Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Medical Costs in Patients with Hyperkalemia on Long-Term Sodium Zirconium Cyclosilicate Therapy: The RECOGNIZE II Study
Authors Agiro A, Dwyer JP , Oluwatosin Y, Desai P
Received 9 May 2023
Accepted for publication 11 September 2023
Published 21 September 2023 Volume 2023:15 Pages 691—702
DOI https://doi.org/10.2147/CEOR.S420217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Lin Feng
Abiy Agiro,1 Jamie P Dwyer,2 Yemisi Oluwatosin,3 Pooja Desai3
1US Evidence, US Medical Affairs, AstraZeneca, Wilmington, DE, USA; 2Department of Internal Medicine, University of Utah Health, Salt Lake City, UT, USA; 3US Renal, US Medical Affairs, AstraZeneca, Wilmington, DE, USA
Correspondence: Abiy Agiro, AstraZeneca, US Medical Affairs, 1800 Concord Pike, Wilmington, DE, 19850, USA, Tel +1 302-886-1898, Email [email protected]
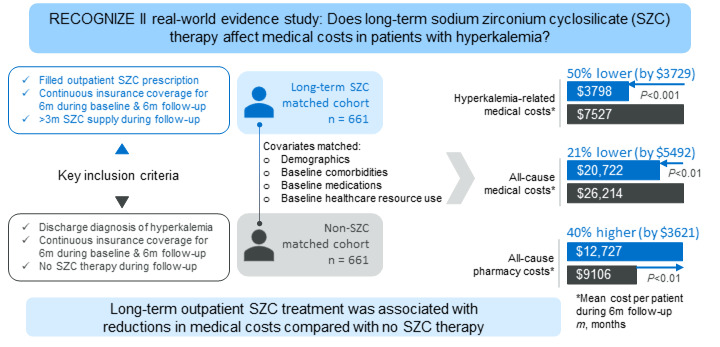
Purpose: Hyperkalemia, defined as abnormally high serum potassium levels of ≥ 5.1 mmol/L, is associated with increased medical costs. This real-world study evaluated the impact of long-term sodium zirconium cyclosilicate (SZC) therapy on medical costs in patients with hyperkalemia.
Patients and Methods: This retrospective, comparative study used claims data from IQVIA PharMetrics® Plus. Patients aged ≥ 18 years with hyperkalemia who had outpatient SZC fills (> 3-month supply over 6 months) between July 2019 and December 2021 and continuous insurance coverage 6 months before and 6 months after the first SZC fill were included. These patients (SZC cohort) were 1:1 exact- and propensity score-matched on baseline variables with patients with hyperkalemia who did not receive SZC (non-SZC cohort). The primary endpoint was hyperkalemia-related medical costs to payers over 6 months.
Results: Each cohort included 661 matched patients. Mean per-patient hyperkalemia-related medical costs were reduced by 49.5% ($3728.47) for the SZC versus non-SZC cohort ($3798.04 vs $7526.51; P< 0.001), whereas mean all-cause medical costs were reduced by 21.0% ($5492.20; $20,722.23 vs $26,214.43; P< 0.01). A 39.8% ($3621.03) increase in all-cause pharmacy costs ($12,727.20 vs $9106.17; P< 0.01) was offset by the medical cost savings.
Conclusion: This study demonstrated that long-term (> 3 months) outpatient treatment with SZC was associated with medical cost savings compared with no SZC therapy.
Plain Language Summary: Hyperkalemia, in which potassium levels in the blood are abnormally high, can lead to significant health issues, including life-threatening heart problems. Patients with kidney disease, heart failure, high blood pressure, and diabetes have an increased risk of experiencing hyperkalemia. It can also be caused by a group of drugs, known as renin-angiotensin-aldosterone system inhibitors (RAASis), that is a key part of treatment for conditions including heart failure and high blood pressure. Sodium zirconium cyclosilicate (SZC) is a treatment for hyperkalemia that lowers potassium levels and, by doing so, can also allow patients to continue taking RAASis. Our aim was to evaluate the effect of long-term (> 3 months) SZC treatment on medical costs (sum of costs for inpatient hospital treatment, emergency department visits, and outpatient treatment) paid by health insurance. We compared medical costs over 6 months for patients with hyperkalemia treated with SZC with those for a matching group of patients with hyperkalemia who did not receive SZC (non-SZC group). We found that the average hyperkalemia-related medical cost for each patient was reduced by $3728.47 (49.5%) for patients treated with SZC versus the non-SZC group. The average medical cost per patient for all causes was also lower for patients treated with SZC, with a reduction of $5492.20 (21.0%); this was larger than the increase in average pharmacy cost per patient of $3621.03 (39.8%) for the SZC versus non-SZC group. Our study shows that long-term treatment with SZC is associated with lower medical costs compared with no SZC treatment.
Keywords: anti-hyperkalemia therapy, elevated blood potassium, healthcare resource utilization, pharmacoeconomics, potassium binder, real-world evidence
Graphical Abstract:
Introduction
Hyperkalemia, defined as abnormally high serum potassium levels of ≥5.1 mmol/L, can lead to potentially fatal cardiac arrhythmia.1,2 In the United States (US), the estimated annual prevalence of hyperkalemia is approximately 2% in the general population but varies according to patient characteristics, with the estimated annual prevalence being >3-fold higher among those with chronic kidney disease (CKD) and/or heart failure (HF).2 Although hyperkalemia is most commonly a complication of CKD and acute kidney failure, the risk of hyperkalemia is also increased with the use of renin-angiotensin-aldosterone system inhibitors (RAASis).1,3
Hyperkalemia is associated with a significant healthcare and economic burden.4–7 Previous US-based studies have shown that patients who experience hyperkalemia incur substantially higher all-cause healthcare costs than matched controls, with inpatient visits being the main driver of increased cost.5,7,8 Healthcare resource utilization has also been shown to be significantly higher among patients who experience hyperkalemia than for matched controls.5,7
Until recently, recommendations for the management of chronic hyperkalemia have included dietary modification, the use of diuretics and the potassium binder sodium polystyrene sulfonate (SPS), and dose modification or discontinuation of medications that elevate potassium levels.9,10 However, restriction of dietary potassium intake alone has limited effect on hyperkalemia risk and may be potentially harmful due to the impact of dietary changes on other beneficial and heart-healthy nutrients.9 These treatment strategies have several limitations, including an increased risk of gout, volume depletion, and worsening kidney function with the use of diuretics and the need to use SPS with caution in patients who are unable to tolerate increases in sodium levels.9,11 Furthermore, because RAASi therapy at maximally-tolerated doses reduces cardiorenal risk, discontinuation or dose reduction of RAASi therapy has the potential to lead to worse cardiorenal outcomes9,11,12 and is thus no longer recommended in recent treatment guidelines unless hyperkalemia cannot be corrected with treatment.13–15
Two newer oral anti-hyperkalemia medications (also known as potassium binders or gastrointestinal cation exchangers), patiromer and sodium zirconium cyclosilicate (SZC), have demonstrated efficacy in patients with chronic hyperkalemia, and their use can allow patients to continue RAASi therapy without dose modification.9,13,14 Before the approval of patiromer in 2015 and SZC in 2018, the only available oral anti-hyperkalemia therapy was SPS.16 However, SPS is associated with substantial toxicity, including hypomagnesemia, hypocalcemia, and gastrointestinal adverse events, and is thus rarely used long term.17,18 The newer oral anti-hyperkalemia medications, patiromer and SZC, are recommended for the management of hyperkalemia and to allow optimization of RAASi therapy in patients with CKD14 and/or HF with reduced ejection fraction.13
Long-term use of newer oral anti-hyperkalemia therapies has the potential to reduce healthcare resource utilization and costs for patients with chronic hyperkalemia. A retrospective study demonstrated a 19% reduction in the rate of spending for all-cause medical costs among patients on patiromer therapy compared with those who did not receive any oral anti-hyperkalemia therapy, with a cost reduction of 34% in patients with inpatient or emergency department (ED) visits.19 These reductions offset the increase in all-cause outpatient pharmacy costs in this study.19 In the non-comparative, real-world RECOGNIZE I study in patients with hyperkalemia who received SZC treatment in the outpatient setting, the proportions of patients with hyperkalemia-related and all-cause inpatient hospitalizations were significantly reduced among patients who received long-term (>90 days) versus short-term (≤90 days) SZC therapy.20 This suggests that long-term SZC therapy may also be associated with reduced medical costs in patients with hyperkalemia, although this was not analyzed in the RECOGNIZE I study.
The aim of the current real-world study, RECOGNIZE II (Real-world Cost Outcomes among Patients on Sodium Zirconium Cyclosilicate Therapy for Hyperkalemia), was to evaluate the impact of long-term outpatient SZC therapy on hyperkalemia-related and all-cause medical costs compared with those of a propensity score-matched group of patients with any hyperkalemia (1 or more hyperkalemia events) not treated with SZC therapy.
Materials and Methods
Study Design
RECOGNIZE II was a retrospective, observational, propensity score-matched cohort study that used de-identified medical and pharmacy claims data for patients with commercial insurance. The data source for the study was the PharMetrics® Plus database, representative of the commercially insured US national population for patients under 65 years of age, which was accessed via the Instant Health Data (IHD) platform. The database includes a diverse range of geographic areas, employers, providers, therapy areas, and payers across the US and captures discharge diagnoses from inpatient stays and outpatient diagnoses, outpatient procedures, and outpatient prescriptions.
This study utilized de-identified data; therefore, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514(e).
Study Population
Eligible patients were aged ≥18 years, with a discharge diagnosis of hyperkalemia or a filled prescription for outpatient SZC therapy between July 2019 and December 2021. Patients were required to have continuous insurance enrollment for ≥6 months prior to their index date (baseline) and for ≥6 months after their index date (follow-up). The index date was defined as the date of the first SZC prescription fill for the SZC cohort and as the date of the hyperkalemia discharge diagnosis for the non-SZC cohort. All patients in the SZC cohort had at least one pharmacy claim for an SZC prescription fill and were required to have received >3 months’ supply of SZC over the 6-month follow-up period. Patients in the non-SZC cohort were required to have at least one diagnosis of hyperkalemia and no outpatient anti-hyperkalemia therapy (SPS, patiromer, or SZC) on their index date. The non-SZC cohort had no outpatient SZC therapy during the 6-month follow-up period. Patients with medical costs in the top 0.5 percentile were excluded as outliers from both cohorts before matching.
Outcomes and Objectives
The primary endpoint was hyperkalemia-related medical costs associated with inpatient hospitalizations, ED visits, and outpatient visits during the 6-month follow-up period, identified by the use of a diagnosis code for hyperkalemia in any diagnosis position. Secondary endpoints were hyperkalemia-related medical costs by setting (inpatient hospitalizations, ED visits, and outpatient visits). Exploratory endpoints were all-cause medical costs and all-cause pharmacy costs for payers.
Costs were compared between matched patients in the SZC and non-SZC cohorts. Both exact and propensity score matching were used to 1:1 match eligible SZC patients with non-SZC patients. Costs for payers were based on health plan paid amounts rather than charged or billed amounts. All costs were adjusted for inflation to 2021 US dollars.
Statistical Analysis
Mean and standard deviation (SD) were used to describe continuous variables and counts, and percentages were used for categorical variables.
To adjust for baseline confounding variables, a propensity score (the probability of filling an outpatient prescription for SZC versus not filling an SZC prescription) was estimated, conditioned on baseline covariates, using a multivariable logistic regression model that contained potential confounders at baseline. Patients in the SZC cohort were matched 1:1 with non-SZC-treated patients based on demographic variables and baseline comorbidities, medications, and healthcare resource utilization using a caliper of 0.02 without replacement and the greedy nearest neighbor algorithm. The covariates used for matching are listed in Supplementary Table 1. A standardized mean difference of <0.2 (<20%) indicated that the multivariable regression adjustment was adequate. In addition to propensity score matching, exact matching was done for highly relevant factors (ie, age <65 years, baseline all-cause inpatient hospitalizations and baseline diagnoses of CKD, end-stage kidney disease, and hyperkalemia). The propensity scores were generated first, then exact matching was performed. Finally, the propensity score was used to match patients in the SZC cohort with patients in the non-SZC-treated cohort who met the same exact matching criteria.
The propensity score was generated using baseline covariates that were anticipated to be strongly associated with filling a prescription for SZC and/or independently associated with medical cost outcomes. The baseline covariates were selected a priori based on clinical expertise and the expected availability of the covariates within the data source used for the study.
The risk of hyperkalemia increases with age and it is more prevalent in males, whereas cost outcomes are highly dependent on payer type and geographic location. Therefore, demographic attributes (such as age, sex, payer type, and census region) were included in the propensity score-matching procedure. In addition, because hyperkalemia is strongly associated with increased aldosterone secretion, chronic conditions that can benefit from aldosterone inhibition (such as hypertension, type 2 diabetes, dyslipidemia, CKD, end-stage kidney disease, coronary artery disease, and HF) were relevant covariates. Similarly, prescription fills for medications that treat these chronic conditions, such as RAASis (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor/neprilysin inhibitors, and mineralocorticoid receptor antagonists), glucose-lowering medications (metformin, sulfonylureas, dipeptidylpeptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium–glucose cotransporter-2 inhibitors, insulin, and thiazoli-dinediones), and cardiovascular medications (alpha-blockers, beta-blockers, calcium channel blockers, anticoagulants, antiplatelets, lipid-lowering drugs, loop diuretics, and thiazide diuretics) were pertinent covariates.
The arrhythmogenic effects of hyperkalemia make arrhythmias a potent covariate. Baseline medications (including oral anti-hyperkalemia therapy in the form of SPS, patiromer, or SZC prescription fills) or procedures (such as dialysis) that directly treat hyperkalemia were cogent covariates. Because cost outcomes are affected by baseline healthcare resource utilization (such as prior hospitalizations, prior ED visits, and prior costs) and multimorbidity (as measured by the Charlson Comorbidity Index), these were also well-founded covariates.
Because the data source used in the study comprised administrative insurance claims data collected for the purposes of reimbursements, no laboratory results or electronic medical record data were available. Therefore, it was not possible to include additional covariates that would have been relevant, such as hyperkalemia severity and changes in estimated glomerular filtration rates.
All analyses were performed within the IHD software (Panalgo, Boston, MA) that is integrated with R, version 3.2.1. (R Foundation for Statistical Computing, Vienna, Austria). No analytic dataset was downloaded from the IHD platform.
For all study endpoints, multivariate adjustment was achieved by 1:1 matching within each matched set of patients; therefore, one-way analysis of variance testing was sufficient to detect statistically significant differences in costs without further multivariate regression adjustment.
Results
Patients
In total, 4615 patients who filled an outpatient prescription for SZC and 406,765 patients with a diagnosis of hyperkalemia but no SZC prescription fill were identified from the IQVIA PharMetrics Plus claims database (Figure 1). After exclusion criteria were applied and before matching, the SZC cohort included 668 patients and the corresponding non-SZC cohort included 131,880 patients. After matching, the SZC and non-SZC cohorts each included 661 matched patients; the other 7 patients from the SZC cohort were excluded because they could not be matched with a patient from the non-SZC cohort.
The demographics and clinical characteristics of the cohorts were well balanced after propensity score matching (Table 1). The majority of patients in the non-SZC and SZC cohorts were male (64.3% and 66.9%, respectively) and approximately three-quarters had commercial insurance (72.3% and 77.5%, respectively). The most common comorbidities in the non-SZC and SZC cohorts were hypertension (91.2% and 87.7%, respectively), type 2 diabetes (64.9% and 60.4%, respectively), and CKD (61.9% in both cohorts). The majority of patients (62.5% in both cohorts) had a diagnosis history of recurrent hyperkalemia; the remaining patients (37.5% in both cohorts) had new-onset hyperkalemia. The median time from the diagnosis of hyperkalemia to the initiation of SZC treatment was 15 days. The standardized mean difference in propensity score of all variables was <0.2, indicating a good balance between the matched pairs.
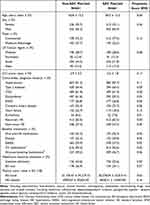 |
Table 1 Patient Demographics, Comorbidities, Baseline Treatments, and Healthcare Resource Utilization After 1:1 Matching |
During follow-up, patients in the SZC cohort received a mean ± SD of 174.1 ± 49.6 days’ supply of SZC. A total of 31 (4.7%) patients in the SZC cohort and 97 (14.7%) patients in the non-SZC cohort had a follow-up indicator for an outpatient prescription for SPS or patiromer. To preserve the real-world applicability of the study results, patients who obtained outpatient SPS or patiromer prescription fills during the follow-up period were retained in both groups.
Hyperkalemia-Related Medical Costs
Hyperkalemia-related medical costs were significantly reduced in the SZC cohort compared with those in the non-SZC cohort. Across all settings (inpatient, ED, and outpatient), mean ± SD hyperkalemia-related medical costs per patient over 6 months were $3798.04 ± $13,277.88 for the SZC cohort versus $7526.51 ± $20,892.48 for the non-SZC cohort. Mean per-patient hyperkalemia-related medical costs were reduced by 49.5% ($3728.47 reduction; 95% confidence interval [CI] $1839.59 to $5617.35; P<0.001) for the SZC cohort versus the non-SZC cohort (Figure 2).
The majority of hyperkalemia-related medical costs were for inpatient admissions, which comprised 65.2% of hyperkalemia-related medical costs in the SZC cohort and 76.9% in the non-SZC cohort. There was a statistically significant 57.2% ($3309.16; 95% CI $1605.54 to $5012.78) reduction in mean medical costs associated with hyperkal-emia-related inpatient admissions for the SZC cohort compared with the non-SZC cohort ($2475.65 ± $10,856.61 vs $5784.81 ± $19,509.48; P<0.001). This difference was the main driver for the reduction in hyperkalemia-related medical costs in the SZC versus non-SZC cohort across all settings.
ED visits accounted for 6.3% of the hyperkalemia-related medical costs in the SZC cohort and 4.7% of these costs in the non-SZC cohort. There was a 32.8% reduction ($116.32; 95% CI –$187.04 to $419.68) in mean hyperkalemia-related ED medical cost in the SZC versus non-SZC cohort (mean ± SD medical costs per patient $237.99 ± $3412.42 vs $354.31 ± $2040.00). However, the difference was not statistically significant (P=0.45).
Outpatient visits accounted for 28.6% of the hyperkalemia-related medical costs in the SZC cohort and 18.4% in the non-SZC cohort. The 21.8% reduction ($302.99; 95% CI –$356.66 to $962.64) in mean outpatient medical costs between the SZC and non-SZC cohorts was also not significant (P=0.37); mean ± SD costs per patient were $1084.40 ± $5524.04 for the SZC cohort versus $1387.39 ± $6649.95 for the non-SZC cohort.
All-Cause Medical and Pharmacy Costs
Mean all-cause medical costs (inpatient, ED, and outpatient) were reduced by 21.0% ($5492.20; 95% CI $540.53 to $9443.87; P<0.01) for the SZC versus the non-SZC cohort (Figure 3). The mean ± SD all-cause medical costs per patient were $20,722.23 ± $32,966.54 in the SZC cohort and $26,214.43 ± $39,940.89 in the non-SZC cohort.
Outpatient visits accounted for the largest proportion of all-cause medical costs in the SZC cohort (66.1%) and approximately half of medical costs in the non-SZC cohort (50.9%). Inpatient hospitalizations represented 29.9% of medical costs in the SZC cohort and 44.7% in the non-SZC cohort, with ED visit medical costs comprising the remaining 4.0% of medical costs in the SZC cohort and 4.4% in the non-SZC cohort.
The mean all-cause inpatient medical cost per patient was significantly lower in the SZC cohort ($6200.65 ± $18,707.88) than that in the non-SZC cohort ($11,716.32 ± $28,429.67), corresponding to a 47.1% reduction in mean all-cause medical cost of $5515.67 (95% CI $2918.84 to $8112.50; P<0.001).
The differences between the SZC and non-SZC cohorts in all-cause ED and outpatient medical costs were not statistically significant. Mean ± SD all-cause ED costs per patient were $824.13 ± $4088.54 in the SZC cohort and $1142.05 ± $3444.80 in the non-SZC cohort (27.8% reduction in mean cost of $317.92; 95% CI –$90.02 to $725.86; P=0.18). Mean ± SD outpatient costs per patient were $13,697.45 ± $23,256.08 in the SZC cohort and $13,356.06 ± $22,632.13 in the non-SZC cohort (2.6% increase of $341.39; 95% CI –$2817.51 to $2134.74; P=0.49).
Mean all-cause pharmacy cost per patient was higher for the SZC cohort ($12,727.20 ± $23,657.17) than that for the non-SZC cohort ($9106.17 ± $23,315.46), with a 39.8% increase in mean cost of $3621.03 (95% CI $1086.57 to $6155.51) per patient (P<0.01).
Discussion
This non-interventional, retrospective, observational, propensity score-matched, comparative cohort study demonstrated a significant reduction in both hyperkalemia-related (49.5%) and all-cause medical (21.0%) costs with long-term SZC treatment compared with costs for no SZC treatment in patients with hyperkalemia. A reduction in medical costs for inpatient hospitalizations was the main driver of the reductions in hyperkalemia-related and all-cause medical costs in the SZC cohort versus the matched non-SZC control cohort. The RECOGNIZE II study findings are supported by those of a previous noncomparative, single-arm, real-world study, RECOGNIZE I, which found that long-term outpatient SZC treatment (>90 days) was associated with a reduction in the proportions of patients with hyperkalemia-related hospital-izations compared with short-term SZC therapy (≤90 days).20
A similar reduction in all-cause medical costs with long-term anti-hyperkalemia therapy was also reported in an earlier, US-based, real-world insurance claims database study of long-term treatment with patiromer.19 In this matched cohort study, 6 months of continuous patiromer exposure was associated with a significant 19% reduction in all-cause medical costs ($5212.00 per patient) compared with no oral anti-hyperkalemia therapy (P<0.05).19
Although previous studies have shown that hyperkalemia is associated with increases in both ED visits and outpatient care,7 long-term SZC treatment was not associated with significant differences in hyperkalemia-related or all-cause ED or outpatient medical costs in the current study. In the RECOGNIZE II study, ED visits that were not followed by inpatient admission accounted for only a small proportion (<7%) of hyperkalemia-related and all-cause medical costs, which made it less likely that differences in ED medical costs between cohorts would be statistically significant. The non-significant difference in hyperkalemia-related outpatient visits may suggest similar levels of engagement with outpatient healthcare providers in the SZC and non-SZC cohorts. Because SZC is only indicated to treat hyperkalemia, medical costs for all-cause outpatient visits were not expected to differ between cohorts.
An important advantage of long-term treatment with a newer oral anti-hyperkalemia medication such as SZC is the potential to enable optimal RAASi therapy.13,14 Although the current study did not evaluate the impact of long-term SZC treatment on the use of RAASi therapy in patients with hyperkalemia, other studies have found that patients treated with newer oral anti-hyperkalemia therapies have been able to continue RAASi treatment. A real-world study found that long-term patiromer treatment (>6 months) was associated with high rates (approximately 80%) of RAASi therapy continuation.21 Long-term clinical studies of SZC treatment in patients with hyperkalemia demonstrated that 89% of patients who were on RAASi therapy at baseline continued RAASi therapy during one year of SZC treatment.22,23 In addition, the OPTIMIZE I real-world study found that approximately 83% of patients with hyperkalemia were able to maintain RAASi therapy during long-term SZC treatment.24,25
The RECOGNIZE II study has some limitations associated with the use of a retrospective insurance claims database, particularly as these databases were not designed for research use. The IQVIA PharMetrics Plus medical and pharmacy claims database did not cover laboratory results. There is potential for misclassification of disease status, study outcomes, and covariates in claims data because of coding limitations. Electronic medical record data, including clinical and laboratory data, are not captured in claims data. It was therefore not possible to account for the effects of hyperkalemia severity and changes in estimated glomerular filtration rates on medical costs. As such, not all possible variables that can determine where a patient would receive SZC therapy were included in the propensity score matching procedure. Additionally, although the patient characteristics of the study cohorts appeared to be well balanced after propensity score matching, residual confounding may be possible. Therefore, it is possible that reductions in medical costs in patients receiving long-term SZC treatment were because of factors other than long-term SZC treatment that were not controlled for in the matching procedure.
The lack of serum potassium values in claims data has limited the options to identify alternative index dates for patients not treated with SZC and remains as a key limitation with unmeasured confounding. Because the diagnosis code for hyperkalemia can cover a wide range of severity with regard to elevated serum potassium values (≥5.1 mmol/L) and because the severity of hyperkalemia determines healthcare resource utilization, including the decision to hospitalize a patient, relying on the timing of diagnosis and treatment to define index dates for the non-SZC and SZC cohorts, respectively, can introduce differential bias in exposure between the two cohorts. Therefore, future research that can index and match patients on serum potassium values is suggested.
Because continuous insurance coverage was required throughout the baseline and follow-up periods, the results of this study are not generalizable to uninsured patients and did not capture patients who discontinued or changed insurance plans during follow-up. In addition, the claims data source used in this study is not generalizable to the Medicare (age ≥65 years) population, and caution is required when applying the study results to older patients.
The primary endpoint, hyperkalemia-related medical cost, was based on claims with hyperkalemia in any diagnosis position; hyperkalemia is not usually listed as the primary diagnosis in claims data. Thus, hyperkalemia was not necessarily the primary reason for hospitalization or ED or outpatient treatment. Given that the reimbursement landscape in the US induces a practice pattern of placing medical procedures or diagnosis codes that generate higher payments in the primary diagnosis position, hyperkalemia is rarely coded in the primary diagnosis position. Considering the extent of multimorbidity that exists in patients with hyperkalemia,26 the results of the current study should be interpreted in light of the limitation that it is not feasible to use claims data to fully dissociate hyperkalemia from underlying chronic conditions. Furthermore, in the study of multimorbidity, the ten most common comorbid conditions among adult patients with hyperkalemia included four conditions that have less clinically plausible associations with hyperkalemia (low back pain, chronic thyroid disease, chronic obstructive pulmonary disease, and nonspecific gastritis/dyspepsia).26
The strengths of this study include the diverse representation of geographic areas, employers, providers, and payers in the IQVIA PharMetrics Plus database, which provides a broadly representative population of patients with hyperkalemia in real-world clinical practice in the US. Because this study used real-world data, the results are likely to be more representative of the patient population and medical cost patterns in clinical practice than those of studies using models based on clinical trial data. Furthermore, this study adjusted for multimorbidity that has a plausible connection with hyperkalemia (such as hypertension, type 2 diabetes, dyslipidemia, CKD, coronary artery disease, and HF). The use of propensity score matching and exact matching improves the internal validity of the study by mitigating some potential biases associated with the use of insurance claims data.
Conclusions
In patients with hyperkalemia, long-term SZC treatment (for >3 months over a 6-month follow-up period) was associated with a significant reduction in hyperkalemia-related and all-cause medical costs compared with no SZC treatment. These reductions were driven by significant reductions in inpatient medical costs. Although there was a significant increase in all-cause pharmacy cost in the SZC compared with the non-SZC cohort, this was offset by a larger reduction in all-cause medical cost. The results of this research study demonstrated that long-term SZC treatment is associated with reductions in medical costs in patients with hyperkalemia.
Abbreviations
CI, confidence interval; CKD, chronic kidney disease; ED, emergency department; HF, heart failure; IHD, Instant Health Data; RAASi, renin-angiotensin-aldosterone system inhibitor; SD, standard deviation; SPS, sodium polystyrene sulfonate; SZC, sodium zirconium cyclosilicate; US, United States.
Data Sharing Statement
This was a claims database analysis using IQVIA PharMetrics® Plus closed claims data obtained under license from IQVIA Inc. The raw data cannot be publicly shared because it was obtained from IQVIA and as per signed agreement between AstraZeneca and IQVIA Inc. However, we have provided all relevant data in the manuscript that supports the research objectives and conclusions. We confirm that interested researchers can reach out to IQVIA Inc. to access the data. For further information on data access, please contact IQVIA.
Ethics Approval and Informed Consent
This study utilized de-identified data; therefore, no institutional review board waiver of informed consent approval or exemption was required, as per article 45 §CFR 164.514(e).
Acknowledgments
Raewyn M. Poole of inScience Communications, Springer Healthcare, and Marie Cheeseman, on behalf of inScience Communications, Springer Healthcare, provided medical writing support in accordance with Good Publication Practice, funded by AstraZeneca.
Jamie P. Dwyer, Abiy Agiro, Pooja Desai, Yemisi Oluwatosin. Medical costs in patients with hyperkalemia on long-term sodium zirconium cyclosilicate therapy: RECOGNIZE II study. Poster presentation at the NKF 2023 Spring Clinical Meeting, April 11–15, 2023, Austin, TX, USA.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The RECOGNIZE II study was funded by AstraZeneca. AstraZeneca employees were involved in study concept and design, statistical analysis, and data interpretation. The final version of the manuscript underwent AstraZeneca medical and legal review prior to submission.
Disclosure
Jamie P. Dwyer has acted as a scientific consultant for AstraZeneca and received fees from AstraZeneca for the conduct of this study; has received fees from Sanofi and CSL Behring as part of a steering committee; has received fees from Novo Nordisk for outcome adjudication for a clinical trial; has received fees from Boehringer Ingelheim and Lilly for study design; and has received personal fees from Bayer, CinCor, Caladrius Biosciences, Inversago, GlaxoSmithKline LLC, and ProKidney. Abiy Agiro and Pooja Desai are employees and stockholders of AstraZeneca. Yemisi Oluwatosin was an employee and stockholder of AstraZeneca at the time of the study.
References
1. Montford JR, Linas S. How dangerous is hyperkalemia? J Am Soc Nephrol. 2017;28(11):3155–3165. doi:10.1681/ASN.2016121344
2. Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34(6):971–978. doi:10.1080/03007995.2018.1433141
3. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–284. doi:10.1016/j.ijcard.2017.07.035
4. Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36(8):1333–1341. doi:10.1080/03007995.2020.1775072
5. Betts KA, Woolley JM, Mu F, et al. Postdischarge health care costs and readmission in patients with hyperkalemia-related hospitalizations. Kidney Int Rep. 2020;5(8):1280–1290. doi:10.1016/j.ekir.2020.06.004
6. Dashputre AA, Gatwood J, Sumida K, et al. Association of dyskalemias with short-term health care utilization in patients with advanced CKD. J Manag Care Spec Pharm. 2021;27(10):1403–1415. doi:10.18553/jmcp.2021.27.10.1403
7. Betts KA, Woolley JM, Mu F, Xiang C, Tang W, Wu EQ. The cost of hyperkalemia in the United States. Kidney Int Rep. 2018;3(2):385–393. doi:10.1016/j.ekir.2017.11.003
8. Sharma A, Alvarez PJ, Woods SD, Fogli J, Dai D. Healthcare resource utilization and costs associated with hyperkalemia in a large managed care population. J Pharm Health Serv Res. 2021;12(1):35–41. doi:10.1093/jphsr/rmaa004
9. Palmer BF, Carrero JJ, Clegg DJ, et al. Clinical management of hyperkalemia. Mayo Clin Proc. 2021;96(3):744–762. doi:10.1016/j.mayocp.2020.06.014
10. Clase CM, Carrero J-J, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(1):42–61. doi:10.1016/j.kint.2019.09.018
11. Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S220.
12. Johnson M, Morrison FJ, McMahon G, Su M, Turchin A. Outcomes in patients with cardiometabolic disease who develop hyperkalemia while treated with a renin-angiotensin-aldosterone system inhibitor. Am Heart J. 2023;258:49–59. doi:10.1016/j.ahj.2023.01.002
13. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–e894. doi:10.1161/CIR.0000000000001062
14. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3 Suppl):S1–S87. doi:10.1016/j.kint.2020.11.003
15. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5 Suppl):S1–S127. doi:10.1016/j.kint.2022.06.008
16. Shrestha DB, Budhathoki P, Sedhai YR, et al. Patiromer and sodium zirconium cyclosilicate in treatment of hyperkalemia: a systematic review and meta-analysis. Curr Ther Res Clin Exp. 2021;95:100635. doi:10.1016/j.curtheres.2021.100635
17. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518–1526. doi:10.1093/ndt/gfz150
18. Rahman S, Marathi R. Sodium polystyrene sulfonate. In: StatPearls [Internet]. Treasure Island (FL): StatsPearls Publishing; Updated July 4, 2022.
19. Desai NR, Alvarez PJ, Golestaneh L, Woods SD, Coca SG, Rowan CG. Healthcare utilization and expenditures associated with hyperkalemia management: a retrospective study of Medicare Advantage patients. J Med Econ. 2021;24(1):1025–1036. doi:10.1080/13696998.2021.1965389
20. Pollack CV Jr, Agiro A, Mu F, et al. Impact on hospitalizations of long-term versus short-term therapy with sodium zirconium cyclosilicate during routine outpatient care of patients with hyperkalemia: the RECOGNIZE I study. Expert Rev Pharmacoecon Outcomes Res. 2023;23(2):241–250. doi:10.1080/14737167.2023.2161514
21. Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States veterans. Postgrad Med. 2020;132(2):176–183. doi:10.1080/00325481.2019.1706920
22. Roger SD, Spinowitz BS, Lerma EV, et al. Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol. 2019;50(6):473–480. doi:10.1159/000504078
23. Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month Phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. doi:10.2215/CJN.12651018
24. Agiro A, Amin A, Mu F, et al. Real-world RAAS inhibitor use and its predictors among patients initiating sodium zirconium cyclosylicate.
25. Agiro A, Amin A, Mu F, et al. Real-world use of renin-angiotensin-aldosterone system inhibitors in patients with heart failure and hypertension initiating sodium zirconium cyclosilicate: the OPTIMIZE study [abstract]. Circulation. 2021;144(Suppl 1):Abstract 10620. doi:10.1161/circ.144.suppl_1.10620
26. Dai D, Sharma A, Alvarez PJ, Woods SD. Multiple comorbid conditions and healthcare resource utilization among adult patients with hyperkalemia: a retrospective observational cohort study using association rule mining. J Multimorb Comorb. 2022;12:26335565221098832. doi:10.1177/26335565221098832
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



