2014 showed that treatment effectiveness related was about 83%, of which suboptimal drug therapy and untreated indication were about 55% and 27% respectively.20 Furthermore, a study done in USA on outpatient heart failure showed that treatment effectiveness related problems was about 36.8%.32 The discrepancy could be due to difference in methods of MRPs classification, study setting, sample size, or study design.
About 91% of patients were compliant to medication which was comparable with a study done in Netherlands (98.6%).33 Non-adherence was about 9%, which was in line with studies done at ambulatory care of JUSH (9%), Harar 12%,34 Barcelona and Spain (14%).20,31 However, study done at TASH showed that non-compliance was about 45%.19 The difference could be due to difference in compliance assessment method and in our study patients may have more access to information about medication from health professional and caregiver, a problem in patients’ adherence to self-care activities or non-pharmacological therapy, and the patient was not given instruction in or did not understand non-pharmacological therapy or self-care advice in previous studies.
In our study, one third of MRPs was due to inappropriate drug selection and about 21% was a dose selection related problem. Indication (need additional drug therapy) was case of about 60% of inappropriate drug selection, which was higher than study conducted at GUH which showed inappropriate drug selection and new indication were about 36% and 59% respectively30 and in India inappropriate drug selection 34% and dose selection 27%.35 However, study on general medical conditions of admitted geriatric patients at JUMC showed that inappropriate drug selection was about 54% and the main causes of it was about 36%.36 This discrepancy may be due to difference in study population, medical conditions, sample size and study design.
In the present study, the most common classes of drugs implicated in MRPs were BBs (35%) and ACEIs (25.3%) which was inconsistent with studies conducted in JUSH, which BBs and ACEI were 34.4% and 24.8% respectively,20 in Taiwan showed that ACEI was about (21%),37 at ambulatory clinic of TASH and in chronic follow up HTN patients at JUSH showed that BBs, ACEIs and antithrombotic were the most common implicated drug classes in MRPs likewise our findings.19,38
The result of multivariate logistic regression showed that alcoholism, comorbidity, stage II HTN and poly-pharmacy were independent predictors of MRPs. Study conducted in southern India supports our findings which showed that having history of alcoholism put patients at high risk of MRPs. The plausible argument is social drug use (alcoholism) causes patients’ financial issues to be disrupted.35 Stage II HTN was one of the independent predictors of MRPs among HTN patients follow their treatment at JUMC. This was supported by studies conducted in Saudi and Nepal.38–42 This might be due to the likelihood of increasing the stage of HTN increases the number the drugs the patient need, which in turn will increase the likelihood of MRPs.
Comorbidity was other independent risk factors of MRPs in HTN patients followed their care at JUMC. This is augmented by studies carried out at ambulatory clinic of GUH and JUMC.5,19,20,43,44 This could be due to patients with comorbidity being more likely to take more drugs to treat other diseases, causing disease-disease interaction, drug-drug interaction, or drug- disease interaction which in turn makes patients more vulnerable to MRPs. Moreover, polypharmacy was also an independent predictor of MRPs, which was also supported by several studies conducted in different settings.5,19,20,39,43,45,46,47,23 This could be due to the fact that the greater the number of medications prescribed, the more drug-drug interactions, risk for adverse events, difficulties for adherence and cost.
Clinical pharmacists’ interventions among chronic patients play a vital role in effectively identifying, resolving and preventing MRPs. According to our study clinical pharmacists’ intervention acceptance rate was about 91.92%, of which about 87.55% interventions were fully implemented and 82% of interventions totally solved the problem. This result was comparable with studies carried out in Southern India and Karnataka, India which revealed that clinical pharmacists’ acceptance were about 97% and 96% respectively.28,24 Moreover clinical pharmacists’ intervention and acceptance rate were about two thirds of MRPs.25,48,49
Limitation of Study
The study involved patients being admitted at a single hospital, not community-based, and thus may not reflect the real picture of the general population. Besides the study was not conducted to assess effectiveness of the intervention.
Conclusion
Our study showed that the prevalence of MRPs was high among HTN patients at JMC. The most common identified MRPs were treatment effectiveness related problems which mainly includes suboptimal effect of drug and untreated indication. Prolonged hospital stay, comorbidity and polypharmacy were found to be independent predictors of MRPs. Clinical intervention and its acceptance rate were high.
Abbreviations
ACEIs, Angiotensin converting enzyme inhibitors; ADR, Adverse drug reaction; AHA, American heart association; ARB, Angiotensin receptor blocker; BBs, Beta blockers; BMQ, Belief about medication questionnaire; CP, Clinical pharmacist; CVD, Cardiovascular disease; MRP, Medication related problems; ESC, European Society of Cardiology; ESTG, Ethiopian Standard treatment guideline; HF, Heart Failure; ICU, Intensive care unit; JMC, Jimma Medical Center; MRA, Mineralocorticoid receptor antagonist; NSAID, Non-steroidal anti-inflammatory drugs; PCNE, Pharmaceutical care Network Europe; TASH, UK, United Kingdom.
Data Sharing Statement
Readers who will require data and materials of the current study can communicate and get from the corresponding author with a reasonable request.
Acknowledgment
We would like to thank Jimma University, data collectors and all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Jimma University Institute of health. The funding body had no any role in the design of the study, data collection, and analysis, interpretation of data and in writing the manuscript.
Disclosure
All authors have no competing interests with the material presented in this manuscript.
References
1. Unger T, Borghi C, Charchar F., et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
2. Kibret KT, Mesfin YM. Prevalence of hypertension in Ethiopia: a systematic meta-analysis. Public Health Rev. 2015;36(1):1–12. doi:10.1186/s40985-015-0014-z
3. Amare F, Hagos B, Sisay M, Molla B. Uncontrolled hypertension in Ethiopia: a systematic review and meta-analysis of institution-based observational studies. BMC Cardiovasc Disord. 2020;20(1):1–9. doi:10.1186/s12872-020-01414-3
4. Schindler E, Richling I, Rose O. Pharmaceutical Care Network Europe (PCNE) drug-related problem classification version 9.00: German translation and validation. Int J Clin Pharm. 2021;43(3):726–730. doi:10.1007/s11096-020-01150-w
5. Srikanth A. Assessment of drug related problems and its associated factors among medical ward patients in University of Gondar teaching hospital, northwest Ethiopia: a prospective cross-sectional study. J Basic Clin Phar. 2017;5:8.
6. Meknonnen GB, Biarra MK, Tekle MT, Bhagavathula AS. Assessment of drug related problems and its associated factors among medical ward patients in University of Gondar teaching hospital, northwest Ethiopia: a prospective cross-sectional study. J Basic Clin Pharma. 2017;8:16–21.
7. Al-Azzam SI, Alzoubi KH, AbuRuz S, Alefan Q. Drug-related problems in a sample of outpatients with chronic diseases: a cross-sectional study from Jordan. Ther Clin Risk Manag. 2016;233–239. doi:10.2147/TCRM.S98165
8. Adem F, Abdela J, Edessa D, Hagos B, Nigussie A, Mohammed MA. Drug-related problems and associated factors in Ethiopia: a systematic review and meta-analysis. J Pharm Policy Pract. 2021;14(1):1–24. doi:10.1186/s40545-021-00312-z
9. Georgiev KD, Hvarchanova N, Georgieva M, Kanazirev B. The role of the clinical pharmacist in the prevention of potential drug interactions in geriatric heart failure patients. Int J Clin Pharm. 2019;41(6):1555–1561. doi:10.1007/s11096-019-00918-z
10. Yates L, Valente M, Wadsworth C. Evaluation of pharmacist medication review service in an outpatient heart failure clinic. J Pharm Pract. 2020;33(6):820–826. doi:10.1177/0897190019842696
11. Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015;15(1):1–8. doi:10.1186/s12889-015-2546-z
12. Viktil KK, Blix HS. The impact of clinical pharmacists on drug‐related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102(3):275–280. doi:10.1111/j.1742-7843.2007.00206.x
13. Dixon-Woods M, Baker R, Charles K, et al. Culture and behaviour in the English national health service: overview of lessons from a large multimethod study. BMJ Qual Saf. 2014;23(2):106–115. doi:10.1136/bmjqs-2013-001947
14. Mannesse CK, Derkx F, De Ridder M, Man In’t Veld A, Van der Cammen T. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29(1):35–39. doi:10.1093/ageing/29.1.35
15. Salvi F, Marchetti A, D’Angelo F, Boemi M, Lattanzio F, Cherubini A. Adverse drug events as a cause of hospitalization in older adults. Drug Safety. 2012;35(1):29–45. doi:10.1007/BF03319101
16. van den Bemt PM, Egberts TC, Brouwers JR, Brouwers JRBJ. Drug-related problems in hospitalised patients. Drug Safety. 2000;22(4):321–333. doi:10.2165/00002018-200022040-00005
17. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41(2):192–199. doi:10.1016/s1086-5802(16)31229-3
18. Al Hamid A, Aslanpour Z, Aljadhey H, Ghaleb M. Hospitalisation resulting from medicine-related problems in adult patients with cardiovascular diseases and diabetes in the United Kingdom and Saudi Arabia. Int J Environ Res Public Health. 2016;13(5):479. doi:10.3390/ijerph13050479
19. Seid E, Engidawork E, Alebachew M, Mekonnen D, Berha AB, Brunner-La Rocca H-P. Evaluation of drug therapy problems, medication adherence and treatment satisfaction among heart failure patients on follow-up at a tertiary care hospital in Ethiopia. PLoS One. 2020;15(8):e0237781. doi:10.1371/journal.pone.0237781
20. Niriayo YL, Kumela K, Kassa TD, Angamo MT, Frey R. Drug therapy problems and contributing factors in the management of heart failure patients in Jimma University specialized hospital, Southwest Ethiopia. PLoS One. 2018;13(10):e0206120. doi:10.1371/journal.pone.0206120
21. Tsianou K, Giannakeas N, Tsipouras M, et al. Accessing patient views about medication in chronic conditions using the Beliefs about Medicine Questionnaire (BMQ): a review study. J Drug Res Dev. 2017;3(2):1–9.
22. Tox L. Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): national institute of diabetes and digestive and kidney diseases; 2012.
23. Yusuff KB, Tayo F. Frequency, types and severity of medication use-related problems among medical outpatients in Nigeria. Int J Clin Pharm. 2011;33(3):558. doi:10.1007/s11096-011-9508-z
24. Celin A, Seuma J, Ramesh A. Assessment of drug related problems in stroke patients admitted to a South Indian tertiary care teaching hospital. Indian J Pharm Pract. 2012;5(4):28–33.
25. Acheampong F, Nkansah FA, Anto BP. Drug-related problems and their clinical interventions in a Ghanaian teaching hospital. Safety in Health. 2016;2(1):1–7. doi:10.1186/s40886-016-0050-5
26. Rohilla A, Yadav S. Adverse drug reactions: an overview. Int J Pharmacol Res. 2013;3(1):10–12.
27. Beezer J, Al Hatrushi M, Kurdi A, Forsyth P. Polypharmacy definition and prevalence in heart failure: a systematic review. Heart Fail Rev. 2021;2021:1–28.
28. Shareef J, Sandeep B, Shastry C. Assessment of drug related problems in patients with cardiovascular diseases in a tertiary care teaching hospital. J Pharm Care. 2014;2012:70–76.
29. Biradar SM, Indu P, Kalyane N, et al. Impact of drug-related problems and clinical pharmacist interventions on therapeutic outcomes of the patients admitted to a tertiary care hospital. Int J Med Sci Public Health. 2017;6(5):867–873. doi:10.5455/ijmsph.2017.1164412122016
30. Abdela OA, Bhagavathula AS, Getachew H, Kelifa Y. Risk factors for developing drug-related problems in patients with cardiovascular diseases attending Gondar University Hospital, Ethiopia. J Pharm Bioallied Sci. 2016;8(4):289. doi:10.4103/0975-7406.199335
31. Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes-Genis A. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail. 2011;17(3):217–223. doi:10.1016/j.cardfail.2010.10.009
32. Dempsey JT, Matta LS, Carter DM, et al. Assessment of drug therapy-related issues in an outpatient heart failure population and the potential impact of pharmacist-driven intervention. J Pharm Pract. 2017;30(3):318–323. doi:10.1177/0897190016641491
33. van Der Wal MH, Jaarsma T, Moser DK, Veeger NJ, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J. 2006;27(4):434–440. doi:10.1093/eurheartj/ehi603
34. Gelchu T, Abdela J. Drug therapy problems among patients with cardiovascular disease admitted to the medical ward and had a follow-up at the ambulatory clinic of Hiwot fana specialized university hospital: the case of a tertiary hospital in eastern Ethiopia. SAGE Open Med. 2019;7:2050312119860401. doi:10.1177/2050312119860401
35. Gona OJ, Shambu SK, Madhan R. Frequency and nature of drug‐related problems in patients with acute coronary syndrome: role of the clinical pharmacist in coronary care practice. J Pharm Pract Res. 2021;51(1):36–42. doi:10.1002/jppr.1684
36. Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 2020;20(1):1–8. doi:10.1186/s12877-020-1413-7
37. Hsu W-T, Shen L-J, Lee C-M. Drug-related problems vary with medication category and treatment duration in Taiwanese heart failure outpatients receiving case management. J Formo Med Assoc. 2016;115(5):335–342. doi:10.1016/j.jfma.2015.11.014
38. Wendie TF, Angamo MT. Drug-therapy problems and predictors among hospitalized heart-failure patients: a prospective observational study. Drug Healthc Patient Saf. 2020;12:281. doi:10.2147/DHPS.S268923
39. Murtaza G, Khan MYG, Azhar S, Khan SA, Khan TM. Assessment of potential drug–drug interactions and its associated factors in the hospitalized cardiac patients. Saudi Pharm J. 2016;24(2):220–225. doi:10.1016/j.jsps.2015.03.009
40. Sharma S, Chhetri HP, Alam K. A study of potential drug-drug interactions among hospitalized cardiac patients in a teaching hospital in Western Nepal. Indian J Pharmacol. 2014;46(2):152. doi:10.4103/0253-7613.129303
41. Wright S, Verouhis D, Gamble G, Swedberg K, Sharpe N, Doughty R. Factors influencing the length of hospital stay of patients with heart failure. Eur J Heart Fail. 2003;5(2):201–209. doi:10.1016/S1388-9842(02)00201-5
42. Urbina O, Ferrández O, Luque S, et al. Patient risk factors for developing a drug-related problem in a cardiology ward. Ther Clin Risk Manag. 2015;11:9. doi:10.2147/TCRM.S71749
43. Sewagegn N, Fekadu S, Chanie T. Adherence to self-care behaviours and knowledge on treatment among heart failure patients in Ethiopia: the case of a tertiary teaching hospital. J Pharm Care Health Sys. 2015;10:2376–2419.
44. Mateti U, Rajakannan T, Nekkanti H, Rajesh V, Mallaysamy S, Ramachandran P. Drug-drug interactions in hospitalized cardiac patients. J Young Pharm. 2011;3(4):329–333. doi:10.4103/0975-1483.90246
45. Mohammed S, Poudel S, Laloo F, Madhur A, Robert R, Mathew B. Assessment of drug-related problems in a tertiary care teaching hospital, India. Asian J Pharm Clin Res. 2017;10(2):310–313. doi:10.22159/ajpcr.2017.v10i2.15678
46. Garedow AW, Mulisa Bobasa E, Desalegn Wolide A, et al. Drug-related problems and associated factors among patients admitted with chronic kidney Disease at Jimma University Medical Center, Jimma Zone, Jimma, Southwest Ethiopia: a hospital-based prospective observational study. Int J Nephrol. 2019;2019:1–9. doi:10.1155/2019/1504371
47. Tigabu BM, Daba D, Habte B. Drug-related problems among medical ward patients in Jimma university specialized hospital, Southwest Ethiopia. J Res Pharm Pract. 2014;3(1):1. doi:10.4103/2279-042X.132702
48. Babelghaith SD, Wajid S, Alrabiah Z, et al. Drug-related problems and pharmacist intervention at a general hospital in the Jazan Region, Saudi Arabia. Risk Manag Healthc Policy. 2020;13:373. doi:10.2147/RMHP.S247686
49. Gökçekuş L, Mestrovic A, Basgut B. Pharmacist intervention in drug-related problems for patients with cardiovascular diseases in selected community pharmacies in Northern Cyprus. Trop J Pharm Res. 2016;15(10):2275–2281. doi:10.4314/tjpr.v15i10.29
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

